Abstract
Estrogen receptor ERα and ERβ heterodimerization has been implicated in cancer chemoprevention. The discovery, structural elucidation and total synthesis of a new natural product, actinopolymorphol A (1), from Actinopolymorpha rutilus (YIM45752) that preferentially induces ERα/β heterodimerization is reported. Total synthesis of 1 has allowed us to determine its absolute stereochemistry and that of a previously known deacetylated congener, and 1 represents the first member of a new class of natural products not previously recognized to modulate ER function.
The estrogen receptors (ERs) are ligand-inducible transcription factors implicated in a wide array of biological processes. Binding of 17β-estradiol (E2) and other estrogenic ligands triggers receptor dimerization and subsequent control of gene transcription. ERs exist in two forms, ERα and ERβ, which have opposing roles in regulating estrogen action: ERα promotes, whereas ERβ, inhibits E2-dependent cell growth.1 Notably, the coexpression of ERβ with ERα leads to reduced proliferation of breast cancer cells and ~60% of all breast tumors express both receptors.2 Coexpression of ERβ has been correlated with a more favorable prognosis relative to tumors expressing ERα alone, and many data now suggest that ERα/β heterodimers function to regulate distinct E2-responsive genes.3 However, facile homodimerization has obscured a clear understanding of heterodimer function.
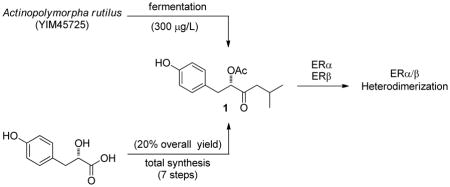
We recently reported a bioluminescent resonance energy transfer (BRET) assay to monitor the ERα/β heterodimer and its respective homodimer formation in live cells, setting the stage to screen for homo- and heterodimer selective ER modulators.4 We now report the discovery and total synthesis of a new natural product, actinopolymorphol A (1), that preferentially induces ERα/β heterodimerization relative to either homodimer. Actinopolymorphol A represents the first member of a new class of natural products not previously recognized to modulate ER function.
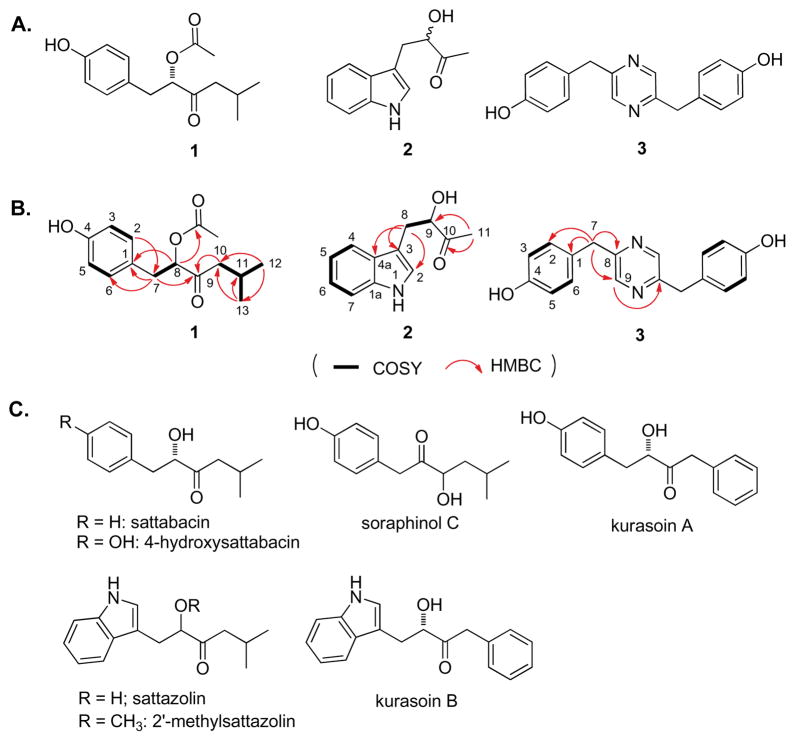
During the course of our efforts to identify novel natural products from microorganisms from diverse and unique ecological niches, we first subjected the crude extract from a recently identified Actinopolymorpha rutilus (YIM45725)5 originating from a soil sample from the Yunnan province of China to the BRET assay. Initial assays clearly indicated the presence of potential ERα/β heterodimerization inducers in the crude extract. Subsequent large-scale fermentation (5 L) and BRET assay-guided fractionation afforded an active fraction, from which 1, actinopolymorphol B (2) and C (3) were purified. The 1H and 13C NMR spectra of purified 1, 2, and 3 were fully assigned on the basis of extensive 1D and 2D NMR (gCOSY, gHSQC, and gHMBC) and APCI-MS and high-resolution ESI-MS analyses (Figure 1B and also see Supporting Information). Dereplication revealed 1, 2, and 3 to be new natural products (Figure 1A) although a relationship to previously reported natural products (Figure 1C)6–10 is clearly evident for 1 and 2.
Figure 1.
Actinopolymorphol A (1), B (2), and C (3) from ERα/β heterodimerizing fraction of A. rutilus crude extract: (A) structures of 1, 2, and 3; (B) COSY and HMBC correlations observed for 1, 2, and 3; and (C) the sattabacin, sattazolin and kurasoin classes of natural products bearing molecular scaffolds similar to 1 or 2.6–10
Second, we determined, using the BRET assay and pure 1, 2, 3, that 1 was solely responsible for ERα/β heterodimerization induction (EC50 = 19 μM) and 2 and 3 were devoid of any ER dimerization inducing activity.11 Consequently, further studies focused exclusively on 1. Significantly, none of the previously reported natural products to which 1 bears a structural resemblance have been noted as ER modulators. Thus, the finding that 1 induces ERα/β heterodimerization may have bearing on the scope of previously reported bioactivities for this class of natural products (Figure 1C).6,7,9,10
Compound 1 was isolated as a colorless oil, for which HR-ESIMS established the molecular formula as C15H20O4 (287.12567 [M + Na]+, calcd for C15H20O4Na, 287.12538) possessing six degrees of unsaturation. Analysis of the NMR spectra revealed chemical shifts indicative of a ketone (δC 207.8), acetoxyl (δC 170.9, s; δC 19.3, q), one 1,4-disubstituted benzene ring [(δH 6.68, 2H; δC 115.1, d) and (δH 7.02, 2H; δC 130.3, d)], a secondary alcohol methine (δH 5.08, δC 79.6, d), one methine (δH 2.03, δC 35.7, d), two methylenes (δC 47.8, t; δC 35.7, t), two secondary methyl groups [(δH 0.82, 3H; δC 21.7, q) and (δH 0.84, 3H; δC 21.7, q)] (Table S1). On the basis of the gHMBC correlations from two methylene protons to corresponding carbons and the gCOSY correlations, the connectivity of every carbon was readily established (Figure 1B).
Third, we established the structure of 1 by total synthesis using the optically pure starting material (S)-2-hydroxy-3-(4-hydroxyphenyl)-propionic acid (98% purity) (4, Figure 2A). Conversion of 4 to methyl ester 5 was effected using methanolic HCl followed by treatment with TBSCl and imidazole to afford diprotected methyl ester 6. The methyl ester underwent smooth conversion to the Weinreb amide 7 by treatment with N, O-dimethyl-hydroxylamine hydrochloride in the presence of (CH3)2CHMgCl. Grignard reaction of 7 with (CH3)2CHCH2MgCl proceeded extraordinarily slowly over the course of 24 hrs but ultimately afforded the diprotected isobutyl ketone 8 in 67% yield from the Weinreb amide. Subsequent cleavage of both silyl ethers with tetrabutylammonium fluoride (TBAF) afforded the corresponding diol 9 in 81% yield. Notably, production of 9 from 4 constitutes a total synthesis of the natural product 4-hydroxysattabacin from Sorangium cellulosum (Figure 1C), for which absolute stereochemistry has been unclear.6 The optical rotation of 9 was found to be +18.3 (c 0.21, CH3OH), which compares favorably to the number previously published for 4-hydroxysattabacin [+14 (0.3, CHCl3)],6 assigning 4-hydroxysattabacin as the S-enantiomer. Diacetylation of 9 was accomplished in 72% yield upon subjection of the diol to excess acetic anhydride and pyridine. Selective deacetylation of the phenol moiety was accomplished using neat pyrrolidine at room temperature, affording finally the synthetic 1 as the S-enantiomer. 1H- and 13C NMR spectral data for synthetic 1 were identical to those previously obtained for natural 1 from A. rutilus YIM45725. BRET assay confirmed that natural and synthetic 1 displayed identical (within standard error) abilities to induce ERα/β heterodimerization.11
Figure 2.
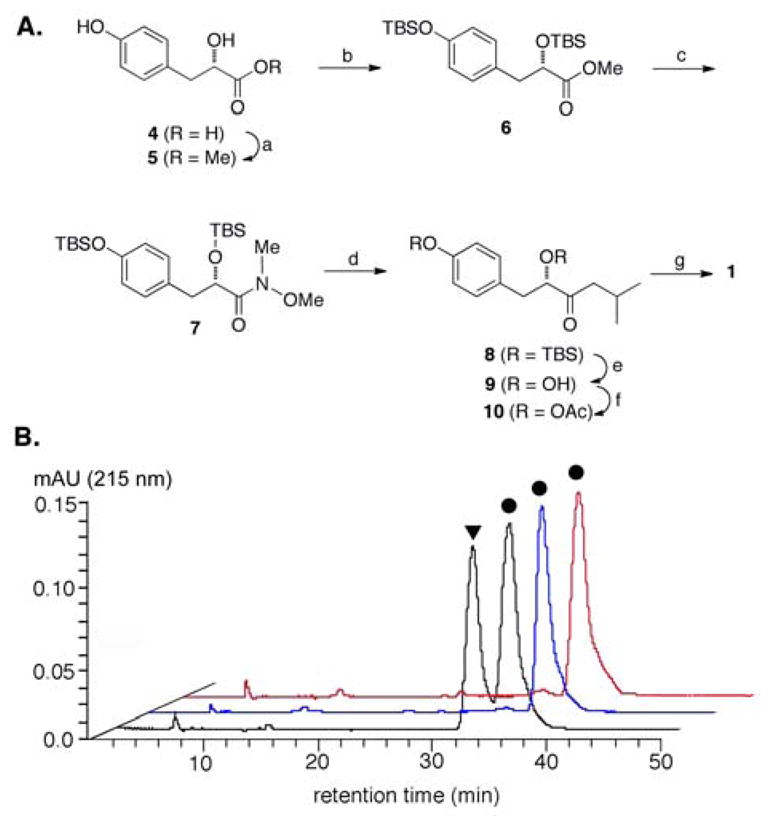
Synthesis and chiral HPLC analysis of natural vs. synthetic 1: (A) reagents and conditions for total synthesis of 1 from the optically pure starting material 4 [(a) MeOH, HCl, rt. quantative (b) TBSCl, imidazole., DMF, rt. 90%; (c) HN(OMe)CH3-HCl, (CH3)2CHMgCl, THF, 0 °C, 81%; (d) (CH3)2CHCH2MgCl, THF, 0°C-rt, 67%; (e) TBAF, THF, rt. 86%; (f) Ac2O, pyridine, rt. 72%; (g) pyrrolidine, 1 min., rt. 66%] and (B) chiral HPLC analysis of racemic 1, 1 from A rutilus (blue), synthetic 1 (red). ●, 1, and ▼ the R-enantiomer of 1.
Finally, the absolute stereochemistry of 1 was confirmed by comparative chiral HPLC and optical rotation analyses. Both the natural and synthetic 1 were found to be identical upon chiral HPLC analyses, possessing the same retention time (Figure 2B) and identical UV-Vis spectra, and to have almost identical optical rotations; +20.9 (c 0.15, CH3OH) for natural 1 and +23.5 (c 0.28, CH3OH) for synthetic 1 (the slight difference in optical rotations is attributed to different concentrations). On the basis of these data, we conclude that 1 from A. rutilus YIM45725 has the S-configuration at C8; this is consistent with the stereochemistries found in the kurasoins and, as unveiled here, 4-hydroxysattabacin.6,8
Selective ER modulators are well established and a number of them are used clinically such as the tamoxifen and raloxifene.12,13 Of particular significance during the development of such agents has been the realization that diols or other agents, able to form H-bonding networks with the ligand binding domains of ER spaced a distance of ~12Å apart, can effectively compete for ER binding with E2 and thereby elicit clinically significant activities. On this basis, we postulate that the phenol moiety of 1 is likely critical to ER heterodimerization activity; an understanding of ER-E2 interactions in which the phenol moiety of E2 is involved in H-bonding supports this hypothesis.13 The small size of 1 yet pronounced ability to modulate ER activity, and relatedness to already established natural products suggests that further study of this class of natural products is clearly warranted. Our ability to produce 1 biosynthetically and via total synthesis provides an outstanding platform to more rigorously elucidate ER heterodimerization by new analogues bearing this privileged molecular scaffold.
Supplementary Material
Acknowledgments
We thank the Analytical Instrumentation Center of the School of Pharmacy, UW-Madison for support in obtaining MS and NMR data. This work is supported in part by NIH grants CA113297 (B.S.), CA125387 (W.X.), MH089442 (W.X.), T32CA009135 (E.P.) and the Chinese Ministry of Education 111 Project B08034 (Y.D.).
Footnotes
Supporting Information Available. Full experimental details for strain cultivation, extraction, compound isolation, spectral data and assignment of all natural products and synthetic intermediates. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Helguero LA, Faulds MH, Gustafsson JA, Haldosen LA. Oncogene. 2005;24:6605–6616. doi: 10.1038/sj.onc.1208807. [DOI] [PubMed] [Google Scholar]
- 2.Pettersson K, Delaunay F, Gustafsson JA. Oncogene. 2000;19:4970–4978. doi: 10.1038/sj.onc.1203828. [DOI] [PubMed] [Google Scholar]
- 3.Chang EC, Frasor J, Komm B, Katzellenbogen BS. Endocrinology. 2006;147:4831–4842. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- 4.Powell E, Xu W. Proc Natl Acad Sci USA. 2008;105:19012–19017. doi: 10.1073/pnas.0807274105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y-X, Zhang YQ, Xu LH, Li WJ. Int J Sys Evol Microbiol. 2008;58:2443–2446. doi: 10.1099/ijs.0.65629-0. [DOI] [PubMed] [Google Scholar]
- 6.Sattabacins & Sattazolins: Lampis G, Deidda D, Maullu C, Madeddu MA, Pompei R, Monache RD, Satta G. J Antibiot. 1995;48:967–972. doi: 10.7164/antibiotics.48.967.
- 7.Soraphinol: Li X, Yu TK, Kwak JH, Son BY, Seo Y, Zee OP, Ahn JW. J Microbiol Biotechnol. 2008;18:520–522.
- 8.Kurasoins: Andrus MB, Hicken EJ, Stephens JC, Bedke DK. J Org Chem. 2006;71:8651–8654. doi: 10.1021/jo061395t.
- 9.Uchida R, Shiomi K, Inokoshi J, Masuma R, Kawakubo T, Tanaka H, Iwai Y, Omura S. J Antibiot. 1996;49:932–934. doi: 10.7164/antibiotics.49.932. [DOI] [PubMed] [Google Scholar]
- 10.Uchida R, Shiomi K, Inokoshi J, Nishizawa A, Hirose T, Tanaka H, Iwai Y, Omura S. J Antibiot. 1996;49:886–889. doi: 10.7164/antibiotics.49.886. [DOI] [PubMed] [Google Scholar]
- 11.Powell E, Huang S-X, Xu Y, Rajski SR, Wang Y, Peters N, Guo S, Xu HE, Hoffmann FM, Shen B, Xu W. Biochem Pharmacol. 2010 doi: 10.1016/j.bcp.2010.06.030. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan VC. J Med Chem. 2003;46:883–908. doi: 10.1021/jm020449y. [DOI] [PubMed] [Google Scholar]
- 13.Jordan VC. J Med Chem. 2003;46:1081–1111. doi: 10.1021/jm020450x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.