Abstract
The cholinergic nervous system has been demonstrated to attenuate the inflammatory response during sepsis via the inhibitory action of acetylcholine (ACh) on macrophages. These findings were largely based on experimental sepsis models using endotoxin as the inducing agent. Herein, however, we report that the specific inhibition of acetylcholinesterase (AChE) renders animals more resistant to infection by a virulent strain of Salmonella enterica serovar Typhimurium, a Gram-negative enteric pathogen. Inhibition of AChE was induced by a subchronic exposure to paraoxon, a potent anti-cholinesterase metabolite of the organophosphorous compound parathion. Our findings indicate that inhibition of AChE enhanced survival of infected mice in a dose-dependent fashion and this correlated with efficient control of bacterial proliferation in target organs. Immunologically, inhibition of AChE enabled the animals to mount a more effective inflammatory anti-microbial response, and to secrete higher levels of interleukin-12, a key T helper type 1-promoting cytokine. The ACh-induced enhancement in resistance to infection was abrogated by co-administration of an oxime which can reactivate AChE. Hence, in a model of Gram-negative bacterial infection, cholinergic stimulation is shown to enhance the anti-microbial immune response leading to effective control of bacterial proliferation and enhanced animal survival.
Keywords: acetylcholine, cholinergic anti-inflammatory pathway, innate immunity, organophosphate, salmonella infection
Introduction
Inflammation is an important defence mechanism protecting the organism from the devastating effects of invading pathogens or tissue injury. This process is mediated by a number of cytokines, including tumour necrosis factor-α (TNF-α), released by macrophages and other cells of the innate immune system. Unrestrained inflammatory reaction, however, has a deleterious effect that can result in systemic inflammation and lethal shock. Therefore, the inflammatory response needs to be tightly regulated. Regulatory mechanisms that act to contain excessive inflammation include humoral factors, e.g. glucocorticoids and anti-inflammatory cytokines such as interleukin-10 (IL-10), and neuronal elements, including both the sympathetic and the parasympathetic nervous systems (for review, see ref. 1). It has been suggested that an ‘inflammatory reflex’ involving the vagus nerve plays a crucial role in modulating immune function and controlling inflammation.2,3 Afferent fibres of the vagus nerve are stimulated by pro-inflammatory cytokines and relay the impulse to the nucleus tractus solitarii in the medulla oblongata and to higher brain centres. From there, efferent vagus nerve fibres innervate lymphatic organs, thereby inhibiting the production of pro-inflammatory cytokines, in particular TNF-α. As the neurotransmitter acetylcholine released by vagus nerve terminals is crucial for inhibiting the inflammatory response, this pathway has been termed the ‘cholinergic anti-inflammatory pathway’.2 Several lines of evidence support this mechanism. During endotoxaemia, electric stimulation of the vagus nerve reduces systemic TNF-α levels, and pro-inflammatory cytokines are elevated after vagotomy.4,5 Vagotomy exacerbates inflammation in murine models of aseptic pancreatitis6 and colitis,7 whereas nicotine treatment attenuates the inflammatory response. Acetylcholine inhibits TNF-α release by binding to the α7 type of the nicotinic acetylcholine receptor, which is expressed by macrophages.5
Whereas cholinergic activation reduces disease activity in sterile inflammation (reviewed in ref. 8), the role of cholinergic modulation in the immune response to microbial infection is still a matter of controversy. Vagotomy significantly increased mortality in murine polymicrobial sepsis9 and nicotine administration improved survival in a murine sepsis model induced by caecal ligation and puncture.10 In contrast, mortality was enhanced by nicotine treatment in a septic peritonitis model induced by intraperitoneal injection of Escherichia coli.11 In this E. coli-induced peritonitis model, although nicotine treatment reduced pro-inflammatory cytokine release, it also resulted in a concurrent reduction in neutrophil influx and diminished anti-microbial activity, leading to impairment of bacterial clearing and consequently heightened mortality.
One way of potentially stimulating the ‘cholinergic anti-inflammatory pathway’ is through the administration of acetylcholinesterase (AChE) inhibitors. This enzyme is responsible for terminating the action of the neurotransmitter acetylcholine, and its inhibition leads to acetylcholine accumulation. Studies assessing the effect of systemic AChE inhibition upon mortality after pathogen-induced sepsis have, however, yielded conflicting results. Using a septic shock model by injecting endotoxin derived from E. coli, one study failed to detect any beneficial effect of additional injections of the AChE inhibitor neostigmine.12 In contrast, another group observed that intraperitoneal injections of either nicotine or the AChE inhibitors physostigmine or neostigmine significantly reduced lethality after sepsis induced by caecal ligation.13 In addition, significant reductions in the concentrations of TNF-α and other circulating pro-inflammatory cytokines as well as in the binding activity of nuclear factor-κB were observed following the injection of AChE inhibitors.13 However, in neither of the two above studies were AChE activities determined, precluding any direct correlation between the observed effects and AChE levels.
Clearly, any modulation of immune system activity must be performed with the view of maintaining an efficient immune response against infections while reducing/minimizing the harmful effects of dysregulated production of pro-inflammatory mediators. The differences in the reported findings between the models are a reflection of whether the experimental systems use live pathogenic organisms or non-viable bacterial products. Using a different approach, we have recently inhibited AChE activity by administering the organophosphorous compound (OPC) paraoxon over a period of 6 weeks.14 The OPCs include a wide variety of substances with a broad range of physical, chemical and biological properties. They are toxic because they irreversibly inhibit AChE and exposure to lethal OPC doses leads to death as the result of an acute cholinergic crisis resulting in cardiovascular and respiratory failure.15 The current standard antidotal treatment includes a combination of atropine and oxime-type AChE reactivators, which cleave the phosphyl moiety from the AChE molecule, thereby restoring its enzymatic function. The therapeutic usefulness of the established oximes pralidoxime and obidoxime is still controversial,16–18 so new asymmetric bispyridinium oximes (K-27 and K-48) have been synthesized,19 which possess low toxicity and promising reactivation parameters.18,20,21
In a previous study, we demonstrated that a 6-week administration of low doses (0·5 mg/kg body weight) of the OPC paraoxon, the active metabolite of the insecticide parathion, had no effect on the survival of mice exposed to a systemic infection with Salmonella enterica serovar Typhimurium (hereafter S. typhimurium).14 However, during this treatment regimen, blood AChE activity was only reduced during the first 3 weeks and returned to normal levels thereafter. The aim of the present study therefore was to determine whether exposure to low doses of paraoxon for 1, 2 or 3 weeks, time-points at which AChE activity is significantly decreased, had any effect on survival following infection with a pathogenic strain of S. typhimurium. In this animal model, control of the infection requires a robust T helper type 1 (Th1) response as well as neutralizing anti-Salmonella antibodies.22–24 The essential roles of pro-inflammatory cytokines, including IL-12, interferon-γ (IFN-γ) and TNF-α, in resistance to Salmonella infection in mice as well as in humans is well documented.25–28 The present data demonstrate that cholinergic activation of splenic macrophages, through the inhibition of AChE by subchronic exposure to paraoxon, confers enhanced protection against a lethal infection with a virulent strain of S. typhimurium. Survival of paraoxon-exposed animals correlated with an enhanced immune response to infection by splenic macrophages, the ability to produce higher IL-12 serum cytokine levels, and more efficient control of bacterial growth in the target organs. The decreased mortality of paraoxon-exposed mice was directly dependent on AChE levels because the co-administration of the AChE reactivator, K-27, completely abolished the protective effect elicited by paraoxon.
Material and methods
Experimental animals
BALB/c mice were purchased from Harlan Olac (Bicester, UK) and bred in the animal facility of the Faculty of Medicine and Health Sciences, UAE University. Male mice aged 8–10 weeks (weight range 20·2–26·9 g) were used for the experiments. Mice received rodent chow and water ad libitum. All studies involving animals were carried out in accordance with, and after approval of the animal research ethics committee of the Faculty of Medicine and Health Sciences, UAE University (Protocol no. AE/06/81).
Chemicals
Paraoxon (Sigma Chemical Co., St Louis, MO) stock solution (100 mmol/l) was prepared in dry acetone. Working solution for intraperitoneal (i.p.) injection was prepared ex tempore in pyrogen-free saline to a final concentration of 80 nmol/ml. Each mouse received 40 nmol/day of paraoxon in 0·5 ml volume (equivalent to 0·44 mg/kg body weight). Control mice received an equivalent volume of pyrogen-free saline (Sigma). The K-27 oxime was generously donated by Dr Kuca (University of Defence, Hradec Kralove, Czech Republic). A 10 mm solution of K-27 was prepared ex tempore in pyrogen-free saline. Each mouse received a daily injection of 0·5 ml, equivalent to 5 μm/day of K-27.
Experimental protocol
Twenty age-matched BALB/c mice were randomly assigned into two groups (10 animals per group). Group I served as control and received daily injection of sterile saline. Group II mice received daily injection of 40 nmol paraoxon. All injections were given i.p. daily for 5 days, followed by a 2-day break, and this cycle was repeated for a total of 3 weeks. Blood from the tail vein was collected on day 5 of the 7-day cycle, routinely 30 min after paraoxon or saline injection, for the determination of red blood cell (RBC) AChE activity. Mice were also weighed at this time. At the end of the treatment, all animals were killed and blood, thymus and spleen were collected. Serum was prepared and stored frozen until further analysis. In some experiments, treated mice were infected (routinely following the 2-day break) via the oral route with a virulent strain of S. typhimurium and either killed at specific time-points, as indicated below, or followed for survival for up to 60 days.
Bacterial strain and growth conditions
The characteristics and the preparation of log-phase bacterial suspensions of S. typhimurium strain SL1344 have been described elsewhere29 and the procedure for oral inoculation was detailed previously.30 For all experiments, bacterial doses were confirmed by colony-forming units (CFU) plate counts.
Enumeration of bacteria in organ homogenates
To determine the bacterial load in target organs, mesenteric lymph nodes (MLN), spleens and livers were removed aseptically and homogenized in 2 ml cold sterile saline, as previously described.29 A 100-μl aliquot of the homogenate, or an appropriate dilution, was plated on T-soy agar plates and viable CFUs were determined after an overnight incubation. Duplicate plates were set up for each dilution or experimental group.
Cell preparation
Erythrocyte-depleted spleen cell suspensions were prepared in supplemented RPMI-1640 medium with 5% fetal calf serum (GibcoBRL, Paisley, UK), essentially as previously described.22 Cells were cultured in the presence or absence of Concanavalin A (Con A) or lipopolysaccharide (LPS) at 2·5 and 10 μg/ml concentrations, respectively. Cells were incubated for 24–48 hr at 37° with 5% CO2. Culture supernatants were collected, spun free of any cells, and kept at −20° until assayed for cytokines.
Acetylcholinesterase activity of red blood cells
The detailed procedure for determining AChE enzyme activity in RBC was recently described.14 Briefly, freshly drawn, diluted, venous blood samples were incubated with 5,5’-dithio-bis 2-nitrobenzoic acid (DTNB; 10 mm) and ethopropazine (6 mm) for 20 min at 37° before the addition of acetylthiocholine. The change in the absorbance of DTNB was measured at 436 nm. The AChE activity was calculated using an absorption coefficient of TNB− at 436 nm (ε = 10·6/mm/cm). The values were normalized to the haemoglobin (Hb) content (determined as cyanmethaemoglobin) and expressed as mU/μm/Hb.31 All enzyme activities were expressed as percentage of the baseline activity (100%).
K-27 studies
Age-matched BALB/c mice were assigned randomly to four groups of 10 animals each. Group I received saline, Group II received paraoxon, Group III received the K-27 oxime and Group IV received K-27 oxime plus paraoxon, simultaneously. Mice received daily injections for a period of 3 weeks at the end of which they were infected with virulent Salmonella strain to follow survival.
In vivo survival studies
Twenty age-matched BALB/c mice were randomly assigned into the control and experimental groups (10 animals per group) following the same treatment per group as detailed above. After 1, 2 or 3 weeks of treatment, animals were orally infected with a lethal dose (∼ 1·5 × 104) of a virulent strain of S. typhimurium (SL1344). Survival of animals was recorded for up to 60 days after challenge. Simultaneously, another 10 mice (five mice/group) which were also exposed to paraoxon for 3 weeks were identically infected and blood was collected and analysed for cytokine content at days 3 and 9 post-infection. Spleen, liver and MLN were analysed for bacterial load at day 9.
Cytokine analysis
Culture supernatants of ex-vivo-cultured spleen cells and serum samples were analysed for cytokine content and for nitric oxide production as detailed previously.22,32 Production of IL-2 and IFN-γ was quantified following culture of spleen cells for 24 hr (IL-2) or 48 hr (IFN-γ) with or without Con A. Production of IL-6, IL-12/IL-23p40 and TNF-α was determined in supernatants of splenocytes cultured with or without LPS for 24 hr. Levels of IL-12/IL-23p40 were also determined in serum at different times post-infection. The sensitivity of detection was 30 pg/ml for IFN-γ and TNF-α, and 15 pg/ml for IL-2, IL-6 and IL-12/IL-23p40. Accumulation of nitrite was used to determine the production of nitric oxide (NO) according to the Griess method. The nitrite concentration was determined from a standard curve prepared using sodium nitrite (5–100 μm), 5 μm being the lower limit of detection.
Flow cytometric analysis
At the end of treatment, single spleen cell suspensions were prepared from treated animals as detailed previously.14,22 Briefly, cells were double-stained, according to the following combinations, with a pair of directly conjugated monoclonal antibodies specific to: T-cell receptor Cβ chain (H57) and B220 (CD45R), CD4 and CD8, Gr-1 and F4/80, CD3 and CD69, B220 and Sca-1 (Ly6A/E), H57 and Sca-1, and F4/80 and Sca-1 (all antibodies were purchased from eBioscience, San Diego, CA). Cell staining was performed for 30 min on ice and washed cells were analysed on a FACScan (Becton Dickinson, Mountain View, CA). Data collected on 20 000 cells were analysed using cellquest software (Becton Dickinson).
Statistical analysis
To test statistical differences of the changes in body weight and in RBC AChE activity over time, Mann–Whitney U-test was performed at the individual time-points (1, 2 and 3 weeks). In addition, the Mann–Whitney U-test was performed over the entire period, during which time significant differences were demonstrable. Differences in spleen weight and in its cellular composition were analysed statistically after 3 weeks using Student’s t-test. For survival analysis, the log rank (Mantel–Cox) test for Kaplan–Meier functions was applied using the spss 15·0 program (IBM, Chicago, IL). Differences between experimental groups were considered significant when P-values were ≤ 0·05.
Results
AChE activity in mice exposed to paraoxon for 3 weeks
We first tested the effect of paraoxon exposure on RBC AChE activity. After 1 week of exposure to paraoxon, the AChE enzymatic activity was reduced to 38% of baseline value (Fig. 1). The reduced AChE activity continued during weeks 2 and 3 of exposure. In contrast, the enzyme levels in saline-treated controls were not significantly different from baseline values throughout the 3-week period of treatment. Differences in the AChE activity between paraoxon and saline-treated groups were highly significant (P ≤ 0·005) at all time-points. These results are in line with the expected mechanism of action of paraoxon as a specific inhibitor of AChE enzyme.
Figure 1.
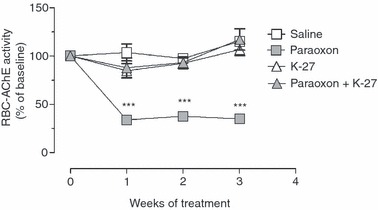
Reduction of red blood cell acetylcholinesterase (RBC AChE) activity in paraoxon-treated mice. Mice were treated for 3 weeks with saline, paraoxon, K-27 oxime, or paraoxon + K-27. Enzyme activity was measured following Ellman’s method, as detailed in the Materials and methods. All enzyme activities are expressed as percentage of the baseline activity (100%). Depicted are the mean values ± SEM of 17 animals per group pooled from two independent experiments. Asterisks denote significant differences between saline and paraoxon groups (***P < 0·001).
Effect of paraoxon exposure on lymphoid tissue
Next, we evaluated the effect of paraoxon exposure on peripheral and central lymphoid organs by examining the size and cellularity of the spleen and thymus. The results demonstrated that paraoxon induced a 48% increase in spleen weight compared with controls, which was significant (Fig. 2a; P = 0·0037). Similarly, organ cellularity, which reflects the total number of viable cells per organ, was increased by 30% in spleens of paraoxon-exposed mice (Fig. 2b; P = 0·016). In contrast, there was no significant effect on the weight or cellularity of the thymus in paraoxon-treated mice (data not shown). The influence of paraoxon on specific populations of lymphoid and myeloid cells in the spleen was analysed by flow cytometry. The results of this analysis are presented in Fig. 2(c–e) as individual graphs depicting the percentages of resting and activated (Sca-1+) B lymphocytes (Fig. 2c), resting and activated (Sca-1+) T lymphocytes (Fig. 2d), and splenic macrophages (F4/80+ cells) as a percentage of the total spleen pool (Fig. 2e). There was no significant change in the percentage of splenic B cells (resting or activated) following paraoxon treatment (Fig. 2c). Similarly, no significant changes were observed in the ratios of resting T-cell subpopulations following paraoxon exposure; however, a small but significant decrease was observed in the percentage of activated Sca-1+ T cells, from 10·5 ± 1·0% in control group to 7·4 ± 0·7% in paraoxon-treated mice (Fig. 2d). Finally, paraoxon exposure induced a small but significant increase in the percentage of F4/80+ macrophages, from 5·3 ± 0·3 to 7·7 ± 0·1%, (Fig. 2e). These findings indicate that, overall, paraoxon had only a limited effect on spleen cell subpopulations following a subchronic, 3-week, exposure.
Figure 2.
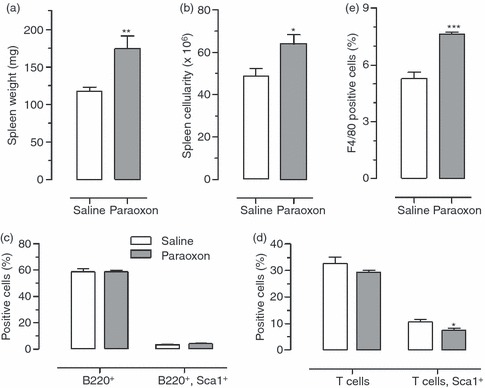
Changes in lymphoid tissue following paraoxon treatment. Following 3 weeks of exposure to saline or paraoxon, mice were analysed for changes in (a) spleen weight, (b) splenocyte count, (c) percentage of resting and activated (Sca-1+) B lymphocytes, (d) T lymphocytes and (e) splenic F4/80+ macrophages. For (a) and (b), the data represent the mean ± SEM of 20 mice per group pooled from six independent experiments. For (c–e), each bar represents the mean ± SEM of four mice per group and is representative of four independent experiments. Asterisks denote significant differences between saline and paraoxon groups (*P < 0·05; **P < 0·01; ***P < 0·001).
Functional in vivo and ex vivo responsiveness
Next, we studied the ex vivo response of spleen cells of saline or paraoxon-exposed mice to Con A or LPS mitogens. The results of this analysis are shown in Fig. 3(a–f). The functional response of macrophages was determined by the production of inflammatory cytokines, including IL-6, TNF-α and IL-12, as well as NO, a key anti-microbial effector molecule (Fig. 3a–d). The results showed that in response to LPS induction, splenocyte cultures from paraoxon-exposed animals produced significantly higher amounts of IL-6, TNF-α and NO compared with saline controls (Fig. 3a–c). Interestingly, however, no differences in IL-12/IL-23p40 levels were observed (Fig 3d). The functional response of T lymphocytes was measured by the levels of IL-2 and IFN-γ (Fig. 3e,f) produced following mitogenic stimulation with Con A, a T-cell mitogen. As shown, Con A-induced spleen T cells of saline or paraoxon-exposed mice produced almost indistinguishable levels of IL-2 and IFN-γ cytokines. As a control, spleen cells cultured in the absence of Con A produced no detectable cytokines. Finally, the effect of paraoxon exposure on cytokine levels in vivo was also evaluated by measurement of serum concentrations of IL-12/IL-23p40, a component of the pro-inflammatory cytokines IL-12 and IL-23, in treated mice immediately after the 3-week exposure period. The data presented in Fig. 3(g) indicate that the levels of IL-12/IL-23p40 protein in the sera of both groups of mice were equivalent. Taken together, these data demonstrate that paraoxon exposure had no effect on the in vivo activity of spleen cells, as ex vivo cultured cells failed to secrete any cytokines constitutively. However, in mitogen-stimulated cultures, splenocytes from paraoxon-treated mice produced higher amounts of IL-6, TNF-α and NO (all of which being products of macrophages) while secreting equivalent levels of IL-2 and IFN-γ (products of T lymphocytes) to saline-treated animals.
Figure 3.
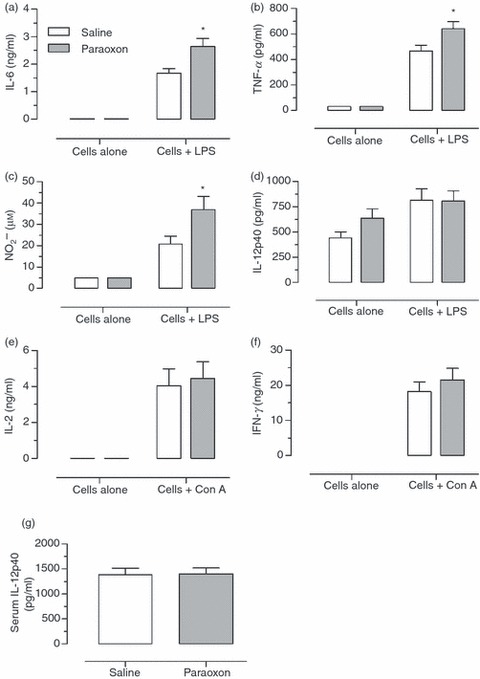
Functional responsiveness of lymphoid and myeloid cells. At the end of a 3-week exposure to saline or paraoxon, single spleen cell suspensions were cultured in the presence or absence of lipopolysaccharide (LPS; a–d), or concanavalin A (Con A; e,f). Culture supernatants were collected and tested for interleukin-6 (IL-6) (a), tumour necrosis factor-α (TNF-α) (b), nitric oxide (c), IL-12/IL-23p40 (d), IL-2 (e) or interferon-γ (IFN-γ) (f). Blood was also collected from each mouse and the serum level of IL-12/IL-23p40 protein was determined (g). Each data point represents the mean ± SEM of nine individually assayed mice per group. Asterisks denote significant differences between control and paraoxon groups (*P < 0·05). The results represent pooled data from three independent experiments.
AChE-dependent enhancement of anti-bacterial immune responses
Next, we tested whether paraoxon could influence the host immune response to infection. Mice were exposed to paraoxon or saline for 1, 2 or 3 weeks and then infected with a virulent strain of S. typhimurium and followed for survival for up to 60 days. After 1 week of paraoxon exposure, overall survival of treated mice was slightly increased compared with saline controls (40% versus 20%, respectively; Fig. 4a). This difference, however, was not statistically significant and the median survival for both groups was identical (18 days). In mice in which exposure to paraoxon was extended for 2 weeks, per cent survival was enhanced to 50% with a median of 42 days compared with 20% and a median of 17 days in controls (Fig. 4b). Lastly, mice exposed to paraoxon for 3 weeks showed a dramatic enhancement in their overall survival with 80% of mice surviving the lethal infection and a median of > 60 days, the maximum period of observation (Fig. 4c). This is in sharp contrast to the saline-treated group where per cent survival was only 18·2% with a median of 13 days. The differences between paraoxon-exposed and control mice were statistically significant (P = 0·007). The enhanced resistance to infection is directly linked to paraoxon-induced inhibition of AChE level, because the co-administration of an oxime (known as K-27), which acts as a cholinesterase reactivator, negated the enhanced survival (Fig. 4d). Co-administration of K-27 with paraoxon effectively blocked AChE inhibition (see Fig. 1). Mice given K-27 alone exhibited no alteration in RBC AChE enzyme activity compared with baseline over the 3 week-exposure period (Fig. 1). As can be seen in Fig. 4(d), the enhanced survival exhibited by paraoxon-treated mice (69·3% survival and a median of > 60 days) was completely reversed by the co-administration of K-27, with mice in this group showing a survival of 14·3% with a median of 14 days (Fig. 4d; paraoxon + K-27 group). Mice treated with K-27 alone showed an overall survival of 28% with a median of 15 days, which was not statistically significantly different from saline controls. These data strongly suggest that the activity of AChE enzyme may directly or indirectly influence host resistance to bacterial infection.
Figure 4.
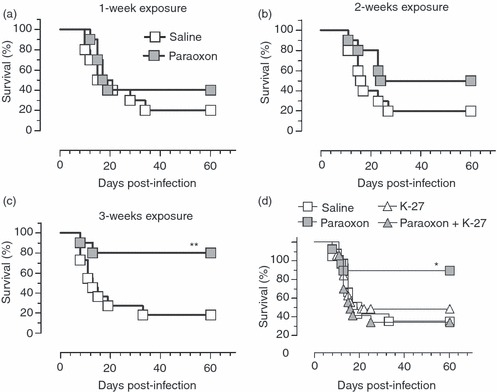
Exposure to paraoxon improves resistance to virulent infections. Mice were treated for 1 week (a) 2 weeks (b) or 3 weeks (c) with paraoxon or saline by daily intraperitoneal injections. At the end of the treatment period, mice were infected orally with a dose of 1·0 × 104 to 1·5 × 104 organisms of SL1344 strain of Salmonella typhimurium and followed for survival up to day 60 post-infection. To determine the effect of acetylcholinesterase on enhanced survival, mice were co-administered the K-27 oxime, with or without paraoxon, (d). Results are representative of two independent experiments. Asterisks denote significant differences between control and paraoxon groups (*P < 0·05; **P < 0·01). Chi squared (Mantel–Cox) statistical test was used for this analysis.
Serum cytokine analysis and organ bacterial load in infected animals
Resistance to Salmonella infection is mediated through the induction of an appropriate pro-inflammatory Th1 response and activation of macrophages, the main host target cells for Salmonella organisms.33,34 To understand the basis of the enhanced survival of paraoxon-treated mice, the serum levels of IL-12/IL-23p40, a component of the critical Th1-inducing cytokine IL-12, were assessed in infected mice at 3, 6 and 9 days after Salmonella inoculation. As shown in Fig. 5(a), equivalent baseline levels of IL-12/IL-23p40 were detected in the sera of mice pre-exposed to saline or paraoxon before infection (day 0) or at day 3 post-infection. However, at day 9 post-infection, paraoxon-exposed mice had 2·2-fold higher cytokine levels than the saline controls (2295 ± 274 pg/ml in paraoxon group versus 1054 ± 109 pg/ml for saline group; P = 0·0036). These results demonstrate that mice pre-exposed to paraoxon have a higher capacity to respond to a lethal bacterial infection by mounting an effective Th1 response (mediated through the release of IL-12) in comparison with the saline pre-treated group. This was confirmed by the significantly higher bacterial loads detected in spleens (Fig. 5b) and MLNs (Fig. 5c) of infected mice. Enumeration of bacterial CFUs at day 9 post-inoculation revealed > 15-fold increase in splenic CFUs in the saline-pretreated group compared with the paraoxon group (P = 0·046). Similarly, > 29-fold higher CFUs were observed in bacterial loads in MLNs (P = 0·009). Hence, paraoxon exposure enhances the ability of animals to mount an effective immune response against a lethal bacterial pathogen.
Figure 5.
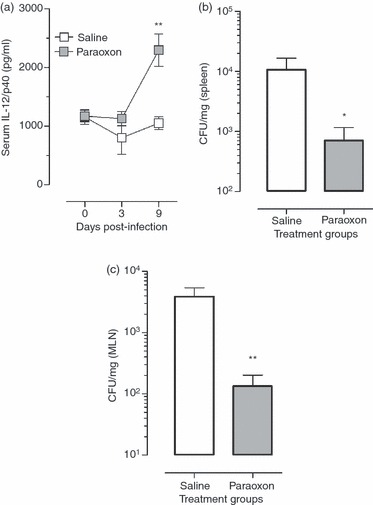
Serum cytokine levels and bacterial loads in paraoxon-treated, infected mice. Mice were treated with paraoxon or saline for 3 weeks and then infected with strain SL1344 of Salmonella typhimurium. Serum was collected at days 3, 6 and 9 after infection and levels of interleukin-12 (IL-12)/IL-23p40 were analysed (a). The levels of bacterial load in spleen (b) and mesenteric lymph nodes (c) were investigated on day 9 after inoculation with 1·1 × 104 SL1344 organisms/mouse. Each data-point represents the mean ± SEM of six to nine mice per group (a) or five mice per group (b,c). Asterisks denote significant differences between the control and experimental groups (*P < 0·05, **P < 0·01). The results are pooled from two independent experiments.
Discussion
The initial aim of the current study was to investigate potential toxic effects of exposure to paraoxon on the immune system. The findings reported herein demonstrate that the administration of up to 40 nmol paraoxon daily for a total period of 3 weeks had no deleterious effects on the immune system, either phenotypically or functionally. Contrary to expectations, this level of paraoxon exposure resulted in an apparent enhancement of immune system function, in particular as it pertains to mounting an effective and protective immune response against a lethal Gram-negative bacterial infection. Our data suggest that paraoxon exerts its influence on the immune system rather indirectly, most likely via its inhibitory action on AChE enzyme which, in turn, leads to a build-up in the levels of ACh. Our data suggest that paraoxon does not induce immune effector functions directly. Instead, paraoxon appears to induce changes in major lymphoid organs, such as the spleen, that evidently allow the animals to respond more efficiently to bacterial infections.
Several lines of evidence have demonstrated that the nervous system plays a role in the regulation of immune responses. Electron microscopic studies provided structural evidence that the vagus nerve, the major parasympathetic nerve, innervates lymphoid tissues such as spleen and thymus and that the nerve terminals form synaptic contacts with lymphoid cells.35 Moreover, muscarinic and nicotinic ACh receptors (AChR) are expressed on many cell types of the immune system, including lymphocytes, macrophages and dendritic cells,36,37 suggesting that ACh may act as a neuroimmunomodulator in interactions between the nervous and immune systems. Several functional studies demonstrated that the interaction between the nervous and immune systems is vital for modulating innate immune responses and controlling inflammation.4,38 Previous studies had shown that ACh, the principal parasympathetic neurotransmitter, attenuates the release of cytokines form LPS-activated macrophages in vitro via the α7 nicotinic AChR.5,39 Furthermore, stimulation of the vagus nerve protects against septic shock in experimental septicaemia.4,13 Currently, it is thought that cholinergic stimulation of splenic macrophages via the vagus nerve results in the induction of an anti-inflammatory pathway that counteracts endotoxaemia-induced inflammation.40–42
One of the main consequences of a dysregulated immune system is increased susceptibility to microbial infections, as we have previously demonstrated in animals following a chronic exposure to lead.30 To determine if paraoxon exposure had any effect on susceptibility to infection, mice were treated with paraoxon for 3 weeks and then inoculated with a lethal dose of the facultative enteric pathogen S. typhimurium. Protection against S. typhimurium requires Th1-type immune responses.22–24,43 Moreover, a robust antibody response is known to be required for resistance against virulent Salmonella infection.22,44,45 Our results demonstrated that, contrary to expectations, paraoxon treatment rendered the animals more resistant to Salmonella infections. Importantly, the effect of paraoxon on anti-bacterial immunity was shown to directly correlate with the extent of inhibition of AChE enzyme activity. Based mainly on studies using animal sepsis models, cholinergic activation has been shown to induce an anti-inflammatory response, acting to attenuate the extent of inflammation and hence promoting better animal survival.2 Many of these studies use purified endotoxin to induce sepsis in what has come to be known as sterile inflammation models.4 In reality, human sepsis is caused by bacterial infections and it is therefore critical to study the role of the cholinergic system in animal models of infection with viable bacterial pathogens. The attenuation of inflammatory responses may be desirable, and even life-saving, in conditions where non-viable agents are used to induce sepsis but this would likely be counterproductive in situations where the pro-inflammatory immune system is required to control the proliferation of viable microbial pathogens.
In the present study, the administration of paraoxon, and the concomitant inhibition of AChE, had no apparent effect on macrophage cellular functions, as shown by the lack of activity of splenocytes cultured ex vivo in the absence of any added stimulatory agents. However, our findings demonstrate that paraoxon exposure results in modest but significant increases in the spleen weight and the percentage of splenic macrophages. This may underlie the higher levels of pro-inflammatory and anti-microbial compounds, including IL-6, TNF-α and NO, released by macrophages following mitogenic stimulation with LPS. Given the fact that the spleen is a major target organ in Salmonella infections, and the critical role of macrophages both as target as well as effector cells, paraoxon-induced changes in the spleen may well be responsible for the enhanced survival rates following infection with virulent Salmonella organisms. These findings are somewhat at variance with recent data demonstrating that in a septic peritonitis model induced by viable E. coli bacteria, nicotine treatment exacerbated the disease and heightened animal mortality as the result of an inhibition of anti-microbial responses and a failure to control bacterial burden.11 However, the experimental design of this study differed from our current study in several respects. First, in the peritonitis study, animals were treated with nicotine in their water for 4 days whereas our model used sub-chronic cholinergic activation produced by AChE inhibition over a period of 3 weeks. Second, AChE inhibition results in the activation of both muscarinic and nicotinic AChR, unlike the former study where nicotinic receptors were the primary targets. Moreover, in our model, live Salmonella organisms were administered through the oral route, whereas in the peritonitis model E. coli bacteria were injected intraperitoneally. Finally, and perhaps most importantly, the septic peritonitis study was essentially looking at acute-phase events occurring within a few hours of bacterial administration. In contrast, the current study was designed to investigate the effect of AChE inhibition on longer-term events in the immune response to infection.
Paraoxon-mediated inhibition of AChE was effectively blocked by the co-administration of a reactivator of AChE (designated K-27), a recently developed oxime-type cholinesterase reactivator that does not cross the blood–brain barrier.20,46 Animals that were given paraoxon plus K-27 were as susceptible to Salmonella infection as saline-treated control mice, demonstrating a direct correlation between the enhancement in anti-bacterial immune defences and the extent of inhibition of AChE enzyme activity. These results are also in line with our previous data showing no enhancement in anti-bacterial immune responses in mice exposed to paraoxon for a period of 6 weeks, a time at which AChE levels have returned to normal.14 The gradual normalization of AChE enzymatic activity despite continued dosing with paraoxon is believed to be the result of the increased synthesis of AChE enzyme, a naturally triggered response in the body to the paraoxon-induced decrease in AChE levels.
The observed enhancement in the level of resistance to bacterial infections is probably mediated through increased ACh levels. This conclusion is supported by a recent study, which reported that control of overwhelming inflammation and improvement in host survival were dependent on the activation of the cholinergic anti-inflammatory pathway by systemic cholinesterase inhibition.13 It is intriguing that continuous dosing with paraoxon was apparently required to be maintained for an extended time (∼ 2 weeks) before the consequences on resistance to infection were manifested. In studies in which mice were exposed to paraoxon for 1, 2 or 3 weeks, the level of AChE activity reached a nadir by 1 week of treatment and persisted at this low level for another 2 weeks. However, when mice exposed for 1, 2 or 3 weeks were challenged with Salmonella organisms, the per cent survival among the treated groups was 40%, 50% and 80%, respectively. Hence, the longer the period of enzyme inactivation the better is the survival against infection. We believe that the continuous dosing with paraoxon over a period of 3 weeks leads to a cumulative inhibitory effect on AChE enzymatic activity, which in turn leads to increased systemic levels of ACh. Following the cessation of paraoxon treatment AChE activity normalizes gradually, going from a nadir of ∼ 20% of baseline control 30 min after paraoxon administration to 46% and 71% after 24 and 48 hr, respectively.47 By 7 days after the last dosing with paraoxon, RBC AChE enzymatic activity has reached 92·4 ± 8·9% of baseline control, essentially a normal level (unpublished data). Other studies demonstrated that maintenance of low AChE activity correlates with an accumulation of extracellular ACh levels in rats following perfusion with neostigmine.48 In our own studies, reversal of the protective effect by the co-administration of K27 oxime also correlated with reversal of enhanced release of IL-6 and NO by splenic macrophages (M. J. Fernandez-Cabezudo, unpublished observations). Therefore, it is reasonable to hypothesize that prolonged inhibition of AChE activity allows for a build-up in ACh levels, which act via the vagus nerve to promote the splenic macrophage anti-Salmonella response, thereby enhancing animal survival.
The possibility that the enhanced survival of paraoxon-exposed animals might be the result of a potential direct anti-bacterial effect of paraoxon is unlikely for several reasons. First, the half-life of paraoxon is very short, reported in one study to be ∼ 3·3 min in rat plasma,49 and in our experiments mice were infected almost 72 hr after the last dosing with paraoxon. Second, paraoxon administration over 6 weeks does not enhance survival after systemic infection with S. typhimurium.14 Third, bacterial growth in the presence of high concentrations up to 11 μg/ml) of paraoxon was not adversely affected (M. J. Fernandez-Cabezudo, unpublished observations). This, nevertheless, does not preclude the possibility that paraoxon may influence host anti-bacterial resistance via other, yet to be identified, pathways. For example, paraoxon-induced increase in cholinergic stimulation might have affected the outcome of the infection by altering bacterial translocation from the gut following oral infection.
Several studies have demonstrated the importance of the innate immune response in limiting bacterial multiplication and thereby controlling infection, ascribing important roles for macrophages and natural killer cells in the acute phase of the infection.50–52 Macrophages are the host cells for Salmonella and also initiate the innate immune response to pathogens by producing pro-inflammatory cytokines, such as IL-12 and TNF-α. Interleukin-12 is a known growth factor for natural killer cells, which have the capacity to rapidly secrete IFN-γ, a cytokine with a potent macrophage-activating capacity. It has been also shown that in IL-12p40−/− mice infected with S. typhimurium, the levels of IFN-γ were reduced and the bacterial load was increased, resulting in higher mortality rates.26 More recent studies confirmed that IL-12 is essential for the control of infection of susceptible mice with an attenuated strain of S. typhimurium.28 Our results demonstrate that at day 9 after infection with a lethal strain of Salmonella, control mice pre-treated with saline have become morbid because of the high level of bacterial burden within their organs (Fig. 5b,c). This is consistent with survival curves showing that animals begin to succumb to an overwhelming infection by day 8 post-inoculation with virulent Salmonella organisms (Fig. 4). Serum analysis at this relatively late time period indicated significantly higher IL-12 levels in paraoxon-treated mice (Fig. 5a), presumably a reflection of the superior control of bacterial proliferation and enhanced survival observed in these animals. It has been suggested that susceptibility of BALB/c mice, the inbred strain used in our study, to intracellular microbial infections is linked to a defective, IL-12-dependent, natural killer cell activation early in the response.53,54 The present data demonstrate that the defect in BALB/c mice can be overcome through the activation of the cholinergic system following inhibition of AChE by paraoxon.
In conclusion, our data clearly demonstrate that sub-chronic treatment with paraoxon induces an inhibition of the AChE activity that in turn leads to an enhancement in anti-bacterial immune defence in inherently susceptible animals. A more exhaustive and detailed evaluation of the effect of OPCs on the immune system is needed to explore their possibility of being used as therapeutic agents.
Acknowledgments
We would like to thank Dr Kamil Kuca (Centre of Advanced Studies, Faculty of Military Health Sciences, University of Defence, Hradec Kralove, Czech Republic) for providing the K-27 oxime. We also thank S. M. Nurulain for technical assistance. This work was funded by a grant from the Faculty of Medicine and Health Sciences, UAE University, United Arab Emirates to M. J. Fernandez-Cabezudo (#NP/07/17).
Disclosures
The authors declare no competing interests.
References
- 1.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;8:125–34. [PMC free article] [PubMed] [Google Scholar]
- 2.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–96. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallowitsch-Puerta M, Pavlov VA. Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sci. 2007;25:2325–9. doi: 10.1016/j.lfs.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 6.Van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ, Van der Poll T. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–30. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–30. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Van Der Zanden EP, Boeckxstaens GE, De Jonge WJ. The vagus nerve as a modulator of intestinal inflammation. Neurogastroenterol Motil. 2009;21:6–17. doi: 10.1111/j.1365-2982.2008.01252.x. [DOI] [PubMed] [Google Scholar]
- 9.Kessler W, Traeger T, Westerholt A, Neher F, Mikulcak M, Muller A, Maier S, Heidecke CD. The vagal nerve as a link between the nervous and immune system in the instance of polymicrobial sepsis. Langenbecks Arch Surg. 2006;391:83–7. doi: 10.1007/s00423-006-0031-y. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–21. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 11.Van Westerloo DJ, Giebelen IA, Florquin S, Daalhuisen J, Bruno MJ, De Vos AF, Tracey KJ, Van der Poll T. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis. 2005;191:2138–48. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 12.Akinci SB, Ulu N, Yondem OZ, Firat P, Guc MO, Kanbak M, Aypar U. Effect of neostigmine on organ injury in murine endotoxemia: missing facts about the cholinergic antiinflammatory pathway. World J Surg. 2005;29:1483–9. doi: 10.1007/s00268-005-0073-2. [DOI] [PubMed] [Google Scholar]
- 13.Hofer S, Eisenbach C, Lukic IK, et al. Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Crit Care Med. 2008;36:404–8. doi: 10.1097/01.CCM.0B013E31816208B3. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Cabezudo MJ, Azimullah S, Nurulain SM, Mechkarska M, Lorke DE, Hasan MY, Petroianu GA, Al-Ramadi BK. The organophosphate paraoxon has no demonstrable effect on the murine immune system following subchronic low dose exposure. Int J Immunopathol Pharmacol. 2008;21:891–901. doi: 10.1177/039463200802100413. [DOI] [PubMed] [Google Scholar]
- 15.Petroianu G, Toomes LM, Petroianu A, Bergler W, Rufer R. Control of blood pressure, heart rate and haematocrit during high-dose intravenous paraoxon exposure in mini pigs. J Appl Toxicol. 1998;18:293–8. doi: 10.1002/(sici)1099-1263(199807/08)18:4<293::aid-jat509>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 16.Buckley NA, Eddleston M, Szinicz L. Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD005085. CD005085. [DOI] [PubMed] [Google Scholar]
- 17.Peter JV, Moran JL, Graham P. Oxime therapy and outcomes in human organophosphate poisoning: an evaluation using meta-analytic techniques. Crit Care Med. 2006;34:502–10. doi: 10.1097/01.ccm.0000198325.46538.ad. [DOI] [PubMed] [Google Scholar]
- 18.Petroianu GA, Lorke DE. Pyridinium oxime reactivators of cholinesterase inhibited by diisopropyl-fluorophosphate (DFP): predictive value of in vitro testing for in vivo efficacy. Mini Rev Med Chem. 2008;8:1328–42. doi: 10.2174/138955708786369555. [DOI] [PubMed] [Google Scholar]
- 19.Kuca K, Bielavsky J, Cabal J, Kassa J. Synthesis of a new reactivator of tabun-inhibited acetylcholinesterase. Bioorg Med Chem Lett. 2003;13:3545–7. doi: 10.1016/s0960-894x(03)00751-0. [DOI] [PubMed] [Google Scholar]
- 20.Lorke DE, Kalasz H, Petroianu GA, Tekes K. Entry of oximes into the brain: a review. Curr Med Chem. 2008;15:743–53. doi: 10.2174/092986708783955563. [DOI] [PubMed] [Google Scholar]
- 21.Petroianu GA, Nurulain SM, Nagelkerke N, Al-Sultan MA, Kuca K, Kassa J. Five oximes (K-27, K-33, K-48, BI-6 and methoxime) in comparison with pralidoxime: survival in rats exposed to the organophosphate paraoxon. J Appl Toxicol. 2006;26:262–8. doi: 10.1002/jat.1143. [DOI] [PubMed] [Google Scholar]
- 22.al-Ramadi BK, Fernandez-Cabezudo MJ, Ullah A, El-Hasasna H, Flavell RA. CD154 is essential for protective immunity in experimental Salmonella infection: evidence for a dual role in innate and adaptive immune responses. J Immunol. 2006;176:496–506. doi: 10.4049/jimmunol.176.1.496. [DOI] [PubMed] [Google Scholar]
- 23.Eisenstein TK, Killar LM, Sultzer BM. Immunity to infection with Salmonella typhimurium: mouse-strain differences in vaccine- and serum-mediated protection. J Infect Dis. 1984;150:425–35. doi: 10.1093/infdis/150.3.425. [DOI] [PubMed] [Google Scholar]
- 24.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun. 1993;61:3981–4. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mastroeni P, Harrison JA, Robinson JH, Clare S, Khan S, Maskell DJ, Dougan G, Hormaeche CE. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent salmonellae in BALB/c mice: role of gamma interferon and macrophage activation. Infect Immun. 1998;66:4767–76. doi: 10.1128/iai.66.10.4767-4776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehmann J, Bellmann S, Werner C, Schroder R, Schutze N, Alber G. IL-12p40-dependent agonistic effects on the development of protective innate and adaptive immunity against Salmonella enteritidis. J Immunol. 2001;167:5304–15. doi: 10.4049/jimmunol.167.9.5304. [DOI] [PubMed] [Google Scholar]
- 27.Filipe-Santos O, Bustamante J, Chapgier A, et al. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18:347–61. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Price JD, Simpfendorfer KR, Mantena RR, Holden J, Heath WR, Van Rooijen N, Strugnell RA, Wijburg OL. Gamma interferon-independent effects of interleukin-12 on immunity to Salmonella enterica serovar Typhimurium. Infect Immun. 2007;75:5753–62. doi: 10.1128/IAI.00971-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.al-Ramadi BK, Al-Dhaheri MH, Mustafa N, Abouhaidar M, Xu D, Liew FY, Lukic ML, Fernandez-Cabezudo MJ. Influence of vector-encoded cytokines on anti-Salmonella immunity: divergent effects of interleukin-2 and tumor necrosis factor α. Infect Immun. 2001;69:3980–8. doi: 10.1128/IAI.69.6.3980-3988.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Cabezudo MJ, Ali SAE, Ullah A, Hasan MY, Kosanovic M, Fahim MA, Adem A, al-Ramadi BK. Pronounced susceptibility to infection by Salmonella enterica serovar Typhimurium in mice chronically exposed to lead correlates with a shift to Th2-type immune responses. Toxicol Appl Pharmacol. 2007;218:215–26. doi: 10.1016/j.taap.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Van Kampen EJ, Zijlstra WG. Standardization of hemoglobinometry II. The hemoglobincyanide method. Clin Chim Acta. 1961;6:538–44. doi: 10.1016/0009-8981(61)90145-0. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez-Cabezudo MJ, Mechkarska M, Azimullah S, al-Ramadi BK. Modulation of macrophage proinflammatory functions by cytokine-expressing Salmonella vectors. Clin Immunol. 2009;130:51–60. doi: 10.1016/j.clim.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–93. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mastroeni P, Sheppard M. Salmonella infections in the mouse model: host resistance factors and in vivo dynamics of bacterial spread and distribution in the tissues. Microbes Infect. 2004;6:398–405. doi: 10.1016/j.micinf.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Waxman S. Clinical Neuroanatomy. 25th edn. New York: McGraw Hill Medical; 2003. pp. 117–20. [Google Scholar]
- 36.Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007;25:2314–9. doi: 10.1016/j.lfs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 37.Saeed RW, Varma S, Peng-Nemeroff T, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–23. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavlov VA, Tracey KJ. Neural regulators of innate immune responses and inflammation. Cell Mol Life Sci. 2004;61:2322–31. doi: 10.1007/s00018-004-4102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 40.De Jonge WJ, Van der Zanden EP, The FO, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–51. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 41.Huston JM, Ochani M, Rosas-Ballina M, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–8. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci USA. 2008;105:11008–13. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez-Cabezudo MJ, Ullah A, Flavell RA, Al-Ramadi BK. Evidence for the requirement for CD40–CD154 interactions in resistance to infections with attenuated Salmonella. J Endotoxin Res. 2005;11:395–9. doi: 10.1179/096805105X67319. [DOI] [PubMed] [Google Scholar]
- 44.Mastroeni P, Simmons C, Fowler R, Hormaeche CE, Dougan G. Igh-6−/− (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect Immun. 2000;68:46–53. doi: 10.1128/iai.68.1.46-53.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar typhimurium. Infect Immun. 2000;68:3344–8. doi: 10.1128/iai.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lorke DE, Hasan MY, Nurulain SM, Sheen R, Kuca K, Petroianu GA. Entry of two new asymmetric bispyridinium oximes (K-27 and K-48) into the rat brain: comparison with obidoxime. J Appl Toxicol. 2007;27:482–90. doi: 10.1002/jat.1229. [DOI] [PubMed] [Google Scholar]
- 47.Petroianu GA, Hasan MY, Nurulain SM, Arafat K, Sha Ullah M, Naseer O. Protective agents in acute high-dose organophosphate exposure: comparison of ranitidine with pralidoxime in rats. J Appl Toxicol. 2005;25:68–73. doi: 10.1002/jat.1037. [DOI] [PubMed] [Google Scholar]
- 48.Joosen MJ, Van Helden HP. Correlations between acetylcholinesterase inhibition, acetylcholine levels and EEG changes during perfusion with neostigmine and N6-cyclopentyladenosine in rat brain. Eur J Pharmacol. 2007;3:122–8. doi: 10.1016/j.ejphar.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Eigenberg DA, Pazdernik TL, Doull J. Hemoperfusion and pharmacokinetic studies with parathion and paraoxon in the rat and dog. Drug Metab Dispos. 1983;11:366–70. [PubMed] [Google Scholar]
- 50.Richter-Dahlfors A, Buchan AM, Finlay BB. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–80. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schafer R, Eisenstein TK. Natural killer cells mediate protection induced by a Salmonella aroA mutant. Infect Immun. 1992;60:791–7. doi: 10.1128/iai.60.3.791-797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.al-Ramadi BK, Mustafa N, AbouHaidar M, Fernandez-Cabezudo MJ. Induction of innate immunity by IL-2-expressing Salmonella confers protection against lethal infection. Mol Immunol. 2003;39:763–70. doi: 10.1016/s0161-5890(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 53.Laskay T, Diefenbach A, Rollinghoff M, Solbach W. Early parasite containment is decisive for resistance to Leishmania major infection. Eur J Immunol. 1995;25:2220–7. doi: 10.1002/eji.1830250816. [DOI] [PubMed] [Google Scholar]
- 54.Scharton-Kersten T, Afonso LC, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol. 1995;154:5320–30. [PubMed] [Google Scholar]


