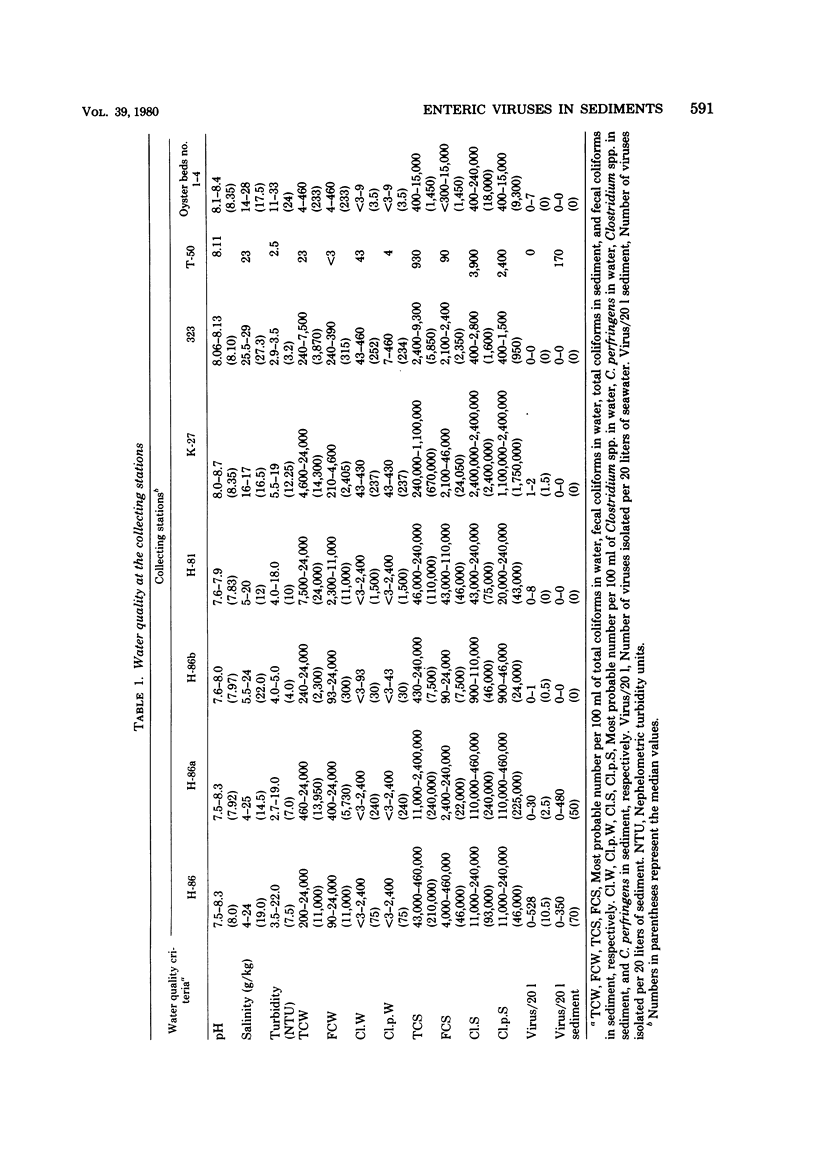
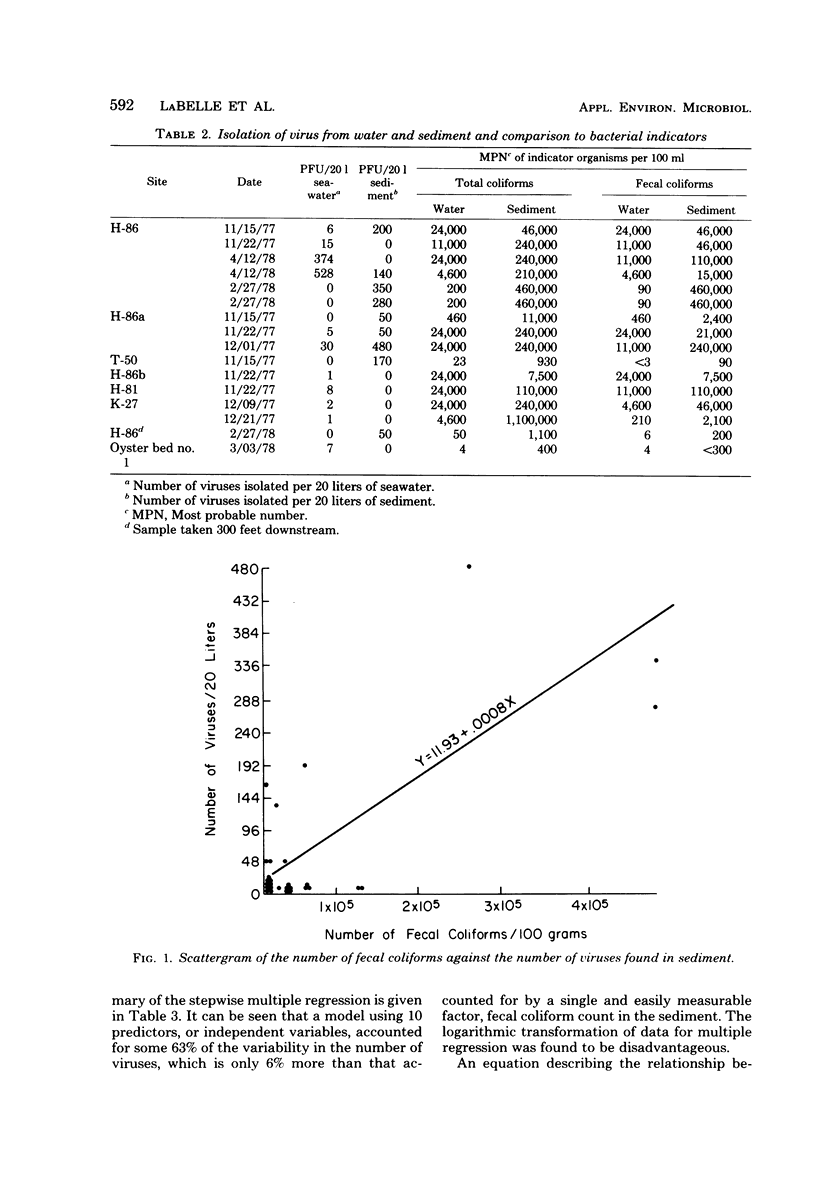
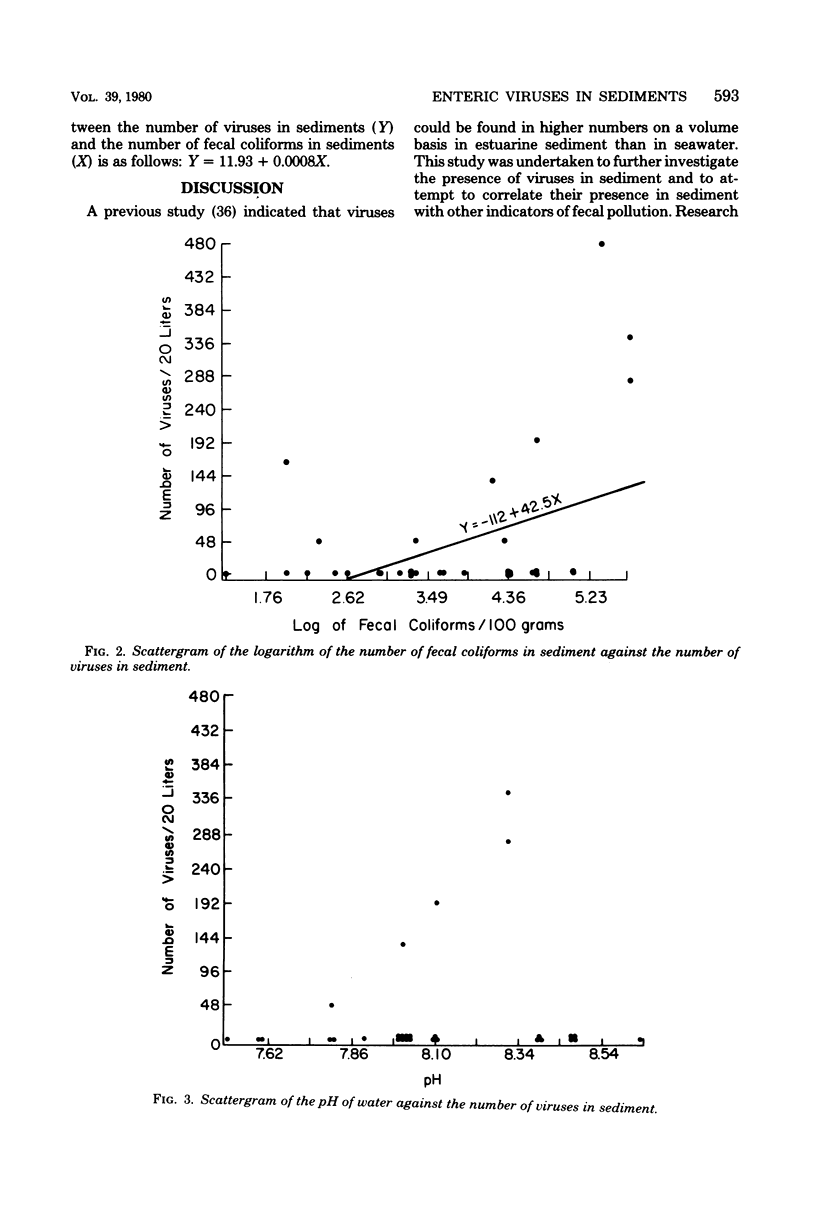
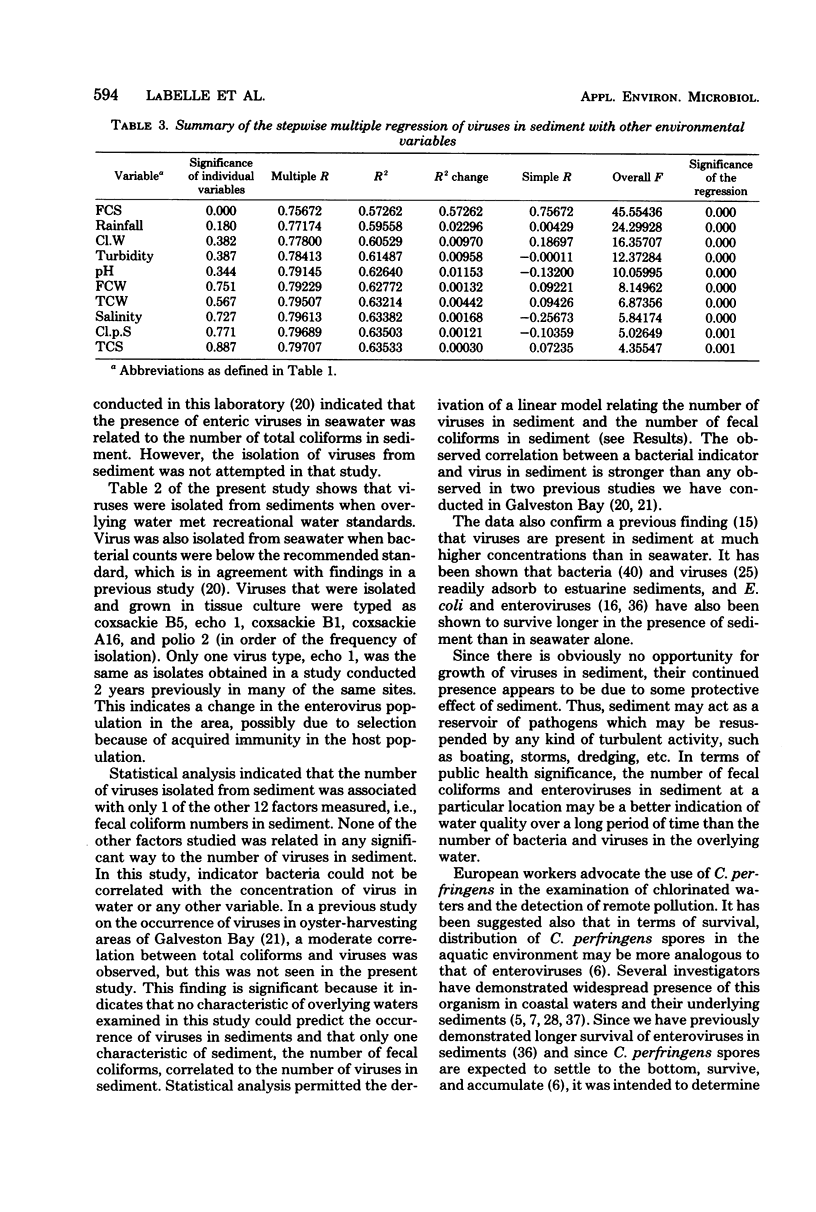
Abstract
Current standards for evaluation of the public health safety of recreational and shellfish-harvesting waters are based upon bacteriological analysis, but do not include an evaluation of the number of viruses. The objective of this study was to determine the occurrence of enteric viruses in estuarine sediments and to find a relationship, if any, between the presence of viruses in seawater or sediment or both and various biological and physicochemical characteristics of the environment. Viruses were found in greater numbers in sediment than in overlying seawater on a volume basis. Several types of enteroviruses were isolated: coxsackievirus types A16, B1, and B5, echovirus type 1, and poliovirus type 2. On several occasions, viruses were isolated from sediments when overlying seawaters met bacteriological water quality standards for recreational use. Statistical analysis of the relationship between viruses in seawater or in sediment and other variables measured yielded only one significant association: the number of viruses in sediment was found to be positively correlated with the number of fecal coliforms in sediment. No other physical, chemical, or biological characteristic of seawater or sediment that was measured showed statistically significant association with viral numbers. No correlation was found between bacterial indicators and virus in the overlying waters. The data indicated that evaluation of the presence of bacteria and viruses in sediment may provide additional insight into long-term water quality conditions and that indicator bacteria in water are not reflective of the concentration of enteric viruses in marine waters.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN L. A., GRINDLEY J., BROOKS E. Some chemical and bacterial characteristics of bottom deposits from lakes and estuaries. J Hyg (Lond) 1953 Jun;51(2):185–194. doi: 10.1017/s0022172400015618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avies J. A. Isolation and identification of clostridia from North Sea sediments. J Appl Bacteriol. 1969 Jun;32(2):164–169. doi: 10.1111/j.1365-2672.1969.tb00962.x. [DOI] [PubMed] [Google Scholar]
- Bisson J. W., Cabelli V. J. Membrane filter enumeration method for Clostridium perfringens. Appl Environ Microbiol. 1979 Jan;37(1):55–66. doi: 10.1128/aem.37.1.55-66.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Flora S., De Renzi G. P., Badolati G. Detection of animal viruses in coastal seawater and sediments. Appl Microbiol. 1975 Sep;30(3):472–475. doi: 10.1128/am.30.3.472-475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Gerba C. P., Wallis C., Melnick J. L. Concentration of viruses from large volumes of tap water using pleated membrane filters. Appl Environ Microbiol. 1976 Feb;31(2):221–226. doi: 10.1128/aem.31.2.221-226.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldreich E. E., Clarke N. A. Bacterial pollution indicators in the intestinal tract of freshwater fish. Appl Microbiol. 1966 May;14(3):429–437. doi: 10.1128/am.14.3.429-437.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., Farrah S. R., Goyal S. M., Wallis C., Melnick J. L. Concentration of enteroviruses from large volumes of tap water, treated sewage, and seawater. Appl Environ Microbiol. 1978 Mar;35(3):540–548. doi: 10.1128/aem.35.3.540-548.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., McLeod J. S. Effect of sediments on the survival of Escherichia coli in marine waters. Appl Environ Microbiol. 1976 Jul;32(1):114–120. doi: 10.1128/aem.32.1.114-120.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., Smith E. M., Melnick J. L. Development of a quantitative method for detecting enteroviruses in estuarine sediments. Appl Environ Microbiol. 1977 Aug;34(2):158–163. doi: 10.1128/aem.34.2.158-163.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S. M., Gerba C. P., Melnick J. L. Human enteroviruses in oysters and their overlying waters. Appl Environ Microbiol. 1979 Mar;37(3):572–581. doi: 10.1128/aem.37.3.572-581.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S. M., Gerba C. P., Melnick J. L. Occurrence and distribution of bacterial indicators and pathogens in canal communities along the Texas coast. Appl Environ Microbiol. 1977 Aug;34(2):139–149. doi: 10.1128/aem.34.2.139-149.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes D. J. Release of sediment-bound fecal coliforms by dredging. Appl Microbiol. 1975 Jan;29(1):109–111. doi: 10.1128/am.29.1.109-111.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks C. W. Increased recovery rate of salmonellae from stream bottom sediments versus surface waters. Appl Microbiol. 1971 Feb;21(2):379–380. doi: 10.1128/am.21.2.379-380.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff J. C., Becker R. C. The accumulation and elimination of crude and clarified poliovirus suxpensions by shellfish. Am J Epidemiol. 1969 Jul;90(1):53–61. doi: 10.1093/oxfordjournals.aje.a121049. [DOI] [PubMed] [Google Scholar]
- LaBelle R. L., Gerba C. P. Influence of pH, salinity, and organic matter on the adsorption of enteric viruses to estuarine sediment. Appl Environ Microbiol. 1979 Jul;38(1):93–101. doi: 10.1128/aem.38.1.93-101.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak P. A., Caraway C. T., Portnoy B. L. Oyster-associated hepatitis: lessons from the Louisiana experience. Am J Epidemiol. 1976 Feb;103(2):181–191. doi: 10.1093/oxfordjournals.aje.a112216. [DOI] [PubMed] [Google Scholar]
- Magnusson S., Hedström C. E., Lycke E. The virus inactivating capacity of sea water. Acta Pathol Microbiol Scand. 1966;66(4):551–559. doi: 10.1111/apm.1966.66.4.551. [DOI] [PubMed] [Google Scholar]
- Matches J. R., Liston J. Mesophilic clostridia in Puget Sound. Can J Microbiol. 1974 Jan;20(1):1–7. doi: 10.1139/m74-001. [DOI] [PubMed] [Google Scholar]
- Matson E. A., Hornor S. G., Buck J. D. Pollution indicators and other microorganisms in river sediment. J Water Pollut Control Fed. 1978 Jan;50(1):13–19. [PubMed] [Google Scholar]
- Smith E. M., Gerba C. P., Melnick J. L. Role of sediment in the persistence of enteroviruses in the estuarine environment. Appl Environ Microbiol. 1978 Apr;35(4):685–689. doi: 10.1128/aem.35.4.685-689.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. D. The clostridial flora of marine sediments from a productive and from a non-productive area. Can J Microbiol. 1968 Dec;14(12):1301–1304. doi: 10.1139/m68-218. [DOI] [PubMed] [Google Scholar]
- Wallis C., Grinstein S., Melnick J. L., Fields J. E. Concentration of viruses from sewage and excreta on insoluble polyelectrolytes. Appl Microbiol. 1969 Dec;18(6):1007–1014. doi: 10.1128/am.18.6.1007-1014.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


