Abstract
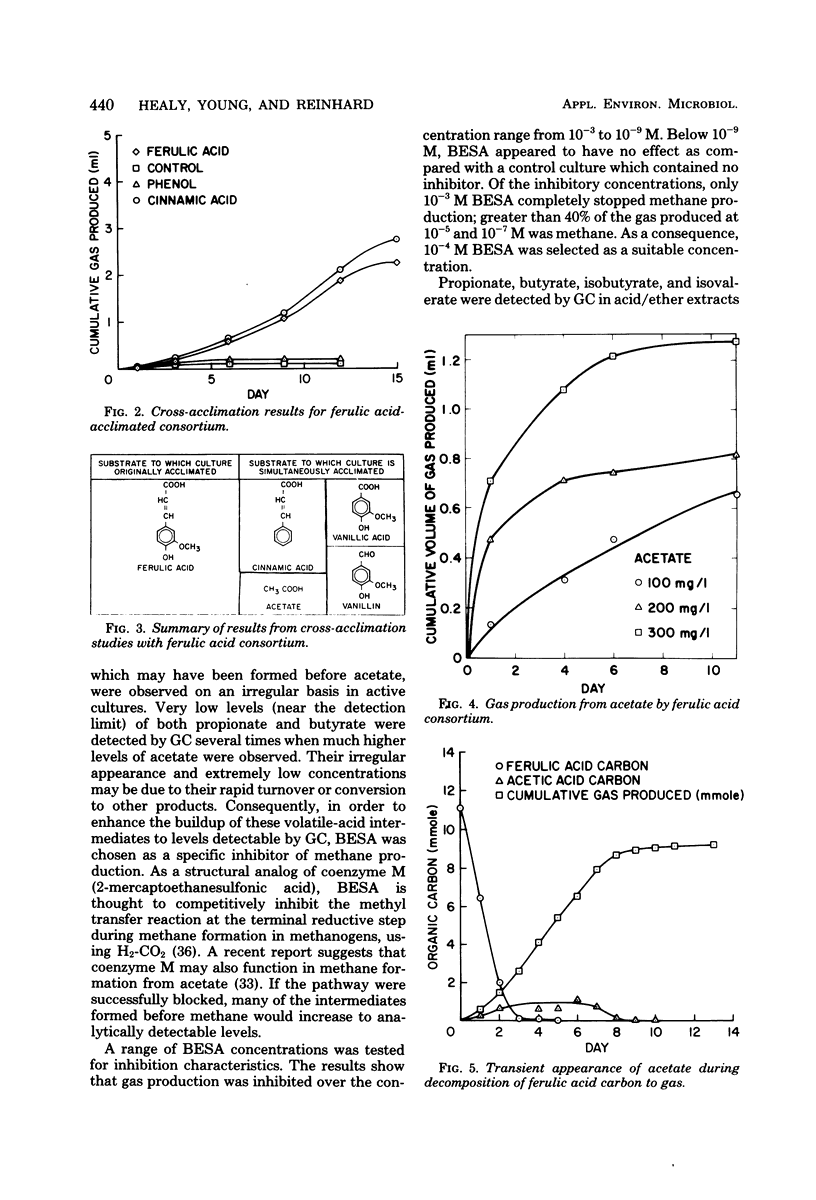
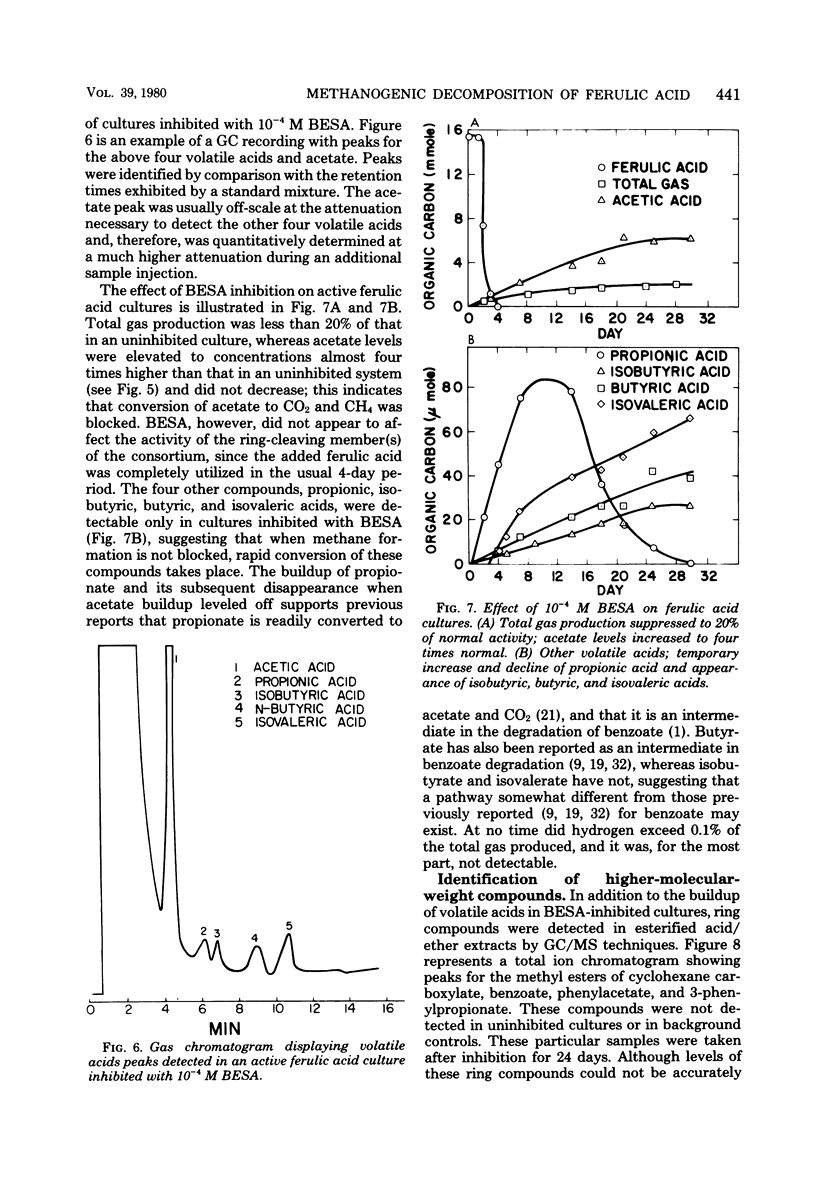
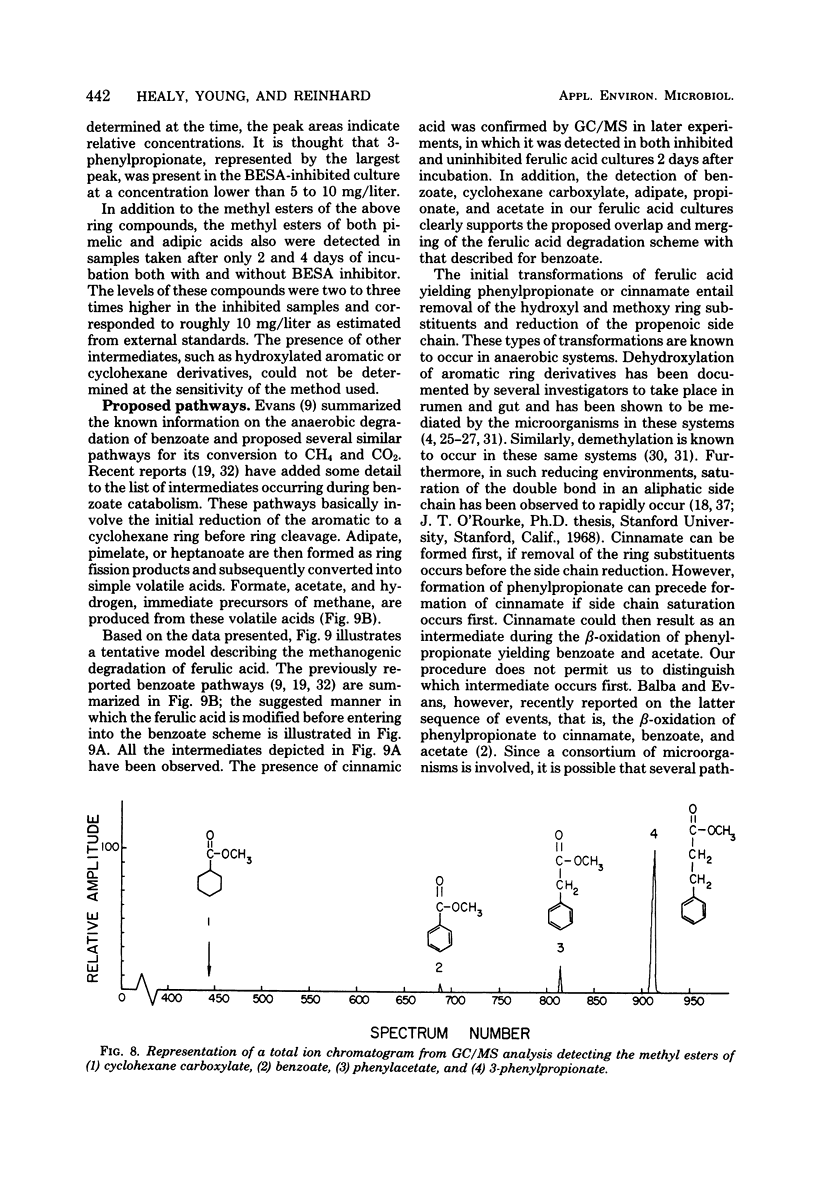
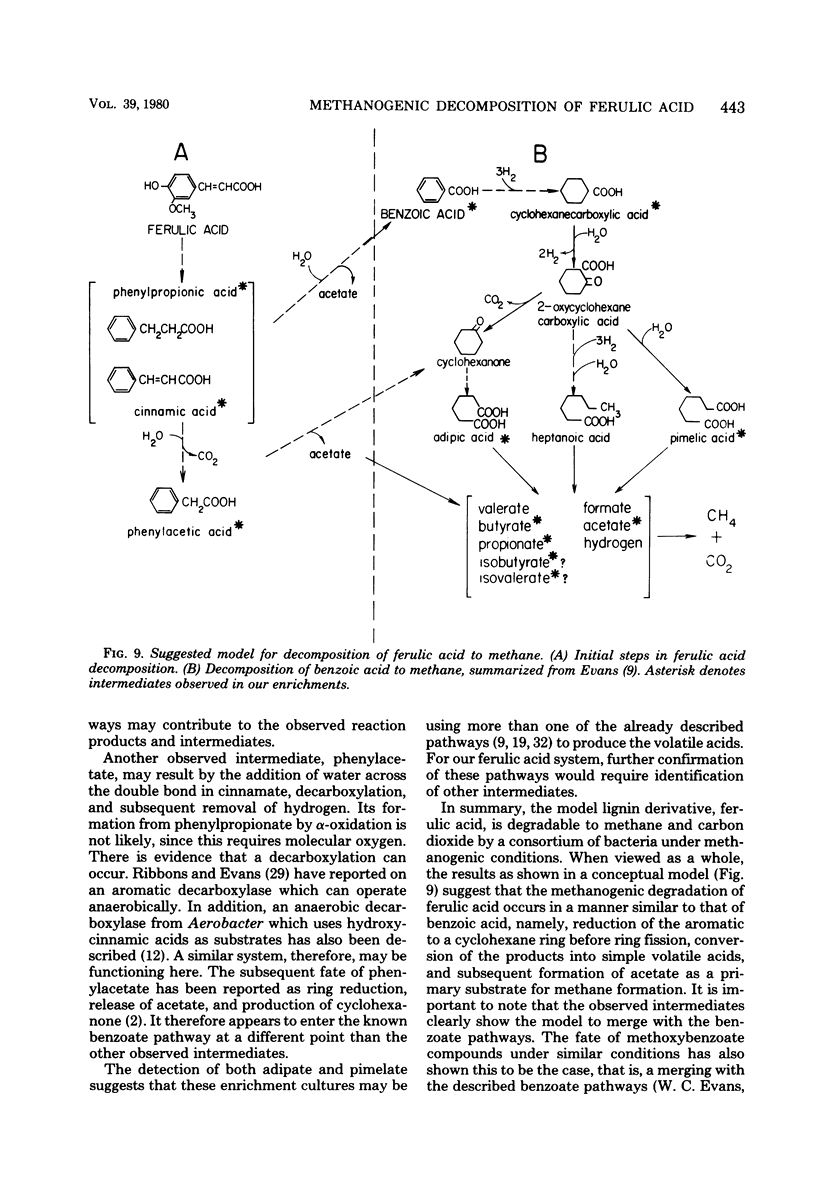
Ferulic acid, a model lignin derivative, was observed to be biodegradable to methane and carbon dioxide under strict anaerobic conditions. This conversion appears to be carried out by a consortium of bacteria similar to that previously described for the methanogenic degradation of benzoic acid. A temporary buildup of acetate in these cultures indicates that it is a likely intermediate and precursor for methane formation. An analog of coenzyme M, 2-bromoethanesulfonic acid (BESA), inhibited gas production and enhanced the buildup of propionate, butyrate, isobutyrate, and isovalerate. Phenylacetate, cinnamate, 3-phenylpropionate, benzoate, cyclohexane carboxylate, adipate, and pimelate were also detected in BESA-inhibited cultures. A pathway is proposed which includes these various acids as possible intermediates in the methanogenic degradation of ferulic acid. This model overlaps previously described benzoic acid degradation pathways, suggesting that this type of anaerobic degradation may be common for aromatic compounds.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balba M. T., Evans W. C. The methanogenic fermentation of aromatic substrates. Biochem Soc Trans. 1977;5(1):302–304. doi: 10.1042/bst0050302. [DOI] [PubMed] [Google Scholar]
- Balba M. T., Evans W. C. The methanogenic fermentation of omega-phenylalkane carboxylic acids [proceedings]. Biochem Soc Trans. 1979 Apr;7(2):403–405. doi: 10.1042/bst0070403. [DOI] [PubMed] [Google Scholar]
- CLARK F. M., FINA L. R. The anaerobic decomposition of benzoic acid during methane fermentation. Arch Biochem Biophys. 1952 Mar;36(1):26–32. doi: 10.1016/0003-9861(52)90374-3. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Evans W. C. The metabolism of aromatic compounds by Rhodopseudomonas palustris. A new, reductive, method of aromatic ring metabolism. Biochem J. 1969 Jul;113(3):525–536. doi: 10.1042/bj1130525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS W. C. THE MICROBIOLOGICAL DEGRADATION OF AROMATIC COMPOUNDS. J Gen Microbiol. 1963 Aug;32:177–184. doi: 10.1099/00221287-32-2-177. [DOI] [PubMed] [Google Scholar]
- Evans W. C. Biochemistry of the bacterial catabolism of aromatic compounds in anaerobic environments. Nature. 1977 Nov 3;270(5632):17–22. doi: 10.1038/270017a0. [DOI] [PubMed] [Google Scholar]
- FINA L. R., FISKIN A. M. The anaerobic decomposition of benzoic acid during methane fermentation. II. Fate of carbons one and seven. Arch Biochem Biophys. 1960 Dec;91:163–165. doi: 10.1016/0003-9861(60)90483-5. [DOI] [PubMed] [Google Scholar]
- FINKLE B. J., LEWIS J. C., CORSE J. W., LUNDIN R. E. Enzyme reactions with phenolic compounds: formation of hydroxystyrenes through the decarboxylation of 4-hydroxycinnamic acids by Aerobacter. J Biol Chem. 1962 Sep;237:2926–2931. [PubMed] [Google Scholar]
- Ferry J. G., Wolfe R. S. Anaerobic degradation of benzoate to methane by a microbial consortium. Arch Microbiol. 1976 Feb;107(1):33–40. doi: 10.1007/BF00427864. [DOI] [PubMed] [Google Scholar]
- Guyer M., Hegeman G. Evidence for a reductive pathway for the anaerobic metabolism of benzoate. J Bacteriol. 1969 Sep;99(3):906–907. doi: 10.1128/jb.99.3.906-907.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett W. F., Connors W. J., Kirk T. K., Zeikus J. G. Microbial decomposition of synthetic C-labeled lignins in nature: lignin biodegradation in a variety of natural materials. Appl Environ Microbiol. 1977 Jan;33(1):43–51. doi: 10.1128/aem.33.1.43-51.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy J. B., Jr, Young L. Y. Catechol and phenol degradation by a methanogenic population of bacteria. Appl Environ Microbiol. 1978 Jan;35(1):216–218. doi: 10.1128/aem.35.1.216-218.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy J. B., Young L. Y. Anaerobic biodegradation of eleven aromatic compounds to methane. Appl Environ Microbiol. 1979 Jul;38(1):84–89. doi: 10.1128/aem.38.1.84-89.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith C. L., Bridges R. L., Fina L. R., Iverson K. L., Cloran J. A. The anaerobic decomposition of benzoic acid during methane fermentation. IV. Dearomatization of the ring and volatile fatty acids formed on ring rupture. Arch Microbiol. 1978 Aug 1;118(2):173–176. doi: 10.1007/BF00415726. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974 May;27(5):985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTON L. N., STANIER R. Y. MECHANISM OF BETA-KETOADIPATE FORMATION BY BACTERIA. Nature. 1964 Dec 26;204:1279–1283. doi: 10.1038/2041279a0. [DOI] [PubMed] [Google Scholar]
- Peppercorn M. A., Goldman P. Caffeic acid metabolism by bacteria of the human gastrointestinal tract. J Bacteriol. 1971 Dec;108(3):996–1000. doi: 10.1128/jb.108.3.996-1000.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppercorn M. A., Goldman P. Caffeic acid metabolism by gnotobiotic rats and their intestinal bacteria. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1413–1415. doi: 10.1073/pnas.69.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Silva G., Rodriguez D., Perez-Silva J. Dehydroxylation of caffeic acid by a bacterium isolated from rat faeces. Nature. 1966 Oct 15;212(5059):303–304. doi: 10.1038/212303b0. [DOI] [PubMed] [Google Scholar]
- Ribbons D. W., Evans W. C. Oxidative metabolism of phthalic acid by soil pseudomonads. Biochem J. 1960 Aug;76(2):310–318. doi: 10.1042/bj0760310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheline R. R. Decarboxylation and demethylation of some phenolic benzoic acid derivatives by rat caecal contents. J Pharm Pharmacol. 1966 Oct;18(10):664–669. doi: 10.1111/j.2042-7158.1966.tb07780.x. [DOI] [PubMed] [Google Scholar]
- Scheline R. R. Metabolism of phenolic acids by the rat intestinal microflora. Acta Pharmacol Toxicol (Copenh) 1968;26(2):189–205. doi: 10.1111/j.1600-0773.1968.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Mah R. A. Growth and methanogenesis by Methanosarcina strain 227 on acetate and methanol. Appl Environ Microbiol. 1978 Dec;36(6):870–879. doi: 10.1128/aem.36.6.870-879.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. F., Campbell W. L., Chinoy I. Anaerobic degradation of the benzene nucleus by a facultatively anaerobic microorganism. J Bacteriol. 1970 May;102(2):430–437. doi: 10.1128/jb.102.2.430-437.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. Structure and methylation of coenzyme M(HSCH2CH2SO3). J Biol Chem. 1974 Aug 10;249(15):4879–4885. [PubMed] [Google Scholar]
- Wilde P. F., Dawson R. M. The biohydrogenation of alpha-linolenic acid and oleic acid by rumen micro-organisms. Biochem J. 1966 Feb;98(2):469–475. doi: 10.1042/bj0980469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. J., Evans W. C. The metabolism of benzoate by Moraxella species through anaerobic nitrate respiration. Evidence for a reductive pathway. Biochem J. 1975 Apr;148(1):1–10. doi: 10.1042/bj1480001a. [DOI] [PMC free article] [PubMed] [Google Scholar]



