Abstract
Selenomonas ruminantium accumulated large quantities of intracellular polysaccharide when grown in simple defined medium in a chemostat, particularly at low dilution rate under NH3 limitation when the carbohydrate content of the cells was greater than 40% of the dry weight. This polysaccharide was used as a source of energy under conditions of energy starvation. Abundant, densely staining cytoplasmic granules were observed by electron microscopy in sections stained by the periodic acid-thiocarbohydrazide-osmium technique. The polysaccharide was extracted in 30% KOH followed by precipitation with 60% ethanol and was found to be a glucose homopolymer. Sepharose 4B gel filtration and iodine-complex spectroscopy showed that the polysaccharide was of the glycogen type with a molecular weight of 5 X 10(5) to greater than 20 X 10(5) and an average chain length of 12 glucose residues.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. G., Lindberg B., Cheng K. J. Characterization of a reserve glucan from Megasphaera elsdenii. Can J Microbiol. 1975 Oct;21(10):1657–1659. doi: 10.1139/m75-242. [DOI] [PubMed] [Google Scholar]
- Brown R. G., Lindberg B., Laishley E. J. Characterization of two reserve glucans from Clostridium pasteurianum. Can J Microbiol. 1975 Jul;21(7):1136–1138. doi: 10.1139/m75-168. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Brown R. G., Costerton J. W. Characterization of a Cytoplasmic Reserve Glucan from Ruminococcus albus. Appl Environ Microbiol. 1977 Mar;33(3):718–724. doi: 10.1128/aem.33.3.718-724.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Hironaka R., Roberts D. W., Costerton J. W. Cytoplasmic glycogen inclusions in cells of anaerobic gram-negative rumen bacteria. Can J Microbiol. 1973 Dec;19(12):1501–1506. doi: 10.1139/m73-244. [DOI] [PubMed] [Google Scholar]
- Clark B., Holms W. H. Control of the sequential utilization of glucose and fructose by Escherichia coli. J Gen Microbiol. 1976 Aug;96(2):191–201. doi: 10.1099/00221287-95-2-191. [DOI] [PubMed] [Google Scholar]
- DOETSCH R. N., HOWARD B. H., MANN S. O., OXFORD A. E. Physiological factors in the production of an iodophilic polysaccharide from pentose by a sheep rumen bacterium. J Gen Microbiol. 1957 Feb;16(1):157–168. doi: 10.1099/00221287-16-1-156. [DOI] [PubMed] [Google Scholar]
- HANKER J. S., SEAMAN A. R., WEISS L. P., UENO H., BERGMAN R. A., SELIGMAN A. M. OSMIOPHILIC REAGENTS: NEW CYTOCHEMICAL PRINCIPLE FOR LIGHT AND ELECTRON MICROSCOPY. Science. 1964 Nov 20;146(3647):1039–1043. doi: 10.1126/science.146.3647.1039. [DOI] [PubMed] [Google Scholar]
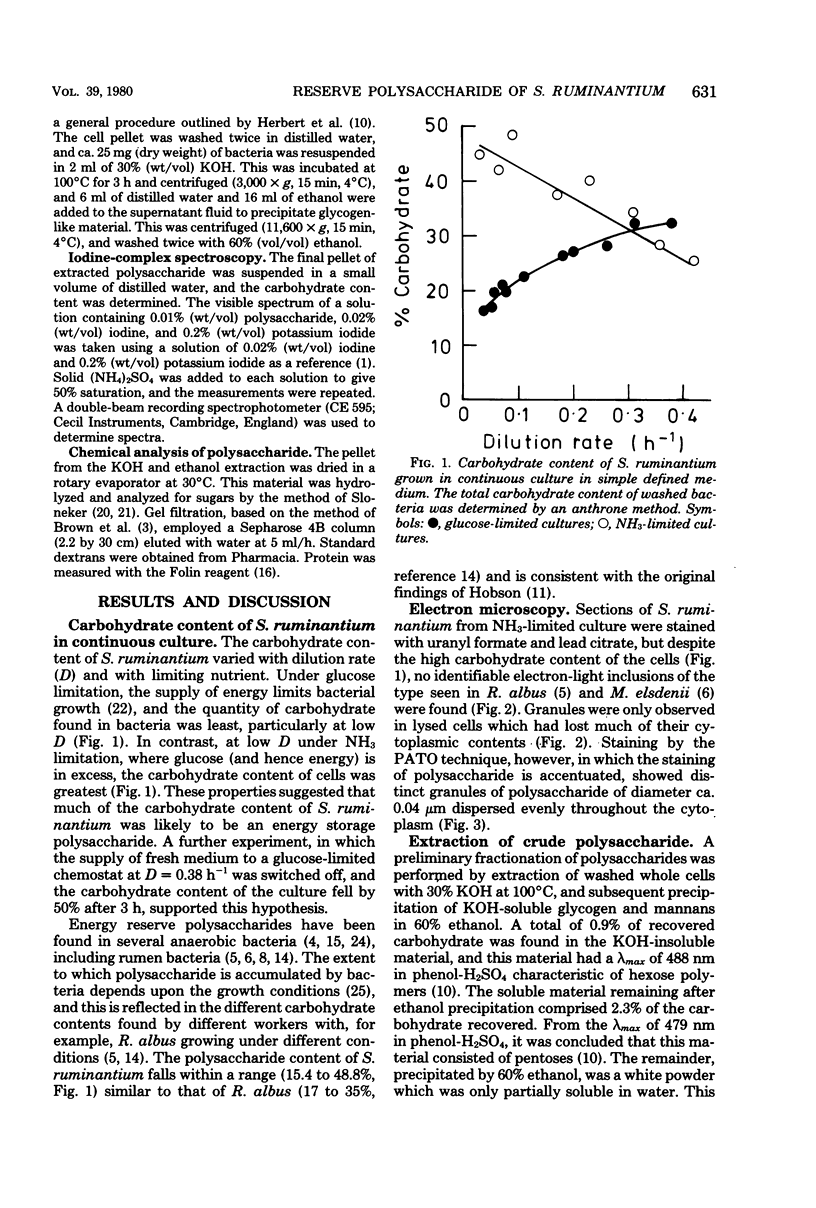
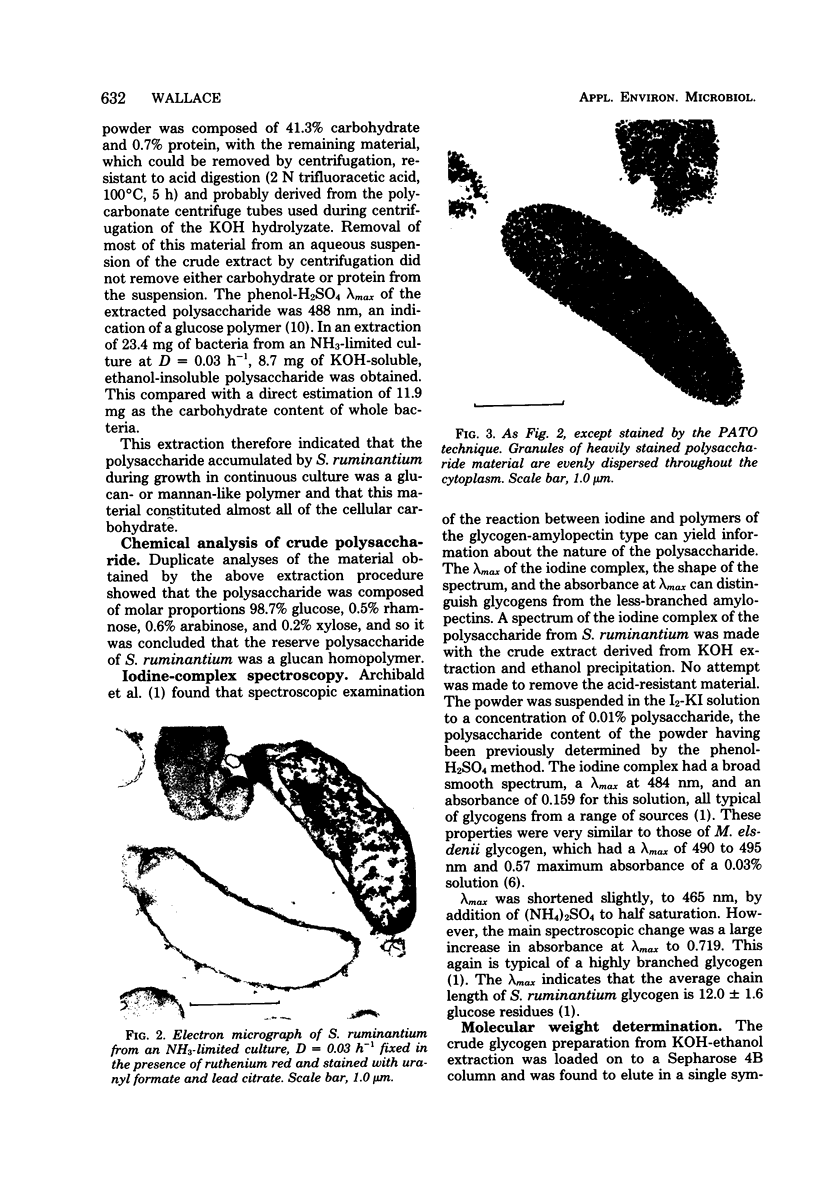
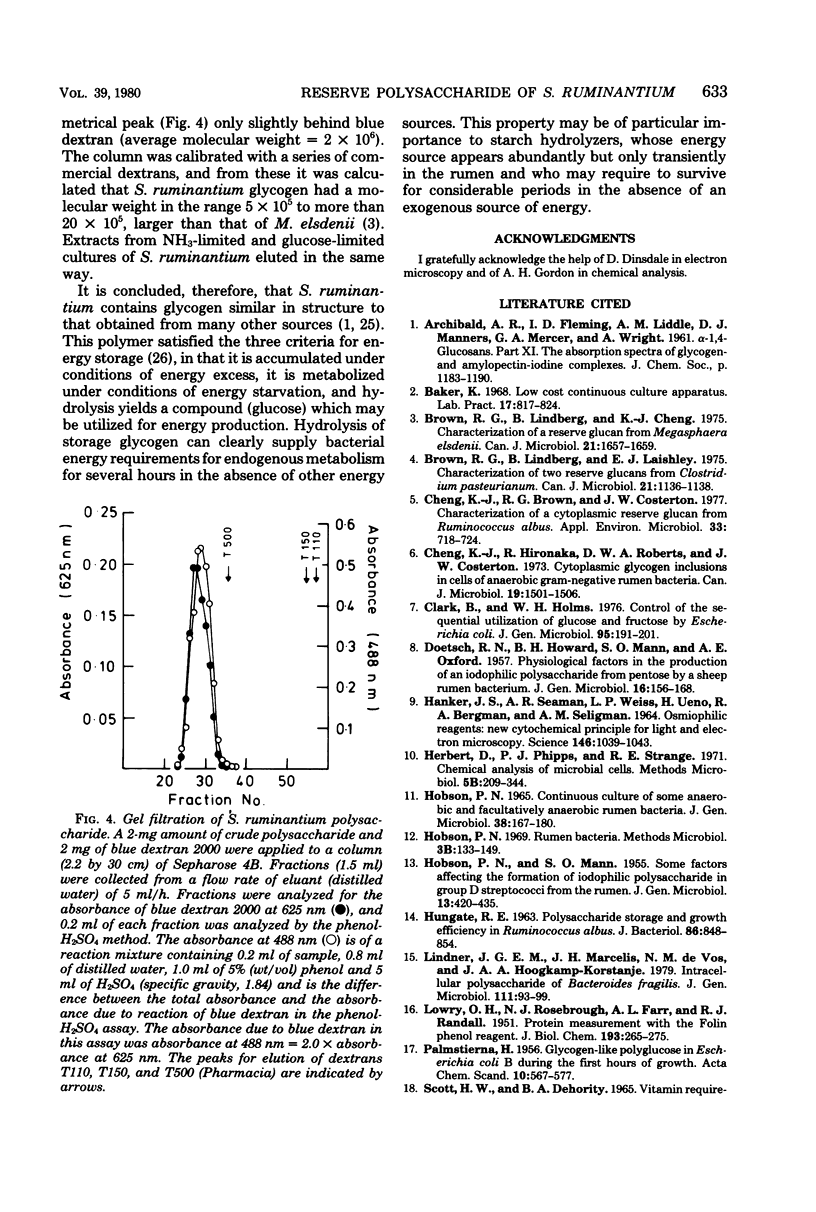
- HOBSON P. N. CONTINUOUS CULTURE OF SOME ANEROBIC AND FACULTATIVELY ANAEROBIC RUMEN BACTERIA. J Gen Microbiol. 1965 Feb;38:167–180. doi: 10.1099/00221287-38-2-167. [DOI] [PubMed] [Google Scholar]
- HOBSON P. N., MANN S. O. Some factors affecting the formation of iodophilic polysaccharide in group D streptococci from the rumen. J Gen Microbiol. 1955 Dec;13(3):420–435. doi: 10.1099/00221287-13-3-420. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. POLYSACCHARIDE STORAGE AND GROWTH EFFICIENCY IN RUMINOCOCCUS ALBUS. J Bacteriol. 1963 Oct;86:848–854. doi: 10.1128/jb.86.4.848-854.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindner J. G., Marcelis J. H., de Vos N. M., Hoogkamp-Korstanje J. A. Intracellular polysaccharide of Bacteroides fragilis. J Gen Microbiol. 1979 Mar;111(1):93–99. doi: 10.1099/00221287-111-1-93. [DOI] [PubMed] [Google Scholar]
- Sloneker J. H. Determination of cellulose and apparent hemicellulose in plant tissue by gas-liquid chromatography. Anal Biochem. 1971 Oct;43(2):539–546. doi: 10.1016/0003-2697(71)90285-5. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Cheng K. J., Dinsdale D., Orskov E. R. An independent microbial flora of the epithelium and its role in the ecomicrobiology of the rumen. Nature. 1979 May 31;279(5712):424–426. doi: 10.1038/279424a0. [DOI] [PubMed] [Google Scholar]
- Wallace R. J. Control of lactate production by Selenomonas ruminantium: homotropic activation of lactate dehydrogenase by pyruvate. J Gen Microbiol. 1978 Jul;107(1):45–52. doi: 10.1099/00221287-107-1-45. [DOI] [PubMed] [Google Scholar]
- Whyte J. N., Strasdine G. A. An intracellular alpha-D-glucan from Clostridium botulinum, type E. Carbohydr Res. 1972 Dec;25(2):435–441. doi: 10.1016/s0008-6215(00)81655-9. [DOI] [PubMed] [Google Scholar]