Abstract
Neural crest cells, the migratory precursors of numerous cell types including the vertebrate peripheral nervous system, arise in the dorsal neural tube and follow prescribed routes into the embryonic periphery. While the timing and location of neural crest migratory pathways has been well documented in the trunk, a comprehensive collection of signals that guides neural crest migration along these paths has only recently been established. In this review, we outline the molecular cascade of events during trunk neural crest development. After describing the sequential routes taken by trunk neural crest cells, we consider the guidance cues that pattern these neural crest trajectories. We pay particular attention to segmental neural crest development and the steps and signals that generate a metameric peripheral nervous system, attempting to reconcile conflicting observations in chick and mouse. Finally, we compare cranial and trunk neural crest development in order to highlight common themes.
Keywords: Neural crest, migration, trunk, segmental, guidance, peripheral nervous system
Introduction
As neural crest cells delaminate from the neural tube, the physical and molecular neighborhood in which they emerge determines where they will migrate. Tissues that surround the length of the neural tube produce distinct positive and negative cues that guide neural crest cells along defined pathways into the periphery. In the head, signals in the cranial mesenchyme sculpt migrating neural crest cells into streams that invade the segmented branchial arches (see accompanying review by Kulesa et al. in this volume). Meanwhile, trunk neural crest development is dominated by the physical structure of the somites. For example, somite-derived signals elicit segmental neural crest migration as well as the metameric organization of the resulting peripheral ganglia (Fig. 1). Building peripheral nervous system (PNS) development on somite pattern ensures that peripheral ganglia and nerves form in register with the somite-derived vertebrae.
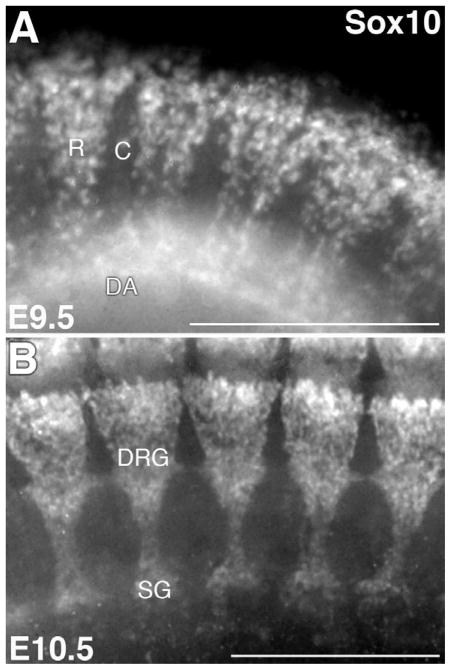
Figure 1. Segmental trunk neural crest migration and dorsal root gangliogenesis.
Images of Sox10 immunostained (Santa Cruz Biotechnologies sc-17342) whole mount mouse embryos at E9.5 and E10.5 illustrate segmental neural crest migration (A) and resulting metameric peripheral ganglia (B). R, rostral; C, caudal; DA, dorsal aorta; DRG, dorsal root ganglia; SG, sympathetic ganglia. Scale bars = 300 microns.
The identity of the signals that pattern trunk neural crest development has intrigued biologists since the many pathways available to migratory trunk neural crest cells were initially traced. In particular, the molecules that guide segmental neural crest migration through the somites (Fig. 1A) have been the subject of intense interest since the phenomenon was first described (Bronner-Fraser, 1986; Rickman et al., 1985). While it has long been clear that the pattern of neural crest migration is dependent upon signals from the somite, identifying these signals proved a challenge (Bronner-Fraser and Stern, 1991; Kalcheim and Teillet, 1989). Although observations initially suggested that multiple, redundant signaling pathways produced a metameric trunk PNS (reviewed in Krull, 2001; Kuan et al., 2004), recent insights allow precise roles to be ascribed to particular signaling pathways during this process. As a result, a molecular cascade of events that guides trunk neural crest migration and differentiation is beginning to emerge.
In this review, we will consider the molecular regulation of trunk neural crest development in chick and mouse. First, we will outline the participating cell types and the resulting trunk neural crest derivatives. Next, we will document the steps involved, from neural crest formation to the generation of a segmented PNS. Then, we will detail the signaling pathways that guide each phase of neural crest migration and differentiation to achieve trunk PNS metamerism. We will contrast results obtained in chick and mouse, attempting to reconcile differences to outline a unified model for trunk neural crest development. Finally, we will compare cranial and trunk neural crest development in order to highlight common mechanisms.
The structures of trunk PNS development
Multiple cell types participate in generating a segmented PNS (Fig. 2A). In addition to the neural tube, from which neural crest cells delaminate along the dorsal side, the somites, metameric mesodermal structures lateral to the neural tube, are crucial as physical impediments, a substrate for migration, and a source of signals. Initially epithelial balls, somites dissociate on their ventral sides, producing two distinct tissue layers: the persistently epithelial dermomyotome dorsally and the loosely packed sclerotome ventrally. Ventral to the somites, the notochord repels migratory neural crest cells, causing them to gather at the dorsal aorta where they form the sympathetic ganglia.
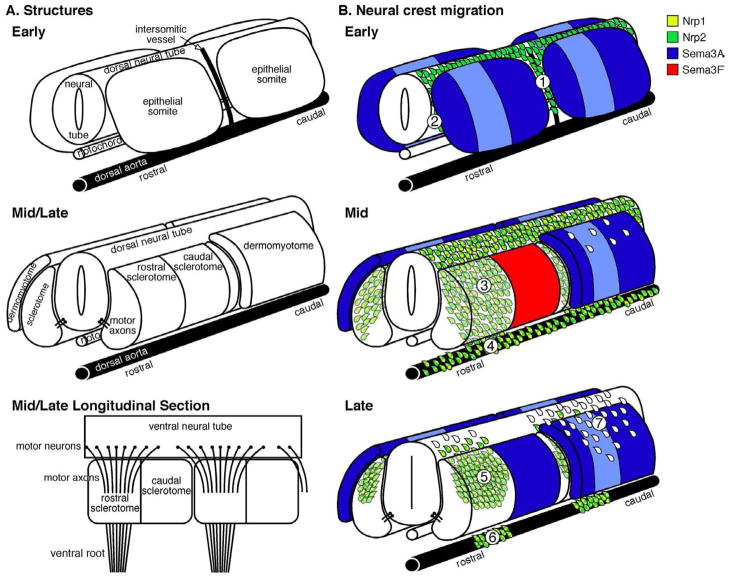
Figure 2. The structures and pathways of trunk neural crest migration.
The vertebrate trunk at the level of the forelimb with the epidermal ectoderm removed is pictured at early (Hamburger and Hamilton (HH) stage 14 in chick and E9.0 in mouse), mid (HH stage 15/16 in chick and E9.5 in mouse), and late (HH stage 20 in chick and E10/10.5 in mouse) stages of neural crest development (Erickson et al., 1992; Serbedzija et al., 1990; Teillet et al., 1987). Rostral to the left, caudal to the right. (A) Neural crest cells encounter a variety of structures as they emigrate from the dorsal neural tube and migrate into the periphery. These structures change as development proceeds. In particular, early, epithelial somites dissociate to give rise to dermomyotome and sclerotome in mid and late stage embryos. (B) As trunk structures develop, the pathways taken by migratory neural crest cells evolve as well. During early migration, neural crest cells migrate ventrally between the somites along intersomitic vessels (1) and between the neural tube and somites (2). In the middle phase of migration, neural crest cells migrate ventrolaterally to invade only the rostral sclerotome (3), with some neural crest cells continuing past the somite to collect at the dorsal aorta, where they disperse along its length (4). In mouse, a few neural crest cells migrate dorsolaterally over the dermomyotome at this stage, while in chick this pathway is closed. In late migration, neural crest cells within the somite condense into segmental dorsal root ganglia (5), while those at the dorsal aorta segregate into segmental sympathetic ganglia (6). As seen in a ventral longitudinal section at this stage, motor neurons in the ventral neural tube/spinal cord project axons through only the rostral sclerotome as well. The dorsolateral pathway opens late in migration, and neural crest cells begin to migrate over the dermomyotome (7). Number labels correspond to the numbers in Table 1. The expression of neuropilin (Nrp) receptors and semaphorin (Sema) ligands are included in B as an example because their dynamic expression patterns and diverse roles during trunk neural crest development illustrate the many steps required to create a metameric PNS. During early migration, neural crest cells express Nrp2 and presumptive dermomyotome within epithelial somites expresses Sema3A (L.S.G. and J.R.A., unpublished observations). As the somites dissociate during the middle phase of migration, neural crest cells upregulate Nrp1 (Schwarz et al., 2009b). As a result Sema3A, which is most abundantly expressed in two stripes in the rostral- and caudal-most dermomyotome and starting to be upregulated in the caudal sclerotome, repels neural crest cells from the intersomitic space (Schwarz et al., 2009b). Neural crest cells maintain Nrp2 expression, restricting their migration to the rostral sclerotome due to Sema3F production by the caudal sclerotome (Gammill et al., 2006). In the late phase of migration, Sema3F is downregulated in the caudal sclerotome while Sema3A expression reaches high levels, driving segmental dorsal root gangliogenesis (Roffers-Agarwal and Gammill, 2009). Because Nrp1Sema−/− neural crest cells migrate ectopically in the dorsolateral path, and Sema3A expression in the dermomyotome persists during late migration, we presume that neuropilin expression is downregulated in late, dorsolaterally migrating neural crest cells, although this has not been shown directly (Roffers-Agarwal and Gammill, 2009; Schwarz et al., 2009b). Nrp2 expression in dorsolaterally migrating neural crest cells has not been determined.
Trunk neural crest cells differentiate into a variety of cell types. These include the neurons and glia of the dorsal root and sympathetic ganglia, Schwann cells that line the ventral roots, adrenal chromaffin cells, and pigment cells (Le Douarin and Kalcheim, 1999). Following neurogenesis, axons project from the dorsal root (sensory) and sympathetic ganglia. These neural crest derived neurites join motor axons that project from the ventral neural tube/spinal cord, fasciculating to form the mixed spinal nerves. “Cardiac” neural crest cells, which migrate from the neural tube between the caudal hindbrain and somite 3, form the aorticopulmonary septum of the heart. Meanwhile, “enteric” neural crest cells that arise at the level of somites 1 through 7 colonize the gut to form the enteric nervous system (ENS). Cardiac neural crest and ENS development, which were recently reviewed elsewhere, are beyond the scope of this review (Heanue and Pachnis, 2007; Hutson and Kirby, 2007).
The stages and features of trunk peripheral nervous system development
Neural crest induction results in the expression of an evolutionarily conserved neural crest gene regulatory network at the borders of the neural plate (reviewed in Sauka-Spengler and Bronner-Fraser, 2008). Following neurulation, these cells with the potential to become migratory neural crest cells ultimately reside within the dorsal neural tube. As development proceeds, maturing somites signal to the dorsal neural tube, leading to downregulation of noggin, activation of BMP signaling, and upregulation of Wnt expression (Burstyn-Cohen et al., 2004; Sela-Donenfeld and Kalchiem, 1999). This triggers neural crest precursors to undergo an epithelial to mesenchymal transition through a variety of mechanisms, and neural crest cells become migratory (Ahlstrom and Erickson, 2009). Neural crest cells leave the neural tube in a rostral to caudal wave. In the trunk, they emerge uniformly along the axis into an adjacent cell-free space bounded by the neural tube, somites, and overlying non-neural ectoderm (Loring and Erickson, 1987; Serbedzija et al., 1990; Weston, 1991). From this site, neural crest cells enter a variety of possible paths (Fig. 2B). Initially, neural crest cells migrate ventrally around the epithelial somites, mostly along blood vessels in the intersomitic space (Fig. 2B1) but also between the somite and the neural tube (Fig. 2B2; (Loring and Erickson, 1987; Schwarz et al., 2009b; Teillet et al., 1987). Upon somite maturation and dissociation into dermomyotome and sclerotome, neural crest cells begin to invade the sclerotome and migrate ventrolaterally (Fig. 2B3), with early arrivals preferring the myotome basal lamina as a substrate for migration (Loring and Erickson, 1987; Teillet et al., 1987; Tosney et al., 1994). Strikingly, neural crest migration through the sclerotome is segmental, with neural crest cells traversing only the rostral portion of each somitic sclerotome (Bronner-Fraser, 1986; Rickman et al., 1985; Serbedzija et al., 1990; Fig. 1A). Finally, as ventral migration ends, neural crest cells migrate along a dorsolateral pathway (Fig. 2B7) between the epidermal ectoderm and dermomyotome (Erickson et al., 1992; Serbedzija et al., 1990; Serbedzija et al., 1989). Although avian neural crest cells delay dorsolateral pathway entrance until a day after the onset of neural crest migration, it appears at least some murine neural crest cells migrate dorsolaterally concomitant with ventrolateral migration in the mouse (Erickson et al., 1992; Serbedzija et al., 1990; Serbedzija et al., 1989).
The pathway a neural crest cell enters predicts its eventual fate. Neural crest cells that migrate in the intersomitic space between the epithelial somites reach the dorsal aorta (Fig. 2B4) and become neurons and glia of the sympathetic ganglia (Fig. 2B6) as well as adrenal chromaffin cells (Kulesa et al., 2009; Thiery et al., 1982). Those that invade the sclerotome and pass all the way through can also contribute to sympathetic ganglia, while those that remain within the sclerotome coalesce to form the sensory neurons and glia of the dorsal root ganglia (Fig. 2B5) and Schwann cells of the ventral roots (Kasemeier-Kulesa et al., 2005; Serbedzija et al., 1990; Teillet et al., 1987). Neural crest cells that wait to enter the dorsolateral pathway become melanocytes (Le Douarin and Kalcheim, 1999). Thus, the migratory pathway limits structures a neural crest cell can become.
Does the path of migration determine or reflect lineage? At least some neural crest cells are multipotent and undergo progressive restrictions in their developmental potential as a result of environmental cues (Bronner-Fraser and Fraser, 1989; Le Douarin and Dupin, 2003; Serbedzija et al., 1994). For example, when individual migratory neural crest cells are labeled in the trunk either before or during migration, some progeny contribute to multiple neural crest-derived structures (Bronner-Fraser and Fraser, 1988, 1989; Collazo et al., 1993; Fraser and Bronner-Fraser, 1991; Serbedzija et al., 1994). In other words, the environment the cell invades determines its fate. However, other evidence suggests that neural crest cell fate is specified prior to migration. Although neural crest cells migrate equally from medial and lateral positions in the dorsal neural tube (Ahlstrom and Erickson, 2009), specifically at the dorsal midline, delaminating neural crest cells are largely fate-restricted (Krispin et al., 2010). In addition, some neural crest cells are biased toward sensory versus autonomic fates (reviewed in Anderson, 2000; Harris and Erickson, 2007), and fate-restricted, nociceptive sensory precursors have been identified (George et al., 2007). Melanocytes in particular are specified before migrating, and only melanocytic precursors are able to enter the dorsolateral path (Erickson and Goins, 1995; Reedy et al., 1998; Wilson et al., 2004). Together, these data argue that migratory pathways are both instructive and reflective of neural crest cell fate determination.
Once neural crest cells have dispersed to the periphery and differentiated as ganglia, axons grow out to connect the spinal cord, peripheral ganglia, and peripheral targets and complete sensory and sympathetic circuits. Like the neural crest cells that came before them, motor axons that extend from the ventral spinal cord also enter only the rostral portion of each sclerotome (Keynes and Stern, 1984; Fig. 2A). Motor axons are thus positionally aligned to join dorsal root ganglia sensory projections, and the sensorimotor circuit is precisely placed between the developing vertebrae.
The signals that guide trunk neural crest development
Neural crest cells are guided along temporally and spatially defined routes by environmental guidance cues. Each pathway is shaped by the expression of a distinct mix of proteins that both attract and repel migrating neural crest cells. In recent years, a large collection of these signals has been defined (Table 1). In the following section, we outline the stepwise process for the molecular regulation of trunk neural crest development, attempting to reconcile conflicting observations from different organisms.
Table 1.
The phases of trunk neural crest migration and the responsible environmental guidance cues.
| Phase | Pathway | Cue | Proposed Role | Reference | |
|---|---|---|---|---|---|
| All | All | Fibronectin, laminin | Permissive, integrin adhesive substrate for neural crest migration | Duband and Thiery, 1987; Newgreen and Thiery, 1980 | |
| Initial | 1. Intersomitic | None known | + | Attracts neural crest cells to intersomitic vessels | Erickson, 1985 |
| 2. Over neural tube | None known | ? | Promotes neural crest migration between the neural tube and somites | ||
| Mid | 3. Ventrolateral Somites | Semaphorin3A in dermomyotome | − | Repels Neuropilin 1 expressing neural crest cells from intersomitic space | Schwarz et al., 2009 |
| Semaphorin3F in caudal sclerotome | − | Restricts Neuropilin 2 expressing neural crest cells and motor axons to rostral sclerotome | Gammill et al., 2006; Roffers-Agarwal and Gammill, 2009 | ||
| ephrins in caudal sclerotome | − | Trigger cell-cell interactions in Eph expressing neural crest cells leading to segmental migration in chick | Krull et al., 1995; Krull et al., 1997; Wang and Anderson, 1997 | ||
| CXCL12 in sclerotome | + | Attracts CXCR4 expressing migratory neural crest cells ventrally into the sclerotome | Belmadani et al., 2005 | ||
| F-spondin in caudal sclerotome | − | Integrin anti-adhesion | Debby-Brafman et al., 1999; Terai et al., 2001 | ||
| Proteoglycans in caudal sclerotome | − | Localize and potentiate semaphorin signaling | De Wit et al., 2005; Kubota et al., 1999; Landolt et al., 1995; Oakley and Tosney, 1991; Perissinotto et al., 2000; Ring et al., 1996 | ||
| Peanut agglutinin (PNA) binding Gal-β(1-3)-GalNAc glycoproteins | − | Glycan moiety is required for segmental neural crest migration, the glycosylated proteins are unknown | Davies et al., 1990; Krull et al., 1995; Oakley and Tosney, 1991; Stern et al., 1986 | ||
| T-cadherin | − | Reduces adhesion to caudal sclerotome | Ranscht and Bronner-Fraser, 1991 | ||
| Thrombospondin in myotome | + | Promotes neural crest migration and proliferation | Tucker et al., 1999 | ||
| 4. Ventrolateral Dorsal aorta | Neuregulin in mesenchyme around dorsal aorta | + | Attracts ErbB2/B3 expressing neural crest cells ventrally past the sclerotome | Britsch et al., 1998 | |
| CXCL12 at the dorsal aorta | + | Attracts CXCR4 expressing migratory neural crest cells to the dorsal aorta | Kasemeier-Kulesa et al., in press | ||
| Artemin in peripheral blood vessels | + | Attraction and proliferation of GFRα expressing sympathetic precursors | Honma et al., 2002 | ||
| Semaphorin3A in limbs, dermomyotome and notochord | − | Restricts Neuropilin1 expressing neural crest cells near the dorsal aorta | Kawasaki et al., 2002 | ||
| Late | 5. Ventrolateral Somites | Semaphorin3A in caudal sclerotome | − | Drives metameric dorsal root gangliogenesis and ventral root formation | Roffers-Agarwal and Gammill, 2009 |
| 6. Ventrolateral Dorsal aorta | Segmental ephrinB1 expression in ventral mesenchyme | − | Sorts EphB2 expressing sympathetic precursors into segmental condensations | Kasemeier-Kulesa et al., 2006 | |
| CXCL12 at the dorsal aorta | + | Targets CXCR4-expressing sympathetic precursors to the ganglia core | Kasemeier-Kulesa et al., in press | ||
| Artemin in peripheral blood vessels | + | Segments GFRα expressing sympathetic precursors | Honma et al., 2002 | ||
| 7. Dorsolateral | Slit expression in dermomyotome during early/mid migration | − | Repels Robo expressing neural crest cells from the dorsolateral path | Jia et al., 2005 | |
| ephrin expression in the dermomyotome | − | Repels EphB3 expressing early/mid phase migratory neural crest cells, attracts EphB3 expressing melanoblasts | Harris et al., 2008; Santiago and Erickson, 2002 | ||
| CHICK: endothelin3 in dermomyotome and ectoderm | + | Attracts EDNRB2 expressing melanoblasts | Harris et al., 2008; Lecoin et al., 1998 | ||
| MOUSE: Kit ligand in dermomyotome | + | Attracts Kit expressing neural crest cells | Wehrle-Haller et al., 2001; Wehrle-Haller and Weston, 1995 | ||
| F-spondin in dermomyotome | − | Integrin anti-adhesion | Debby-Brafman et al., 1999; Terai et al., 2001 | ||
| Peanut agglutinin (PNA) binding Gal-β(1-3)-GalNAc glycoproteins | − | Transiently expressed PNA-binding glycoproteins in the dermomyotome block dorsolateral neural crest migration | Oakley et al., 1994 | ||
Initial migration: the ventral pathway
The first neural crest cells to emerge from the dorsal neural tube migrate around the somites: between adjacent somites (Fig. 2B1) or between the somite and neural tube (Fig. 2B2). It is not clear whether these pathways are the only routes available to neural crest cells at this stage, or whether signals actively drive neural crest cells under and between the somites. Neural crest cells migrating in the intersomitic space follow blood vessels, which may simply provide traction or could be a source of chemotactic factors (Schwarz et al., 2009b). In support of the latter, neural crest cells grafted at the level of epithelial somites preferentially associate with intersomitic vessels (Erickson, 1985). However, the molecular basis for this preference is unknown. One possibility is that Eph/ephrin signaling mediates neural crest/intersomitic vessel attraction. Neural crest cells express Eph receptors, including EphB1, B2 and B3 (Krull et al., 1997; Santiago and Erickson, 2002; Wang and Anderson, 1997). The initial wave of trunk neural crest migration is disorganized in ephrinB2 mutant mice (Davy and Soriano, 2007), and the intersomitic vasculature is abnormal in these mutants (Adams et al., 1999; Oike et al., 2002). Unfortunately the location of misrouted early migrating neural crest cells within ephrinB2 mutants (eg: between the somites and epidermal ectoderm or between the somites and neural tube) was not determined, but is worth investigating (Davy and Soriano, 2007). Artemin, the GDNF family factor that is attractive for sympathetic precursors and later expressed by intercostal vessels, also warrants consideration as a signal that draws neural crest cells into the intersomitic space (Honma et al., 2002).
Somite morphogenesis: opening up the ventrolateral pathway
Neural crest migratory routes are tied to somite development (Loring and Erickson, 1987; Teillet et al., 1987; Tosney et al., 1994). The earliest migrating neural crest cells encounter epithelial somites. Cells of the outer, epithelial layer of early somites are tightly connected to one another through junctional complexes and the external surface is surrounded by a basal lamina (Duband et al., 1987; Newgreen and Thiery, 1980; Solursh et al., 1979). As neural crest cells are unable to cross basal laminae or tight junctions (Erickson, 1987), it is possible that the ventrolateral pathway through the somite is simply not available to neural crest cells upon their initial migration and so pathways under and between the somites are utilized instead (Tosney et al., 1994). Following the epithelial to mesenchymal transition of the ventral somite, sclerotome cells become disconnected, dispersed and accessible to invasive neural crest cells (Duband et al., 1987; Solursh et al., 1979). This undoubtedly reveals uniformly expressed permissive, migration-friendly extracellular matrix signals like fibronectin, laminin, and collagen (Duband and Thiery, 1987; Newgreen and Thiery, 1980), as well as instructive factors that are differentially expressed in the rostral and caudal sclerotome (see below). Environmental cues that regulate the timing of somite invasion could also be involved, as sacral neural crest cells invade the somite before the myotome develops (Erickson and Goins, 2000).
Diverting cells out of the ventral pathway: Nrp1/Sema3A
During the second wave of migration, neural crest cells are actively driven out of the intersomitic space to invade the sclerotome. Early migrating neural crest cells do not express the receptor neuropilin 1 (Nrp1), while later migrating cells do (Eickholt et al., 1999; Schwarz et al., 2009b; Fig. 2B). Meanwhile, semaphorin 3A (Sema3A), the Nrp1 repulsive ligand, is strongly expressed in the dermomyotome adjacent to the intersomitic space, gradually spreading to the caudal-most sclerotome in intermediate phases of migration (Roffers-Agarwal and Gammill, 2009; Schwarz et al., 2009b; Fig. 2B). Trunk neural crest cells avoid Sema3A immobilized substrata in culture (Eickholt et al., 1999), and in Nrp1 and Sema3A mutant mice, neural crest cells continue to migrate between the somites long after they have been redirected to the rostral sclerotome in wildtypes (Roffers-Agarwal and Gammill, 2009; Schwarz et al., 2009b). This indicates that Nrp1/Sema3A signaling actively diverts neural crest cells away from the intersomitic space. Furthermore, this repulsion and the switch to the ventrolateral pathway is important for the formation of peripheral ganglia, as ectopic sensory and sympathetic neurons are observed in Nrp1 mutants (Kawasaki et al., 2002; Schwarz et al., 2009b).
Segmental migration through the rostral sclerotome: Nrp2/Sema3F
Once the sclerotome dissociates, neural crest cells invade the ventrolateral path in a segmental fashion (Fig. 1A; Fig. 2B3). Arguably the most visually striking event during trunk neural crest development, intense effort has focused on identifying the factors that restrict neural crest cells to the rostral sclerotome. Consequently, a number of signals were implicated in segmental migration through in vitro and in vivo assays in the chick, and it was proposed that segmental neural crest migration was guided by a collection of redundantly acting factors (Krull, 2001; Kuan et al., 2004).
On the contrary, the entirely non-segmental migration of neural crest cells in Nrp2 or Sema3F mutant mice established the central importance this single receptor/ligand pair in restricting neural crest cells to the rostral sclerotome (Gammill et al., 2006; Fig. 2B3). Neural crest cells express the Nrp2 receptor, while its secreted ligand Sema3F is expressed by caudal sclerotome (Fig. 2B). Neural crest cells are present uniformly throughout the sclerotome of Nrp2 and Sema3F mutant mice. Furthermore, the rostrocaudal polarity of the somites is intact, and there is a cell-intrinsic requirement for the Nrp2 receptor on neural crest cells to avoid Sema3F in the environment. These results indicate that Nrp2-expressing neural crest cells detect Sema3F as a repulsive cue in the caudal sclerotome, eliciting segmental migration. Nrp2 is expressed in an identical pattern in chick embryos, and preliminary data suggest that Nrp2 is also required for chick segmental trunk neural crest migration (Gammill et al., 2006; R. McLennan and P.M. Kulesa, personal communication).
Other influences on chick neural crest migration through the somite
While numerous other factors have been proposed to guide rostral-only migration through the sclerotome, thus far only Nrp2/Sema3F signaling is essential in the mouse. As all other signaling pathways are intact in Nrp2 and Sema3F mutant mice, the complete absence of segmental migration suggests that these pathways are not sufficient for rostrocaudal patterning of murine neural crest migration. However, striking expression patterns and effects in functional assays indicate they are involved. We consider these additional influences in turn, outlining their possible roles in this process.
Eph/ephrins
Data in the chick implicate Eph/ephrin signaling during neural crest migration through the somite. Chick neural crest cells express a variety of Eph receptors, while ephrin ligands are restricted to the caudal sclerotome (Krull et al., 1997; McLennan and Krull, 2002; Santiago and Erickson, 2002). Intriguingly, application of secreted subclass A and B Eph and ephrin receptors to chick explants causes rostrocaudal migration defects (Krull et al., 1997; McLennan and Krull, 2002). Importantly, this effect is context-dependent. 33% of neural crest cells invade the caudal sclerotome following dimeric ephrin-B1-Fc treatment, which partially stimulates EphB2 receptor activation, while 47% of neural crest cells enter the caudal sclerotome following monomeric ephrin-B1-myc treatment, which does not stimulate and presumably interferes with receptor activation (Krull et al., 1997).
While it was concluded that Eph/ephrin signaling guides segmental neural crest migration, this possibility is thus far not supported by findings in mice. Mouse ephrin-B1 and -B2 are restricted to the caudal sclerotome and trunk neural crest cells express their receptors as in chick (Wang and Anderson, 1997). However, mice mutant for Ephs and ephrins do not exhibit trunk segmental migration phenotypes (Adams et al., 2001; Davy et al., 2004; Orioli et al., 1996; Wang et al., 1998) unless accompanied by somite patterning defects, including misexpression of Sema3F within the anterior sclerotome (Davy and Soriano, 2007). Meanwhile, neural crest cells migrate non-segmentally in Nrp2 and Sema3F mutants despite normal expression of ephrin-B2 in properly patterned somites (Gammill et al., 2006). This suggests that intact Eph/ephrin signaling is not sufficient for guidance of neural crest cells through the rostral sclerotome in Nrp2 and Sema3F mutant animals. Do Ephs and ephrins exhibit identical expression patterns in chick and mouse, but exert functionally divergent roles in these organisms? Do different vertebrates emphasize different signaling pathways in generating a segmental PNS?
One possibility is that Eph/ephrin signaling is not a guidance cue in the sclerotome, but instead triggers migratory trunk neural crest cell-cell interactions essential for guidance. Trunk neural crest cells normally migrate in chains, maintaining filopodial contact with at least one other cell as they migrate (Kasemeier-Kulesa et al., 2005; Krull et al., 1995). If this contact is broken, a neural crest cell will lose directionality, become disoriented, and wander through rostral and caudal sclerotome with apparent insensitivity to repulsive cues (Kasemeier-Kulesa et al., 2005). This behavior is strikingly similar to that observed following secreted ephrin treatment, where neural crest cells lose directionality and exhibit erratic migration trajectories in both rostral and caudal sclerotome (Krull et al., 1997). In addition to triggering repulsion, Eph/ephrin signaling affects cell adhesion and sorting (Arvanitis and Davy, 2008; Pasquale, 2005). For example, mixed EphB2 and ephrin-B2 expressing cells sort into adherent, segregated populations that do not intermingle or exhibit gap junctional communication between them (Davy et al., 2006; Mellitzer et al., 1999). Notably, ephrin-B1 physically interacts with the gap junction protein connexin43, which is required for cell sorting and partially rescues ephrin-B1 sorting defects (Davy et al., 2006). Connexin43 enables junctional communication between neural crest cells, and may be required for adhesion to the substrate during migration (Elias et al., 2007; Huang et al., 1998). Connexin43 is also essential for semaphorin-mediated repulsion of neural crest cells, as connexin43 mutant cardiac neural crest cells do not retract protrusions in response to Sema3A (Xu et al., 2006). Together, these data present the possibility that Eph/ephrin signaling between neural crest cells and the caudal sclerotome promotes connexin43 activity in the neural crest cells, which is necessary for them to respond to guidance cues like semaphorins. In this model, when Eph/ephrin signaling is perturbed in the chick, connexin43 activity is disrupted, causing neural crest cells to break their chain associations, migrate randomly, and lose their ability to respond to repulsive cues like Sema3F. In the mouse, functional redundancy of multiple Ephs and ephrins obscures this phenotype in single mutant animals. Neural crest cells avoid ephrins in vitro (Krull et al., 1997; Wang and Anderson, 1997) because they express Eph receptors for other purposes: to regulate dorsolateral migration (Santiago and Erickson, 2002), sympathetic ganglion segmentation (Kasemeier-Kulesa et al., 2006), and potentially cell-cell interactions as we propose here. However, this does not necessarily mean that neural crest cells use Eph/ephrin signaling as a guidance cue in the somites in vivo. Meanwhile, in addition to possibly triggering cell-cell interactions, ephrin expression in the caudal somite is required for somite polarity, which is necessary for patterned neural crest migration (Davy and Soriano, 2007; Durbin et al., 1998).
CXCL12/CXCR4
CXCL12 (formerly known as stromal cell-derived factor-1 or SDF-1; IUS/WHO Subcommittee, 2003) is one of the few secreted proteins known to attract migratory neural crest cells, and the only attractive secreted factor presently known to be active in the ventrolateral path. Neural crest cells express the receptor CXCR4, while CXCL12 is localized to the sclerotome, dorsal aorta and the region surrounding the gut (Belmadani et al., 2005; Kasemeier-Kulesa et al., in press; Lieberam et al., 2005; Rehimi et al., 2008). CXCL12 is a neural crest cell chemoattractant, drawing neural crest cells ventrally as far as the sympathetic ganglia (Belmadani et al., 2005; Kasemeier-Kulesa et al., in press). CXCR4 loss-of-function disrupts neural crest migration and leads to ectopic sensory and sympathetic neurons dorsal to the respective ganglia (Belmadani et al., 2005; Kasemeier-Kulesa et al., in press). However, CXCL12/CXCR4 signaling is unlikely to be a segmental neural crest chemoattractant, as it is not clear whether CXCL12 is restricted to the rostral sclerotome, and dorsal root ganglia are metameric in CXCR4 mutants (Belmadani et al., 2005).
Extracellular matrix
Neural crest cells migrate through and interact with the extracellular matrix (ECM). A variety of ECM proteins are uniformly or regionally restricted within the somite and exert permissive, positive, or inhibitory effects on neural crest migration.
Permissive ECM factors expressed throughout the embryo along neural crest migratory routes include fibronectin, laminin, and collagen (Duband and Thiery, 1987; Newgreen and Thiery, 1980). Permissive factors are necessary substrates for migratory neural crest cells to attach and migrate in vivo and in vitro, promoting motility without directly guiding it per se (Perris and Perissinotto, 2000). Integrins, which are receptors for fibronectin and laminins, are essential for neural crest migration and the interaction of neural crest cells with permissive substrates (Bronner-Fraser, 1985; Goh et al., 1997; Kil et al., 1998; Lallier and Bronner-Fraser, 1993; Monier-Gavelle and Duband, 1997; Testaz and Duband, 2001). However, mouse mutants suggest neural crest cells rely on multiple, redundant integrins (Haack and Hynes, 2001).
Of the ECM molecules with guidance properties, most is known about inhibitory factors localized to the caudal sclerotome. F-spondin (Debby-Brafman et al., 1999), T-cadherin (Ranscht and Bronner-Fraser, 1991), the proteoglycan aggrecan (Perissinotto et al., 2000), and chondroitin sulfate proteoglycans (CSPGs) (Kubota et al., 1999) including Collagen XI (Ring et al., 1996), chondroitin-6-sulfate (Oakley and Tosney, 1991), and versican (Landolt et al., 1995), are all expressed in the caudal sclerotome during neural crest cell migration. Peanut agglutinin (PNA) binding, cell-surface glycoproteins of unknown identity are also restricted to the caudal sclerotome (Davies et al., 1990; Oakley and Tosney, 1991; Stern et al., 1986). Neural crest cells will avoid these factors when they are presented as substrates in vitro. However, these factors do not act in isolation in the embryo, and likely function by modulating neural crest cells’ adherence to and interaction with the matrix as they migrate. For example, spondins regulate integrin-mediated adhesion, and F-spondin in particular inhibits integrin binding to the ECM (Terai et al., 2001). Furthermore, an in vivo requirement for segmental neural crest migration has been demonstrated only in the case of F-spondin, CSPGs, and PNA binding glycoproteins (Debby-Brafman et al., 1999; Krull et al., 1995; Kubota et al., 1999). In many cases, the localization of these molecules is quite broad. For example, migratory neural crest cells overlap with the versican “caudal” expression domain, and neural crest cells traverse F-spondin positive areas near the neural tube (Debby-Brafman et al., 1999; Dutt et al., 2006). F-spondin in particular is cleaved into two fragments, one attractive and the other repulsive, which are differentially localized (Zisman et al., 2007). These observations suggest that these factors are not strictly inhibitory, and/or that ECM effects on migration are context-dependent.
Along these lines, it is important to consider that ECM molecules interact with guidance cues and modulate their activity (De Wit and Verhaagen, 2007). In particular, class 3 semaphorins bind proteoglycans. In the case of cortical neurons, the association of Sema3A with proteoglycans serves to localize Sema3A protein and potentiate its activity (De Wit et al., 2005). Thus, the segmental expression of CSPGs and other inhibitory ECM proteins in the caudal sclerotome could reflect a role in modulating Nrp/Sema signaling during segmental neural crest migration and dorsal root gangliogenesis.
Thrombospondin is the only rostral somite ECM molecule identified that positively stimulates neural crest migration (Tucker et al., 1999). Thrombospondin is abundantly localized to the basement membrane of the rostral myotome, a preferred substrate for neural crest cells, and promotes neural crest migration in vitro (Tosney et al., 1994; Tucker, 2001). Thrombospondin is a multifunctional protein, binding a variety of receptors including integrins and proteoglycans to modulate ECM interactions in a context dependent manner, but also existing as a soluble, cleaved, antiadhesive peptide (Elzie and Murphy-Ullrich, 2004). The relative contributions of these various activities to neural crest migration remain to be determined.
Migratory neural crest cells themselves also secrete ECM proteins, in particular another positive ECM molecule, the glycoprotein tenascin-C (Stern et al., 1986; Tucker and McKay, 1991). Tenascin-C reduces adhesion to fibronectin and promotes neural crest motility (Halfter et al., 1989; Tucker, 2001). Tenascin-C knockdown prevents neural crest migration in chick (Tucker, 2001); however, two independent tenascin-C mouse null mutations exhibit no apparent neural crest migration defects (Forsberg et al., 1996; Saga et al., 1992). A tenascin isoform that is absent in chick and expressed in the mouse may account for this discrepancy (Tucker, 2001).
Metameric dorsal root gangliogenesis: Nrp1/Sema3A
Once neural crest cells are segmentally arranged along the axis, they differentiate into the metameric peripheral ganglia (Fig. 1B). Within the somites, neural crest cells form sensory dorsal root ganglia (Fig. 2B5). When caudal somites are surgically or genetically ablated, neural crest cells migrate non-segmentally and dorsal root ganglia are fused (Kalcheim and Teillet, 1989; Leitges et al., 2000; Mansouri et al., 2000). Thus, it was concluded that segmental neural crest migration generates dorsal root ganglia segmentation. However, this is not the case. Although Nrp2-deficient neural crest cells migrate uniformly throughout the sclerotome, they still condense to form segmentally individualized dorsal root ganglia (Gammill et al., 2006). These observations indicate that segmental neural crest migration and metameric dorsal root gangliogenesis are separable processes independently patterned by signals in the caudal somite.
Nrp1/Sema3A is the caudal somite-associated signaling pathway that drives dorsal root ganglia segmentation (Fig. 2B). Animals with Nrp1 receptors unable to bind semaphorins (Nrp1Sema−) exhibit segmental neural crest migration and normal, metameric dorsal root gangliogenesis (Gu et al., 2003). However, in a Nrp2 mutant background where neural crest cells migrate non-segmentally, Nrp1Sema−/− dorsal root ganglia are fused (Roffers-Agarwal and Gammill, 2009; Schwarz et al., 2009a). In other words, segmental neural crest migration can substitute for Nrp1/Sema3A signaling, presumably by properly positioning neural crest cells within the rostral sclerotome. However, in the absence of segmental migration, Nrp1/Sema3A is required for metameric dorsal root gangliogenesis. This requirement for Nrp2 and Nrp1 is thought to be sequential (Nrp2 guides migration, Nrp1 patterns dorsal root ganglia condensation) rather than redundant (both Nrp2 and Nrp1 required for segmental gangliogenesis). A sequential requirement is suggested by temporally phased expression of the Sema3F and Sema3A ligands in the sclerotome and the non-additive nature of the Nrp1 and 2 phenotypes (Roffers-Agarwal and Gammill, 2009; Schwarz et al., 2009a; Fig. 2B). Curiously, while dorsal root ganglia are morphologically normal but more compact in Nrp1Sema− mutants, fusions and deformities are apparent in Nrp1 null animals, indicating the alternative Nrp1 ligand vascular endothelial growth factor is also involved in this process (Roffers-Agarwal and Gammill, 2009; Schwarz et al., 2009a; Schwarz et al., 2009b).
It is unlikely that the requirement for Nrp1/Sema3A during dorsal root ganglia formation represents a later manifestation of the requirement for Nrp1/Sema3A to repel neural crest cells from the intersomitic space (see above). First, neural crest migration in Nrp2 and Sema3F mutants is completely uniform and without interruption at the intersomitic fissure, which would be expected if Nrp1/Sema3A signaling in this region were responsible for later segmentation of dorsal root ganglia (Gammill et al., 2006; Roffers-Agarwal and Gammill, 2009; Schwarz et al., 2009a). Second, Sema3A is most abundantly expressed by the dermomyotome at the time neural crest cells are repelled from the intersomitic space, while dorsal root ganglia form in the sclerotome (Roffers-Agarwal and Gammill, 2009; Schwarz et al., 2009b). Sema3A expression begins to spread to the caudal sclerotome at E9.5 (Schwarz et al., 2009b), but is not prominently expressed throughout the caudal sclerotome until E10.0 (Roffers-Agarwal and Gammill, 2009), around the time dorsal root ganglion condensation begins (Marmigere and Ernfors, 2007). Thus, Nrp1/Sema3A signaling is required for two spatially and temporally distinct events during neural crest development within the somite: repulsion from the intersomitic space, and segmentation of dorsal root gangliogenesis.
Multiple signaling pathways influence sympathetic ganglia segmentation
Neural crest cells that continue ventral to the somite form metameric sympathetic ganglia in a multi-step process. First, neural crest cells must collect at the dorsal aorta (Fig. 2B4). Migratory neural crest cells, which express erbB2/B3 receptors as well as the CXCR4 receptor, are attracted ventrally through the dorsal somite by neuregulin (Britsch et al., 1998) and CXCL12, respectively (Kasemeier-Kulesa et al., in press). In the absence of these attractive signaling pathways, neural crest cells do not migrate efficiently to the dorsal aorta (Britsch et al., 1998; Kasemeier-Kulesa et al., in press). Sympathetic precursors also express the GFRα3 receptor, which binds the GDNF-family ligand artemin (Honma et al., 2002). Artemin is produced by peripheral vessels, attracting neural crest cells ventrally and possibly promoting their proliferation, as fewer sympathetic neurons are present in GFRα3−/− and artemin−/− mice (Honma et al., 2002). The repulsive activity of Sema3A produced by the dermomyotome, limbs, and notochord is likewise required to concentrate Nrp1 expressing sympathetic precursors at the dorsal aorta, as sympathetic neurons are widely scattered in the absence of Nrp1 or Sema3A signaling (Bron et al., 2004; Kawasaki et al., 2002).
Second, in a process independent from segmental neural crest migration, metameric sympathetic ganglia form (Gammill et al., 2006; Kasemeier-Kulesa et al., 2005; Fig. 2B6). Once gathered at the dorsal aorta, neural crest cells, which were segmentally arranged upon their arrival, disperse along its length and undergo extensive mixing (Kasemeier-Kulesa et al., 2005; Kasemeier-Kulesa et al., 2006). In fact, lineage analyses indicate that sympathetic precursors travel as far as two segments rostrally and caudally from their segment of origin (Kasemeier-Kulesa et al., 2005; Yip, 1986). Now uniformly distributed along the dorsal aorta, sympathetic precursors are then resegmented into discrete, metameric ganglia (Kasemeier-Kulesa et al., 2005; Kasemeier-Kulesa et al., 2006). Sympathetic ganglia segmentation, which like dorsal root gangliogenesis is dependent upon rostrocaudal somite pattern (Goldstein and Kalcheim, 1991), is due in part to the targeting of earliest arriving sympathetic precursors to the site of the sympathetic ganglia primordia (Kulesa et al., 2009). In addition, interganglionic ephrinB1 expression in the mesoderm surrounding the dorsal aorta sorts EphB2-positive neural crest cells into segmental populations (Kasemeier-Kulesa et al., 2006), while CXCL12/CXCR4 signaling attracts sympathetic precursors to the ganglia core (Kasemeier-Kulesa et al., in press). Sympathetic chain segmentation is also defective in GFRα3 and artemin mutant mice, indicating this signaling pathway is also required (Honma et al., 2002). As a result of N-cadherin mediated adhesion, neural crest cells subsequently condense into discrete, metameric sympathetic ganglia (Kasemeier-Kulesa et al., 2006).
Segmental motor axon outgrowth: another role for neuropilin signaling
Like neural crest cells, which migrate, differentiate, and project axons in a metameric pattern, motor axon outgrowth from the ventral spinal cord is also segmentally arranged (Keynes and Stern, 1984). Motor axons initially project adjacent to rostral sclerotomes, while later projecting caudal-adjacent axons fasciculate with rostral pioneers, creating a metameric, punctuated appearance to motor axon outgrowth along the neural tube (Keynes and Stern, 1984) Fig. 2A). Strikingly, this pattern is lost in Nrp2 mutant mice, and initial motor axon projections are apparent uniformly along the neural tube into rostral and caudal sclerotomes (Roffers-Agarwal and Gammill, 2009). Subsequently, motor axons fasciculate into ventral roots within the rostral domain. While this process is normal in Nrp2 mutants, ventral roots are defasciculated in Nrp1Sema− mutants, indicating a sequential requirement for Nrp2 and Nrp1 during motor axon outgrowth as during neural crest development (Roffers-Agarwal and Gammill, 2009). In Nrp1Sema−/Nrp2 mutant animals, all motor axon and ventral root segmentation is completely abolished, and axons are present uniformly along the axis (Roffers-Agarwal and Gammill, 2009). Thus, sensory (dorsal root ganglia) and motor metamerism are both entirely dependent on Nrp1 and Nrp2 signaling. Distal to the spinal cord, neuropilin signaling is also required for fasciculation of the mixed spinal nerves, with the severity of segmentation defects being background dependent (loss of segmentation in C57/Black6 (Roffers-Agarwal and Gammill, 2009) versus residual segmentation in a mixed background (Schwarz et al., 2009a); see also (Huber et al., 2005)).
Positive and negative signals restrict entry to the dorsolateral path
The final migratory route taken by neural crest cells is one that does not participate in PNS development, but it is a choice faced by all neural crest cells early in their migration: ventral or dorsal pathway? In chick embryos, early migrating neural crest cells are excluded from the dorsolateral path between the epidermal ectoderm and the somite (Fig. 2B7), while late migrating cells preferentially migrate dorsolaterally because they are specified as melanocytes (Erickson et al., 1992; Reedy et al., 1998; Serbedzija et al., 1989). Heterochronic transplantation experiments confirm that the transition from ventral to dorsal pathway preference is neural crest cell autonomous and not due to changes in the environment: early neural crest cells will not migrate dorsolaterally in an older host, while late migrating neural crest cells will invade the “closed” dorsolateral pathway of younger embryos (Erickson et al., 1992; Erickson and Goins, 1995). The dermomyotome is the source of guidance factors, as dermomyotome ablation allows precocious neural crest migration along the dorsolateral route (Erickson et al., 1992).
Both attractive and repulsive cues have been implicated in dorsolateral pathway choice. Inhibitory factors have largely been characterized in chick, and include receptor/ligand pairs and changes in the extracellular matrix. The dermomyotome expresses repulsive slit ligands, while early migrating neural crest cells express Robos, which are slit receptors (Jia et al., 2005). Because of this repulsive interaction, only late migrating neural crest cells, which downregulate Robos, or neural crest cells expressing a dominant negative Robo, can migrate dorsolaterally (Jia et al., 2005). In addition, the dermomyotome expresses ephrins, while neural crest cells express Eph receptors (Krull et al., 1997; Santiago and Erickson, 2002; Wang and Anderson, 1997). Curiously, Eph/ephrin signaling repulses early migrating chick neural crest cells and promotes migration of late migrating, melanocyte precursors (Santiago and Erickson, 2002). Ephrin-mediated repulsion versus attraction appears to be mediated by differential expression of Eph receptors: early migrating neural crest cells express EphB3, while melanoblasts upregulate EphB2 (Harris et al., 2008; Krull et al., 1997; Santiago and Erickson, 2002). Meanwhile, expression of the extracellular matrix protein F-spondin in the dermomyotome is required for dorsolateral pathway delay (Debby-Brafman et al., 1999). Similarly, peanut agglutinin binding cell-surface glycoproteins are transiently expressed along the dorsolateral path (Oakley et al., 1994). Finally, Nrp1Sema mutant mice exhibit increased, ectopic dorsolateral neural crest migration (Roffers-Agarwal and Gammill, 2009; Schwarz et al., 2009b). Because Sema3A expression remains high in the dermomyotome throughout late neural crest migration, this suggests that downregulation of Nrp1 on neural crest cells, or acquisition of VEGF expression along the route, are also crucial to open the dorsolateral pathway.
Differences exist between the signals that attract chick and mouse neural crest cells into the dorsolateral path. This is likely reflective of the distinct timing of dorsolateral migration in mouse and chick; there is no temporal delay in dorsolateral migration in the mouse, while chick neural crest cells enter this pathway only 24 hours after the onset of migration (Erickson et al., 1992; Serbedzija et al., 1990; Serbedzija et al., 1989). Murine melanoblasts express the Kit receptor even while still in the neural tube, and Kit ligand expression in the dermomyotome is essential to attract migratory neural crest cells dorsolaterally (Wehrle-Haller et al., 2001; Wehrle-Haller and Weston, 1995; Wilson et al., 2004). Meanwhile, chick melanoblasts do not upregulate Kit until they are already migrating dorsolaterally, and Kit is not required for chick dorsolateral neural crest migration (Harris et al., 2008; Lecoin et al., 1995). Instead, chick melanocytic precursors exhibit species-specific expression of endothelin receptor B2 (EDNRB2), which is both necessary and sufficient for neural crest migration along the dorsolateral path (Harris et al., 2008; Lecoin et al., 1998).
Common themes along the rostrocaudal axis
At the onset of neural crest migration in the trunk, cranial neural crest cells have already been migrating for several hours. Is migration in the trunk a continuation of the process in the head? Although neural crest cells delaminate from the dorsal neural tube regardless of their position along the axis, cranial and trunk neural crest migration are morphologically distinct events. In the head, cranial neural crest cells are segmentally generated in large numbers, and initially invade an unsegmented mesenchyme with few physical obstacles. In the trunk, neural crest cells delaminate individually and uniformly, becoming metameric by navigating the segmental somites.
In spite of these differences, as the events and signaling pathways that direct cranial (Kulesa et al., this volume) and trunk neural crest development unfold, several universal principals of neural crest migration have become apparent. First is the critical importance of neuropilin signaling in neural crest guidance. In all neural crest cells, Nrp1 and Nrp2 are essential to guide and pattern migratory neural crest cells and their derivatives; in their absence, neural crest migration is non-segmental and neural crest derivatives are fused (Gammill et al., 2007; Gammill et al., 2006; McLennan and Kulesa, 2007; Osborne et al., 2005; Roffers-Agarwal and Gammill, 2009; Schwarz et al., 2009a; Schwarz et al., 2009b; Schwarz et al., 2008; Yu and Moens, 2005). Intriguingly, both semaphorins and vascular endothelial growth factor (VEGF), which is an alternative neuropilin ligand (Gluzman-Poltorak et al., 2000; Soker et al., 1998), are environmental guidance cues that signal through neuropilins in the head and trunk (McLennan et al., 2010; Schwarz et al., 2009b). Thus, neuropilin receptors serve to integrate repulsive (semaphorin) and attractive (VEGF) signals to guide neural crest migration and morphogenesis. Although other signaling pathways, most notably Eph/ephrins, are required during neural crest migration in the head and trunk, their loss-of-function phenotypes are less consistent and generally less severe (Adams et al., 2001; Davy et al., 2004; Davy and Soriano, 2007; Krull et al., 1997; Orioli et al., 1996; Santiago and Erickson, 2002; Wang et al., 1998). This could be due to functional redundancy within this large gene family that obscures general trends.
Second, cranial and trunk neural crest cells exhibit identical migratory behaviors. In particular, all neural crest cells migrate in chains, maintaining filopodial contact with neighboring cells (Kasemeier-Kulesa et al., 2005; Krull et al., 1995; Kulesa and Fraser, 2000; Kulesa and Fraser, 1998; Teddy and Kulesa, 2004). Importantly, these cell-cell contacts influence directionality and guidance in both the head and trunk (Kasemeier-Kulesa et al., 2005; Teddy and Kulesa, 2004). Thus, chain formations are a functionally relevant characteristic of all migratory neural crest cells. Future studies need to address whether other cranially-defined neural crest behaviors, such as differences in cell morphology based upon position within a migratory stream, are common to all neural crest cells (Teddy and Kulesa, 2004; Kulesa et al., this volume).
The third unifying feature of neural crest migration in the head and trunk is that segmental migration and the resulting segmental derivatives are generated independently. In the head, signals that sculpt migratory streams and maintain neural crest-free zones are distinct from the factors that enable invasion of and morphogenesis in the branchial arches (reviewed in Kulesa et al., this volume). In the trunk, segmental neural crest migration and condensation into metameric peripheral ganglia are separable processes (see discussion above). By breaking the process of neural crest migration and morphogenesis into multiple, semi-redundant steps, the reliable segmentation of neural crest-derived structures is ensured. A multi-step process may also be important for patterning neuronal and other subpopulations within differentiated neural crest derivatives (Kulesa et al., 2009).
Conclusion
Through the use of sequential migratory pathways and the establishment of pattern upon existing pattern, a metameric trunk peripheral nervous system forms. Overall, much progress has been made in defining the many steps and signaling pathways that govern each phase of trunk neural crest development. With an extensive list of participating factors now available (Table 1), future experiments will focus on understanding how these diverse signals work together to pattern neural crest cells. For example, do Eph/ephrins and Nrp2/Sema3F function in concert to regulate cell-cell interactions and guidance, respectively? Do ECM molecules enhance these activities? In addition, much remains to be learned about the earliest events in neural crest migration, as neural crest cells migrate in the intersomitic space and over the neural tube. Does a signal(s) actively attract neural crest cells to the intersomitic vessels? Is there a factor that promotes ventral migration between the neural tube and somite? Further investigation into these questions will clarify the mechanisms that dictate patterned neural crest migration and segmental peripheral nervous development.
Acknowledgments
We would like to thank Bridget Jacques-Fricke and Paul Kulesa for stimulating discussions and for critical reading of the manuscript. L.S.G. gratefully acknowledges support from the NIH (K22 DE015309), the March of Dimes Foundation (5-FY2009-39), the American Cancer Society (IRG-58-001-49-IRG32), the Minnesota Medical Foundation (3965-9220-09) and the University of Minnesota Academic Health Center (2009-44) and Graduate School (21393). J.R.A. is supported by F32 DE019755.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Adams R, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand EphrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlstrom JD, Erickson CA. The neural crest epithelial-mesenchymal transition in 4D: a; tail’ of multiple non-obligatory cellular mechanisms. Development. 2009;136:1801–1812. doi: 10.1242/dev.034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ. Genes, lineages and the neural crest: a speculative review. Philos Trans R Soc Lond B Biol Sci. 2000;355:953–964. doi: 10.1098/rstb.2000.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes Dev. 2008;22:416–429. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Assimacopoulos S, Grove EA, Miller RJ. The Chemokine Stromal Cell-Derived Factor-1 Regulates the Migration of Sensory Neuron Progenitors. J Neurosci. 2005;25:3995–4003. doi: 10.1523/JNEUROSCI.4631-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britsch S, Li L, Kirchhoff S, Theuring F, Brinkmann V, Birchmeier C, Riethmacher D. The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes Dev. 1998;12:1825–1836. doi: 10.1101/gad.12.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron R, Eickholt BJ, Vermeren M, Fragale N, Cohen J. Functional knockdown of neuropilin-1 in the developing chick nervous system by siRNA hairpins phenocopies genetic ablation in the mouse. Dev Dyn. 2004;230:299–308. doi: 10.1002/dvdy.20043. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Alterations in neural crest migration by a monoclonal antibody that affects cell adhesion. J Cell Biol. 1985;101:610–617. doi: 10.1083/jcb.101.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M. Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Dev Biol. 1986;115:44–55. doi: 10.1016/0012-1606(86)90226-5. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M, Fraser S. Cell lineage analysis shows multipotentiality of some avian neural crest cells. Nature. 1988;335:161–164. doi: 10.1038/335161a0. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M, Fraser S. Developmental potential of avian trunk neural crest cells in situ. Neuron. 1989;3:755–766. doi: 10.1016/0896-6273(89)90244-4. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M, Stern C. Effects of mesodermal tissues on avian neural crest cell migration. Dev Biol. 1991;143:213–217. doi: 10.1016/0012-1606(91)90071-a. [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen T, Stanleigh J, Sela-Donenfeld D, Kalcheim C. Canonical Wnt activity regulates trunk neural crest delamination linking BMP/noggin signaling with G1/S transition. Development. 2004;131:5327–5339. doi: 10.1242/dev.01424. [DOI] [PubMed] [Google Scholar]
- Collazo A, Bronner-Fraser M, Fraser S. Vital dye labelling of Xenopus laevis trunk neural crest reveals multipotency and novel pathways of migration. Development. 1993;118:363–376. doi: 10.1242/dev.118.2.363. [DOI] [PubMed] [Google Scholar]
- Davies JA, Cook GM, Stern CD, Keynes RJ. Isolation from chick somites of a glycoprotein fraction that causes collapse of dorsal root ganglion growth cones. Neuron. 1990;4:11–20. doi: 10.1016/0896-6273(90)90439-m. [DOI] [PubMed] [Google Scholar]
- Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 2004;18:572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006;4:e315. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Soriano P. Ephrin-B2 forward signaling regulates somite patterning and neural crest cell development. Dev Biol. 2007;304:182–193. doi: 10.1016/j.ydbio.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit J, De Winter F, Klooster J, Verhaagen J. Semaphorin 3A displays a punctate distribution on the surface of neuronal cells and interacts with proteoglycans in the extracellular matrix. Mol Cell Neurosci. 2005;29:40–55. doi: 10.1016/j.mcn.2004.12.009. [DOI] [PubMed] [Google Scholar]
- De Wit J, Verhaagen J. Proteoglycans as modulators of axon guidance cue function. Adv Exp Med Biol. 2007;600:73–89. doi: 10.1007/978-0-387-70956-7_7. [DOI] [PubMed] [Google Scholar]
- Debby-Brafman A, Burstyn-Cohen T, Klar A, Kalchiem C. F-Spondin, expressed in somite regions avoided by neural crest cells, mediates inhibition of distinct somite domains to neural crest migration. Neuron. 1999;22:475–488. doi: 10.1016/s0896-6273(00)80703-5. [DOI] [PubMed] [Google Scholar]
- Duband JL, Dufour S, Hatta K, Takeichi M, Edelman GM, Thiery JP. Adhesion molecules during somitogenesis in the avian embryo. J Cell Biol. 1987;104:1361–1374. doi: 10.1083/jcb.104.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband JL, Thiery JP. Distribution of laminin and collagens during avian neural crest development. Development. 1987;101:461–478. doi: 10.1242/dev.101.3.461. [DOI] [PubMed] [Google Scholar]
- Durbin L, Brennan C, Shiomi K, Cooke J, Barrios A, Shanmugalingam S, Guthrie B, Lindberg R, Holder N. Eph signaling is required for segmentation and differentiation of the somites. Genes Dev. 1998;12:3096–3109. doi: 10.1101/gad.12.19.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt S, Kleber M, Matasci M, Sommer L, Zimmermann DR. Versican V0 and V1 guide migratory neural crest cells. J Biol Chem. 2006;281:12123–12131. doi: 10.1074/jbc.M510834200. [DOI] [PubMed] [Google Scholar]
- Eickholt B, Mackenzie S, Graham A, Walsh F, Doherty P. Evidence for collapsin-1 functioning in the control of neural crest migration in both trunk and hindbrain regions. Development. 1999;126:2181–2189. doi: 10.1242/dev.126.10.2181. [DOI] [PubMed] [Google Scholar]
- Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- Elzie CA, Murphy-Ullrich JE. The N-terminus of thrombospondin: the domain stands apart. Int J Biochem Cell Biol. 2004;36:1090–1101. doi: 10.1016/j.biocel.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Erickson CA. Control of neural crest cell dispersion in the trunk of the avian embryo. Dev Biol. 1985;111:138–157. doi: 10.1016/0012-1606(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Erickson CA. Behavior of neural crest cells on embryonic basal laminae. Dev Biol. 1987;120:38–49. doi: 10.1016/0012-1606(87)90101-1. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Duong TD, Tosney KW. Descriptive and experimental analysis of the dispersion of neural crest cells along the dorsolateral path and their entry into ectoderm in the chick embryo. Dev Biol. 1992;151:251–272. doi: 10.1016/0012-1606(92)90231-5. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Goins TL. Avian neural crest cells can migrate in the dorsolateral path only if they are specified as melanocytes. Development. 1995;121:915–924. doi: 10.1242/dev.121.3.915. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Goins TL. Sacral neural crest cell migration to the gut is dependent upon the migratory environment and not cell-autonomous migratory properties. Dev Biol. 2000;219:79–97. doi: 10.1006/dbio.1999.9597. [DOI] [PubMed] [Google Scholar]
- Forsberg E, Hirsch E, Frohlich L, Meyer M, Ekblom P, Aszodi A, Werner S, Fassler R. Skin wounds and severed nerves heal normally in mice lacking tenascin-C. Proc Natl Acad Sci U S A. 1996;93:6594–6599. doi: 10.1073/pnas.93.13.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser SE, Bronner-Fraser M. Migrating neural crest cells in the trunk of the avian embryo are multipotent. Development. 1991;112:913–920. doi: 10.1242/dev.112.4.913. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Gonzalez C, Bronner-Fraser M. Neuropilin 2/semaphorin 3F signaling is essential for cranial neural crest migration and trigeminal ganglion condensation. Dev Neurobiol. 2007;67:47–56. doi: 10.1002/dneu.20326. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Gonzalez C, Gu C, Bronner-Fraser M. Guidance of trunk neural crest migration requires neuropilin 2/semaphorin 3F signaling. Development. 2006;133:99–106. doi: 10.1242/dev.02187. [DOI] [PubMed] [Google Scholar]
- George L, Chaverra M, Todd V, Lansford R, Lefcort F. Nociceptive sensory neurons derive from contralaterally migrating, fate-restricted neural crest cells. Nat Neurosci. 2007;10:1287–1293. doi: 10.1038/nn1962. [DOI] [PubMed] [Google Scholar]
- Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165. J Biol Chem. 2000;275:18040–18045. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- Goh KL, Yang JT, Hynes RO. Mesodermal defects and cranial neural crest apoptosis in alpha5 integrin-null embryos. Development. 1997;124:4309–4319. doi: 10.1242/dev.124.21.4309. [DOI] [PubMed] [Google Scholar]
- Goldstein RS, Kalcheim C. Normal segmentation and size of the primary sympathetic ganglia depend upon the alternation of rostrocaudal properties of the somites. Development. 1991;112:327–334. doi: 10.1242/dev.112.1.327. [DOI] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack H, Hynes RO. Integrin receptors are required for cell survival and proliferation during development of the peripheral glial lineage. Dev Biol. 2001;233:38–55. doi: 10.1006/dbio.2001.0213. [DOI] [PubMed] [Google Scholar]
- Halfter W, Chiquet-Ehrismann R, Tucker RP. The effect of tenascin and embryonic basal lamina on the behavior and morphology of neural crest cells in vitro. Dev Biol. 1989;132:14–25. doi: 10.1016/0012-1606(89)90200-5. [DOI] [PubMed] [Google Scholar]
- Harris ML, Erickson CA. Lineage specification in neural crest cell pathfinding. Dev Dyn. 2007;236:1–19. doi: 10.1002/dvdy.20919. [DOI] [PubMed] [Google Scholar]
- Harris ML, Hall R, Erickson CA. Directing pathfinding along the dorsolateral path - the role of EDNRB2 and EphB2 in overcoming inhibition. Development. 2008;135:4113–4122. doi: 10.1242/dev.023119. [DOI] [PubMed] [Google Scholar]
- Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth R, Johnson E, Milbrandt J. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron. 2002;35:267–282. doi: 10.1016/s0896-6273(02)00774-2. [DOI] [PubMed] [Google Scholar]
- Huang GY, Cooper ES, Waldo K, Kirby ML, Gilula NB, Lo CW. Gap junction-mediated cell-cell communication modulates mouse neural crest migration. J Cell Biol. 1998;143:1725–1734. doi: 10.1083/jcb.143.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AB, Kania A, Tran TS, Gu C, De Marco Garcia N, Lieberam I, Johnson D, Jessell TM, Ginty DD, Kolodkin AL. Distinct roles for secreted semaphorin signaling in spinal motor axon guidance. Neuron. 2005;48:949–964. doi: 10.1016/j.neuron.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Hutson MR, Kirby ML. Model systems for the study of heart development and disease. Cardiac neural crest and conotruncal malformations. Semin Cell Dev Biol. 2007;18:101–110. doi: 10.1016/j.semcdb.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUS/WHO_Subcommittee. Chemokine/chemokine receptor nomenclature. Cytokine. 2003;21:48–49. doi: 10.1016/s1043-4666(02)00493-3. [DOI] [PubMed] [Google Scholar]
- Jia L, Cheng L, Raper J. Slit/Robo signaling is necessary to confine early neural crest cells to the ventral migratory pathway in the trunk. Dev Biol. 2005;282:411–421. doi: 10.1016/j.ydbio.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Kalcheim C, Teillet MA. Consequences of somite manipulation on the pattern of the dorsal root ganglion development. Development. 1989;106:85–93. doi: 10.1242/dev.106.1.85. [DOI] [PubMed] [Google Scholar]
- Kasemeier-Kulesa J, Kulesa P, Lefcort F. Imaging neural crest cell dynamics during formation of dorsal root ganglia and sympathetic ganglia. Development. 2005;132:235–245. doi: 10.1242/dev.01553. [DOI] [PubMed] [Google Scholar]
- Kasemeier-Kulesa JC, Bradley R, Pasquale EB, Lefcort F, Kulesa PM. Eph/ephrins and N-cadherin coordinate to control the pattern of sympathetic ganglia. Development. 2006;133:4839–4847. doi: 10.1242/dev.02662. [DOI] [PubMed] [Google Scholar]
- Kasemeier-Kulesa JC, McLennan R, Lefcort F, Kulesa PM. CXCR4 controls ventral migration of sympathetic precursor cells. J Neurosci. doi: 10.1523/JNEUROSCI.0892-10.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Bekku Y, Suto F, Kisukawa T, Taniguchi M, Nagatsu I, Nagatsu T, Itoh K, Yagi T, Fujisawa H. Requirement of neuropilin-1-mediated Sema3A signals in patterning of the sympathetic nervous system. Development. 2002;129:671–680. doi: 10.1242/dev.129.3.671. [DOI] [PubMed] [Google Scholar]
- Keynes R, Stern C. Segmentation in the vertebrate nervous system. Nature. 1984;310:786–789. doi: 10.1038/310786a0. [DOI] [PubMed] [Google Scholar]
- Kil S, Krull C, Cann G, Clegg D, Bronner-Fraser M. The alpha4 subunit of integrin is important for neural crest cell migration. Dev Biol. 1998;202:29–42. doi: 10.1006/dbio.1998.8985. [DOI] [PubMed] [Google Scholar]
- Krispin S, Nitzan E, Kassem Y, Kalcheim C. Evidence for a dynamic spatiotemporal fate map and early fate restrictions of premigratory avian neural crest. Development. 2010;137:585–595. doi: 10.1242/dev.041509. [DOI] [PubMed] [Google Scholar]
- Krull C. Segmental organization of neural crest migration. Mech Dev. 2001;105:37–45. doi: 10.1016/s0925-4773(01)00395-1. [DOI] [PubMed] [Google Scholar]
- Krull C, Lansford R, Gale N, Collazo A, Marcelle C, Yancopoulos G, Fraser S, Bronner-Fraser M. Interactions of Eph-related receptors and ligands confer rostrocaudal pattern to trunk neural crest migration. Curr Biol. 1997;7:571–580. doi: 10.1016/s0960-9822(06)00256-9. [DOI] [PubMed] [Google Scholar]
- Krull CE, Collazo A, Fraser SE, Bronner-Fraser M. Segmental migration of trunk neural crest: time-lapse analysis reveals a role for PNA-binding molecules. Development. 1995;121:3733–3743. doi: 10.1242/dev.121.11.3733. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Tannahill D, Cook G, Keynes R. Somite polarity and segmental patterning of the peripheral nervous system. Mech Dev. 2004;121:1055–1068. doi: 10.1016/j.mod.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Morita T, Kusakabe M, Sakakura T, Ito K. Spatial and temporal changes in chondroitin sulfate distribution in the sclerotome play an essential role in the formation of migration patterns of mouse neural crest cells. Dev Dyn. 1999;214:55–65. doi: 10.1002/(SICI)1097-0177(199901)214:1<55::AID-DVDY6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Kulesa P, Fraser S. In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches. Development. 2000;127:1161–1172. doi: 10.1242/dev.127.6.1161. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Fraser SE. Neural crest cell dynamics revealed by time-lapse video microscopy of whole embryo chick explant cultures. Dev Biol. 1998;204:327–344. doi: 10.1006/dbio.1998.9082. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Lefcort F, Kasemeier-Kulesa JC. The migration of autonomic precursor cells in the embryo. Auton Neurosci. 2009;151:3–9. doi: 10.1016/j.autneu.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Lallier T, Bronner-Fraser M. Inhibition of neural crest cell attachment by integrin antisense oligonucleotides. Science. 1993;259:692–695. doi: 10.1126/science.8430321. [DOI] [PubMed] [Google Scholar]
- Landolt RM, Vaughan L, Winterhalter KH, Zimmermann DR. Versican is selectively expressed in embryonic tissues that act as barriers to neural crest cell migration and axon outgrowth. Development. 1995;121:2303–2312. doi: 10.1242/dev.121.8.2303. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Dupin E. Multipotentiality of the neural crest. Curr Opin Genet Dev. 2003;13:529–536. doi: 10.1016/j.gde.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Lecoin L, Lahav R, Martin FH, Teillet MA, Le Douarin NM. Steel and c-kit in the development of avian melanocytes: a study of normally pigmented birds and of the hyperpigmented mutant silky fowl. Dev Dyn. 1995;203:106–118. doi: 10.1002/aja.1002030111. [DOI] [PubMed] [Google Scholar]
- Lecoin L, Sakurai T, Ngo MT, Abe Y, Yanagisawa M, Le Douarin NM. Cloning and characterization of a novel endothelin receptor subtype in the avian class. Proc Natl Acad Sci U S A. 1998;95:3024–3029. doi: 10.1073/pnas.95.6.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N, Kalcheim C. The Neural Crest. 2. Cambridge University Press; 1999. [Google Scholar]
- Leitges M, Neidhardt L, Haenig B, Herrmann B, Kispert A. The paired homeobox gene Uncx4.1 specifies pedicles, transverse processes and proximal ribs of the vertebral column. Development. 2000;127:2259–2267. doi: 10.1242/dev.127.11.2259. [DOI] [PubMed] [Google Scholar]
- Lieberam I, Agalliu D, Nagasawa T, Ericson J, Jessell TM. A Cxcl12-CXCR4 chemokine signaling pathway defines the initial trajectory of mammalian motor axons. Neuron. 2005;47:667–679. doi: 10.1016/j.neuron.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Loring JF, Erickson CA. Neural crest cell migratory pathways in the trunk of the chick embryo. Dev Biol. 1987;121:220–236. doi: 10.1016/0012-1606(87)90154-0. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Voss A, Thomas T, Yokota Y, Gruss P. Uncx4.1 is required for the formation of the pedicles and proximal ribs and acts upstream of Pax9. Development. 2000;127:2251–2258. doi: 10.1242/dev.127.11.2251. [DOI] [PubMed] [Google Scholar]
- Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- McLennan R, Krull CE. Ephrin-as cooperate with EphA4 to promote trunk neural crest migration. Gene Expr. 2002;10:295–305. doi: 10.3727/000000002783992389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan R, Kulesa PM. In vivo analysis reveals a critical role for neuropilin-1 in cranial neural crest cell migration in chick. Dev Biol. 2007;301:227–239. doi: 10.1016/j.ydbio.2006.08.019. [DOI] [PubMed] [Google Scholar]
- McLennan R, Teddy JM, Kasemeier-Kulesa JC, Romine MH, Kulesa PM. Vascular Endothelial Growth Factor (VEGF) regulates cranial neural crest migration in vivo. Dev Biol. 2010;339:114–125. doi: 10.1016/j.ydbio.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellitzer G, Xu Q, Wilkinson DG. Eph receptors and ephrins restrict cell intermingling and communication. Nature. 1999;400:77–81. doi: 10.1038/21907. [DOI] [PubMed] [Google Scholar]
- Monier-Gavelle F, Duband JL. Cross talk between adhesion molecules: control of N-cadherin activity by intracellular signals elicited by beta1 and beta3 integrins in migrating neural crest cells. J Cell Biol. 1997;137:1663–1681. doi: 10.1083/jcb.137.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgreen D, Thiery JP. Fibronectin in early avian embryos: synthesis and distribution along the migration pathways of neural crest cells. Cell Tissue Res. 1980;211:269–291. doi: 10.1007/BF00236449. [DOI] [PubMed] [Google Scholar]
- Oakley RA, Lasky CJ, Erickson CA, Tosney KW. Glycoconjugates mark a transient barrier to neural crest migration in the chicken embryo. Development. 1994;120:103–114. doi: 10.1242/dev.120.1.103. [DOI] [PubMed] [Google Scholar]
- Oakley RA, Tosney KW. Peanut agglutinin and chondroitin-6-sulfate are molecular markers for tissues that act as barriers to axon advance in the avian embryo. Dev Biol. 1991;147:187–206. doi: 10.1016/s0012-1606(05)80017-x. [DOI] [PubMed] [Google Scholar]
- Oike Y, Ito Y, Hamada K, Zhang XQ, Miyata K, Arai F, Inada T, Araki K, Nakagata N, Takeya M, Kisanuki YY, Yanagisawa M, Gale NW, Suda T. Regulation of vasculogenesis and angiogenesis by EphB/ephrin-B2 signaling between endothelial cells and surrounding mesenchymal cells. Blood. 2002;100:1326–1333. [PubMed] [Google Scholar]
- Orioli D, Henkemeyer M, Lemke G, Klein R, Pawson T. Sek4 and Nuk receptors cooperate in guidance of commissural axons and in palate formation. EMBO J. 1996:6035–6049. [PMC free article] [PubMed] [Google Scholar]
- Osborne N, Begbie J, Chilton J, Schmidt H, Eickholt B. Semaphorin/neuropilin signaling influences the positioning of migratory neural crest cells within the hindbrain region of the chick. Dev Dyn. 2005;232:939–949. doi: 10.1002/dvdy.20258. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Perissinotto D, Iacopetti P, Bellina I, Doliana R, Colombatti A, Pettway Z, Bronner-Fraser M, Shinomura T, Kimata K, Morgelin M, Lofberg J, Perris R. Avian neural crest cell migration is diversely regulated by the two major hyaluronan-binding proteoglycans PG-M/versican and aggrecan. Development. 2000;127:2823–2842. doi: 10.1242/dev.127.13.2823. [DOI] [PubMed] [Google Scholar]
- Perris R, Perissinotto D. Role of the extracellular matrix during neural crest migration. Mech Dev. 2000;95:3–21. doi: 10.1016/s0925-4773(00)00365-8. [DOI] [PubMed] [Google Scholar]
- Ranscht B, Bronner-Fraser M. T-cadherin expression alternates with migrating neural crest cells in the trunk of the avian embryo. Development. 1991;111:15–22. doi: 10.1242/dev.111.1.15. [DOI] [PubMed] [Google Scholar]
- Reedy MV, Faraco CD, Erickson CA. The delayed entry of thoracic neural crest cells into the dorsolateral path is a consequence of the late emigration of melanogenic neural crest cells from the neural tube. Dev Biol. 1998;200:234–246. doi: 10.1006/dbio.1998.8963. [DOI] [PubMed] [Google Scholar]
- Rehimi R, Khalida N, Yusuf F, Dai F, Morosan-Puopolo G, Brand-Saberi B. Stromal-derived factor-1 (SDF-1) expression during early chick development. Int J Dev Biol. 2008;52:87–92. doi: 10.1387/ijdb.072374rr. [DOI] [PubMed] [Google Scholar]
- Rickman M, Fawcett J, Keynes R. The migration of neural crest cells and the growth of motor axons through the rostral half of the chick somite. J Embryol Exp Morph. 1985;90:437–455. [PubMed] [Google Scholar]
- Ring C, Hassell J, Halfter W. Expression pattern of collagen IX and potential role in the segmentation of the peripheral nervous system. Dev Biol. 1996;180:41–53. doi: 10.1006/dbio.1996.0283. [DOI] [PubMed] [Google Scholar]
- Roffers-Agarwal J, Gammill LS. Neuropilin receptors guide distinct phases of sensory and motor neuronal segmentation. Development. 2009;136:1879–1888. doi: 10.1242/dev.032920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y, Yagi T, Ikawa Y, Sakakura T, Aizawa S. Mice develop normally without tenascin. Genes Dev. 1992;6:1821–1831. doi: 10.1101/gad.6.10.1821. [DOI] [PubMed] [Google Scholar]
- Santiago A, Erickson CA. Ephrin-B ligands play a dual role in the control of neural crest cell migration. Development. 2002;129:3621–3632. doi: 10.1242/dev.129.15.3621. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- Schwarz Q, Maden CH, Davidson K, Ruhrberg C. Neuropilin-mediated neural crest cell guidance is essential to organise sensory neurons into segmented dorsal root ganglia. Development. 2009a;136:1785–1789. doi: 10.1242/dev.034322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Q, Maden CH, Vieira JM, Ruhrberg C. Neuropilin 1 signaling guides neural crest cells to coordinate pathway choice with cell specification. Proc Natl Acad Sci U S A. 2009b;106:6164–6169. doi: 10.1073/pnas.0811521106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Q, Vieira JM, Howard B, Eickholt BJ, Ruhrberg C. Neuropilin 1 and 2 control cranial gangliogenesis and axon guidance through neural crest cells. Development. 2008;135:1605–1613. doi: 10.1242/dev.015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela-Donenfeld D, Kalchiem C. Regulation of the onset of neural crest emigration by coordinated activity of BMP4 and Noggin in the dorsal neural tube. Development. 1999;126:4749–4762. doi: 10.1242/dev.126.21.4749. [DOI] [PubMed] [Google Scholar]
- Serbedzija G, Fraser S, Bronner-Fraser M. Pathways of trunk neural crest migration in the mouse embryo as revealed by vital dye labelling. Development. 1990;108:605–612. doi: 10.1242/dev.108.4.605. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. A vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Development. 1989;106:809–816. doi: 10.1242/dev.106.4.809. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. Developmental potential of trunk neural crest cells in the mouse. Development. 1994;120:1709–1718. doi: 10.1242/dev.120.7.1709. [DOI] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Solursh M, Fisher M, Meier S, Singley CT. The role of extracellular matrix in the formation of the sclerotome. J Embryol Exp Morphol. 1979;54:75–98. [PubMed] [Google Scholar]
- Stern C, Sisodaya S, Keynes R. Interactions between neurites and somite cells: inhibition and stimulation of nerve growth in the chick embryo. J Embryol Exp Morph. 1986;91:209–226. [PubMed] [Google Scholar]
- Teddy JM, Kulesa PM. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development. 2004;131:6141–6151. doi: 10.1242/dev.01534. [DOI] [PubMed] [Google Scholar]
- Teillet MA, Kalcheim C, Le Douarin NM. Formation of the dorsal root ganglia in the avian embryo: segmental origin and migratory behavior of neural crest progenitor cells. Dev Biol. 1987;120:329–347. doi: 10.1016/0012-1606(87)90236-3. [DOI] [PubMed] [Google Scholar]
- Terai Y, Abe M, Miyamoto K, Koike M, Yamasaki M, Ueda M, Ueki M, Sato Y. Vascular smooth muscle cell growth-promoting factor/F-spondin inhibits angiogenesis via the blockade of integrin alphavbeta3 on vascular endothelial cells. J Cell Physiol. 2001;188:394–402. doi: 10.1002/jcp.1122. [DOI] [PubMed] [Google Scholar]
- Testaz S, Duband JL. Central role of the alpha4beta1 integrin in the coordination of avian truncal neural crest cell adhesion, migration, and survival. Dev Dyn. 2001;222:127–140. doi: 10.1002/dvdy.1181. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Duband JL, Delouvee A. Pathways and mechanisms of avian trunk neural crest cell migration and localization. Dev Biol. 1982;93:324–343. doi: 10.1016/0012-1606(82)90121-x. [DOI] [PubMed] [Google Scholar]
- Tosney KW, Dehnbostel DB, Erickson CA. Neural crest cells prefer the myotome’s basal lamina over the sclerotome as a substratum. Dev Biol. 1994;163:389–406. doi: 10.1006/dbio.1994.1157. [DOI] [PubMed] [Google Scholar]
- Tucker RP. Abnormal neural crest cell migration after the in vivo knockdown of tenascin-C expression with morpholino antisense oligonucleotides. Dev Dyn. 2001;222:115–119. doi: 10.1002/dvdy.1171. [DOI] [PubMed] [Google Scholar]
- Tucker RP, Hagios C, Chiquet-Ehrismann R, Lawler J, Hall RJ, Erickson CA. Thrombospondin-1 and neural crest cell migration. Dev Dyn. 1999;214:312–322. doi: 10.1002/(SICI)1097-0177(199904)214:4<312::AID-AJA4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tucker RP, McKay SE. The expression of tenascin by neural crest cells and glia. Development. 1991;112:1031–1039. doi: 10.1242/dev.112.4.1031. [DOI] [PubMed] [Google Scholar]
- Wang H, Anderson D. Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron. 1997;18:383–396. doi: 10.1016/s0896-6273(00)81240-4. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen ZF, Anderson D. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B, Meller M, Weston JA. Analysis of melanocyte precursors in Nf1 mutants reveals that MGF/KIT signaling promotes directed cell migration independent of its function in cell survival. Dev Biol. 2001;232:471–483. doi: 10.1006/dbio.2001.0167. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B, Weston JA. Soluble and cell-bound forms of steel factor activity play distinct roles in melanocyte precursor dispersal and survival on the lateral neural crest migration pathway. Development. 1995;121:731–742. doi: 10.1242/dev.121.3.731. [DOI] [PubMed] [Google Scholar]
- Weston JA. Sequential segregation and fate of developmentally restricted intermediate cell populations in the neural crest lineage. Curr Top Dev Biol. 1991;25:133–153. doi: 10.1016/s0070-2153(08)60414-7. [DOI] [PubMed] [Google Scholar]
- Wilson YM, Richards KL, Ford-Perriss ML, Panthier JJ, Murphy M. Neural crest cell lineage segregation in the mouse neural tube. Development. 2004;131:6153–6162. doi: 10.1242/dev.01533. [DOI] [PubMed] [Google Scholar]
- Xu X, Francis R, Wei CJ, Linask KL, Lo CW. Connexin 43-mediated modulation of polarized cell movement and the directional migration of cardiac neural crest cells. Development. 2006;133:3629–3639. doi: 10.1242/dev.02543. [DOI] [PubMed] [Google Scholar]
- Yip JW. Migratory patterns of sympathetic ganglioblasts and other neural crest derivatives in chick embryos. J Neurosci. 1986;6:3465–3473. doi: 10.1523/JNEUROSCI.06-12-03465.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HH, Moens C. Semaphorin signaling guides cranial neural crest cell migration in zebrafish. Dev Biol. 2005;280:373–385. doi: 10.1016/j.ydbio.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Zisman S, Marom K, Avraham O, Rinsky-Halivni L, Gai U, Kligun G, Tzarfaty-Majar V, Suzuki T, Klar A. Proteolysis and membrane capture of F-spondin generates combinatorial guidance cues from a single molecule. J Cell Biol. 2007;178:1237–1249. doi: 10.1083/jcb.200702184. [DOI] [PMC free article] [PubMed] [Google Scholar]