Abstract
Background
The purpose of this study was: (1) to document the critical requirement of cystine for growth of human tumor cells in vitro, and (2) to determine the effect of the anticancer agent irinotecan on the cystine transporter xc– in head and neck FaDu xenografts.
Methods
Cell growth was measured by sulforhodamine B assay. xCT protein, glutathione (GSH) and DNA damage were determined using Western blot, spectrophotometry, and immunohistochemistry, respectively.
Results
Depletion of cystine from the medium inhibited tumor cell growth. Treatment of FaDu tumor with a therapeutic dose of irinotecan resulted in depression of xCT protein levels, leading to tumor growth retardation and downregulation of GSH with increased reactive oxygen species (ROS). The accumulation of ROS correlated with increased DNA damage as evidenced by increased H2AX.
Conclusion
Depression of xCT protein by irinotecan resulted in downregulation of GSH and increase in ROS, which could be the other possible mechanisms of DNA damage by irinotecan.
Key Words: Glutathione, Irinotecan, p-H2AX, SN-38, xCT
Introduction
The amino acid transporter system xc– is a heterodimer consisting of the SLC7A11 light chain (xCT) and the SLC3A2 heavy chain (4F2hc, also known as CD98) [1]. The xCT component is unique to system xc–, while 4F2hc is present in many transport systems. System xc– is a cystine-glutamate exchanger associated with cellular response to stress and production of the antioxidant glutathione (GSH). GSH is a tripeptide thiol containing glutamate, cysteine and glycine and is important for the cellular defense against oxidative stress and is a free radical scavenger and detoxifying agent [2]. Glutamate and glycine are amino acids that are abundant intracellulary, whereas cysteine is the rate-limiting amino acid in synthesis of GSH, underscoring the requirement for the functional system xc– to facilitate the uptake of cystine which will reduce to cysteine intracellulary. Many other factors influence the GSH levels, including γ-glutamylcysteine synthetase (γ-GCS) expression and activity, along with oxidative stress and antioxidants [3].
GSH is required by cancer cells to combat the significantly increased reactive oxygen species (ROS) during their growth. In cancer cells, GSH regulates DNA synthesis, cell growth, radiation resistance and also multidrug resistance, making it a target for cancer therapy [4,5]. Depletion of GSH leads to inhibition of drug efflux by multidrug resistance proteins (MRPs) resulting in the increased intracellular accumulation of drugs, and reduction of drug resistance [6,7,8]. Importantly, GSH is responsible for the detoxification of certain drugs [9] and GSH depletion has been linked to the therapeutic efficacy of some anticancer drugs [10,11].
Increased GSH synthesis requires enhanced cystine uptake [12,13,14] by cystine transporter system xc– which consists of xCT, a catalytic light chain. To determine chemical compounds that are susceptible to GSH mediated chemoresistance, Dai et al. applied chemoinformatics analysis with panel 3045 (NCI 3K) anticancer compounds on NCI-60 cells and found ∼15% of these compounds yielded negative correlation (resistance) with the xCT (SLC7A11) [15]. This result indicates that there are tumors in which the resistance is attributed to xCT overexpression, which provides the basis for the development of new anticancer drugs targeting xCT. Previously, Huang et al. demonstrated a correlation of SLC7A11 expression and geldanamycin resistance mediated by GSH [16]. Furthermore, the chemoinformatics analysis revealed that the SLC7A11-drug correlation was significantly greater than the other SLC7A sub family genes, γ-GCS, GSTP1 and other chemoresistance genes like ABCB1 (MDR1) and ABCC1 (MRP1) tested in NCI-60 cells, indicating that SLC7A11 plays an important role in chemomodulation [15]. Recently, xCT has been recognized as an important molecular target for the therapy of various diseases including cancer [5].
Irinotecan is used as a chemotherapeutic agent in the treatment of various solid tumors including colorectal cancer and lung cancer [17,18]. Irinotecan is a topoisomerase I (topo I) inhibitor that induces DNA double strand breaks and cell death. SN-38, the active metabolite of irinotecan, has been reported as the inducer of ROS in gastric cancer cells [19]. Based on this, one can reasonably expect that SN-38 may downregulate intracellular GSH levels. The anticancer drug topotecan belongs to the irinotecan family and exerts its cytotoxic effects through the increased production of ROS [20]. The ROS-generating compounds induce apoptotic pathways in MDA-MB-231 human breast carcinoma cells [21] and rat glioma cells [22]. Given the importance of system xc– in the synthesis of GSH and the key role of GSH in cancer cell growth, we investigated the role of irinotecan in the regulation of GSH in FaDu tumor xenografts and FaDu cells in vitro. FaDu, a human epithelial cell line from squamous cell carcinoma of the hypopharynx was isolated and established from a patient [23]. Additionally, we and others found that xCT of the cystine transporter system xc– played the key role in acquiring cysteine for the synthesis of GSH [24,25,26]. Collectively, our data indicate that the observed reduction of GSH and concomitant increase in ROS level as a consequence of downregulation of xCT by irinotecan represent a potential new mechanism in addition to the inhibition of topo I.
Materials and Methods
Mice
Female athymicnude mice (Foxn1nu; Harlan Sprague-Dawley, Inc. Indianapolis, Ind., USA) that were 8–12 weeks old were maintained in the animal facility. All studies were performed in accordance with protocols approved by the institutional animal care and use committee at Roswell Park Cancer Institute.
Drugs
Irinotecan was purchased from Pfizer (formerly Pharmacia and Upjohn Co., Kalamazoo, Mich., USA) in 5-ml aliquots as ‘ready to use’ clinical formulations containing 100 mg drug (20 mg/ml). SN-38 was purchased from Pharmacia and dissolved in DMSO. The required final drug concentrations for in vitro experiments were obtained by diluting in growth medium. Monosodium glutamate (MSG) and sulfasalazine were obtained from Sigma-Aldrich (St. Louis, Mo., USA).
Determination of External Cystine Requirement for the Growth of Cancer Cells
FaDu and other cancer cells (A253, A549, PC3; purchased from ATCC) free of Mycoplasma tested periodically using Mycoplasma T.C. rapid detection system (Gen-Probe, Inc., San Diego, Calif., USA), were used to determine the cystine requirement for growth. Special RPMI-1640 medium (Cat No. 17-104-Cl; Mediatech, Herndon, Va., USA) without L-cystine, L-glutamine and L-methionine was used. To make the complete medium, 2.05 mM glutamine (300 mg/l) and 100 μM methionine (15 mg/l), the concentrations present in the regular RPMI-1640, were added. In contrast to this, 84 μM of cystine was added at its physiological concentration [27], which is approximately one third of the regular RPMI-1640 medium. Approximately 300–400 cells in each well of a 96-well plate were grown overnight in regular RPMI-1640 medium with 10% fetal bovine serum (FBS). Non-dialyzed FBS was used in all the media and experimental conditions. Cells were washed three times with sterile PBS (pH 7.2, 37°C) and special RPMI-1640 medium with and without cystine and/or methionine was added and cells were allowed to grow for 5 days with fresh media replacement on the third day. Control cells were maintained in complete special RPMI-1640 medium with cystine, glutamine, methionine and 10% FBS. Cell growth inhibition was measured by determining total protein with a sulforhodamine B assay as described previously [28]. Briefly, the cells were fixed with 10% trichloroacetic acid, washed with water, stained with sulforhodamine B assay, and washed again. The dye bound to protein was extracted with Tris base and measured at 570 nm with an automated Bio-Kinetics reader (Bio-Tek Instruments, Winooski, Vt., USA). The effect of cystine and methionine depletion and addition on cell growth was determined by comparing the percent growth inhibition with the control group. Experiments were performed in triplicates, and repeated thrice.
Determination of Specific Inhibitor of Cystine Transporter MSG Effect on the Growth of FaDu Cells
FaDu cells (3 × 105) were plated in each well of a 12-well plate and allowed to grow overnight with regular RPMI-1640 with 10% FBS. Cells were rinsed with PBS three times and special RPMI-1640 with physiological levels of cystine (84 μM) with 100, 150 and 180 mM MSG alone and in combination with 66 μM β-mercaptoethanol (BME) was added. After 24 h of treatment, cells were rinsed with PBS and special RPMI-1640 medium was added and further incubated for 72 h. Photomicrographs were captured with the inverted microscope. Cells were rinsed with PBS, trypsinized, collected and counted with a coulter counter. Percent growth inhibition was compared with the untreated control. The experiment was repeated with duplicate samples.
Effect of Irinotecan on the Growth of FaDu Tumor Xenografts
FaDu tumor xenografts were established using ∼50 mg non-necrotic FaDu tumors pieces in 8–12-week-old female athymic mice (Foxnnu 20–25 g) as described earlier [29]. Mice bearing an established tumor (∼100–200 mg, 7 days after implantation) were treated with the maximum tolerated dose of irinotecan (100 mg/kg i.v. weekly ×2). Tumor size was measured on two axes (in millimeters; L, longest axis, and W, shortest axis) with the aid of Vernier calipers as described previously [30]. Tumor weight (mg or mm3) was calculated by using the formula: tumor weight = 1/2 (L × W2). Twenty-four hours after the first and second dose of irinotecan, tumors were removed from the mice for further analysis.
Western Blot Analysis
FaDu tumor xenografts were collected from nude mice 24 h after the first and second doses of irinotecan (days 8 and 15) and frozen in liquid nitrogen. Tissue homogenates were prepared using a polytron homogenizer in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton ×100) containing a protease inhibitor cocktail (Roche, Indianapolis, Ind., USA). Protein extraction was carried out using lysis buffer with FaDu cells treated with 0.1 μM SN-38 and untreated cells. Thirty to forty micrograms of protein were electrophoresed and transferred to a PVDF membrane. Western blot was performed with a rabbit polyclonal antibody against xCT (antibody preparation described previously [31]) and goat polyclonal antibody against xCT (Abcam, Cambridge, Mass., USA) at 1:500 dilution. Monoclonal p-H2AX antibody (Upstate, Lake Placid, N.Y., USA) was used at 1:1,000 dilution. All the antibodies were used in 5% milk in phosphate buffered saline with tween 20 (PBS-T) and incubated for 1 h followed by a wash with 5% milk in PBS-T. HRP-conjugated anti-rabbit and anti-mouse antibodies at 1:5,000 dilution were added in the PBS-T milk for 1 h followed by washing four times with PBS-T (15 min each wash). The ECL-Plus detection kit (GE Biosciences, Piscataway, N.J., USA) was used to detect the protein.
RT-PCR Analysis
Total RNA was isolated from the control and SN-38-treated FaDu cells using the TRIzol reagent (Invitrogen, Carlsbad, Calif., USA) and cDNA was made using the high capacity RNA-to-cDNA master mix (Applied Biosystems, Foster City, Calif., USA). PCR was performed using the gene-specific primers, and PCR product was separated on 1% agarose gel to determine the expression levels as described earlier [24].
Glutathione Assay
FaDu cells were grown in minimum essential medium with 10% FBS containing 84 μM cystine, a concentration close to physiological levels [27,32], and treated with 0.1 μM SN-38 for 24 and 48 h. To determine whether GSH levels were downregulated by SN-38, cells were cultured with and without 66 μM BME, a reducing agent that facilitate uptake of cystine by cells via formation of a mixed disulfide consisting of cystine and BME, which is then readily taken up by cells via L-transport system when cystine transporter is inhibited or not functional [33]. FaDu tumor xenografts taken 24 h after the second dose of irinotecan and the respective controls were processed immediately after removal from the mouse for measuring GSH levels. Total GSH was determined using the Glutathione Assay Kit (Calbiochem, San Diego, Calif., USA) according to the manufacturer's protocol. Tumor cells were washed with PBS after treatment, homogenized in cold 5% metaphosphoric acid and centrifuged at 3,000 g for 10 min. Supernatant was collected and used for the assay. Tumor xenografts were washed with 0.9% NaCl solution and blotted on tissue paper. The tissue was weighed minced in cold 5% metaphosphoric acid and homogenized using the polytron homogenizer. The homogenate was centrifuged at 3,000 g, at 4°C for 10 min. The clear supernatant was collected and kept at 4°C for the assay. A standard curve was prepared with 0, 20, 40, 80 and 100 μmol/l GSH in metaphosphoric acid solution and buffer (solution 3) was added to 900 μl. 50 μl of each reagent, R1 (4-chloro-1-methyl-7-trifluromethyl-quinolinium methylsulfate) and reagent R2 (30% NaOH) were added, vortexed and incubated at 25 ± 3°C for 10 min in the dark. The absorbance at 400 nm was determined using a spectrophotometer, and a standard curve was obtained. 100 μl of tumor cell extract and tumor xenografts homogenate was added to 900 μl of buffer. The reagents R1 and R2 were added and absorbance measured as mentioned above. The concentration of GSH was calculated using the standard curve. The experiment was repeated with three different tumor samples.
Measurement of Intracellular ROS
Intracellular levels of ROS were measured with a 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (Carboxy-H2DCFDA) (Molecular Probes, Eugene, Oreg., USA) probe as per the manufacturer's instructions described by us [34]. Briefly, FaDu cells were grown overnight in the 96 well plates and then incubated with CM-DCFDA for 30 min before treating with 0.1 μM SN-38. After 2 h of treatment the cells were processed and analyzed by fluorescence (Multi Detection Microplate Reader; BioTek Synergy TM HT, Winooski, Vt., USA) with excitation at 495 nm and emission at 528 nm.
DNA Double-Strand Breaks
DNA double-strand breaks were analyzed in FaDu cells treated with 0.1 μM SN-38 and untreated cells by pulsed-field gel electrophoresis (PFGE) as described previously [35]. Briefly, DNA plugs were prepared by using 5 × 106 cells in 2% low melting agarose at 50°C and poured into molds to obtain 1 × 106 FaDu cells in each mold. The cells were lysed using lysis buffer (0.5 EDTA, pH 8.0, 10 mM Tris, 1% sarkosyl, and 1 mg/ml of proteinase K) for 24 h at 50°C. The DNA plugs were washed with Tris/EDTA buffer treated with RNase A for 1 h and used for electrophoresis on 0.8% agarose gels. Electrophoresis was carried out using Hex-A-Field horizontal gel electrophoresis apparatus at 14°C with buffer circulation. Gels were stained with ethidium bromide and photographed on UV-transilluminator.
Immunohistochemical Detection of Phosphorylated Histone H2AX
FaDu tumor xenograft samples were fixed in 10% neutral buffered formalin overnight and were processed for paraffin embedding, and 4- to 5-μm sections were mounted on charged slides. Following deparaffinization, sections were incubated in citrate buffer (pH 6) in a microwave for 20 min for antigen retrieval. Endogenous peroxidase was quenched with 3% H2O2 and casein (0.03%) was used to block nonspecific binding. Anti-human anti-phospho-H2AX (Ser.139) clone J3W301 mouse Mab (Upstate) in 2 μg/ml concentration was applied for 1 h as primary antibody. Elite biotinylated secondary antibody (Vector Labs, Burlingame, Calif., USA) was applied for 30 min followed by incubation with Elite ABC reagent (Vector Labs) for 30 min. The chromogen DAB (Dako, Carpinteria, Calif., USA) was applied for 5 min for visualization of the immunoreactive product. Nuclear counterstaining was done with hematoxylin. Slides were washed between the various steps with PBS/500 μl/l Tween. Mouse IgG1, an isotype-matched negative control, was used on duplicate slides in place of primary antibody in the same concentration as primary antibody. All immunohistochemical procedures were carried out in automated immunostainer (Dako). The method was developed in our lab, optimized and validated in comparison with pulse field electrophoresis data showing untreated FaDu tumor cells do not have H2AX (known negative control), while SN-38-treated tumor cells show H2AX, a marker of double-strand DNA breaks (known positive control). H2AX expression was assessed semiquantitatively by counting the positive (brown) and negative (unstained) tumor cell nuclei in 6 high-power microscopic fields (400×) and expressed as a percentage of the total nuclei. In order to assess the stained proportion of tumor cell nuclei, the microscopic fields were selected after each other as highly, moderately, less or not labeled areas of the tumor. All studies were reviewed, counted, and interpreted by a board-certified, experienced pathologist (K.T.).
Statistical Analysis
The experimental data presented were obtained from experiments repeated three times with triplicates. Mean values were calculated and significance was determined using the Student's two-tailed t test. Tumor xenograft growth curve data were analyzed using GraphPad prism version 5 (La Jolla, Calif., USA) software and p < 0.05 was considered significant. Tumor growth curves in nude mice were compared using linear regression analysis.
Results
Exogenous Cystine Is Required for Normal Growth of Tumor Cells in vitro
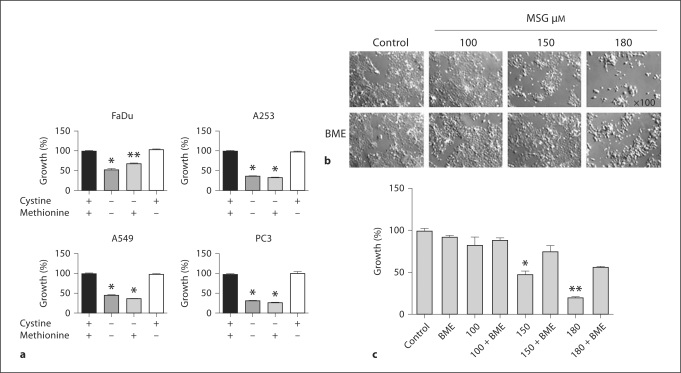
Since some cancer cells and normal cells require the cystine transporter for their growth, the roles of cystine and methionine in support of tumor cells growth were investigated. There was a significant growth inhibition in four tumor cell lines, poorly differentiated human head and neck squamous cell carcinoma (HNSCC) FaDu, well-differentiated HNSCC A253, non-small cell lung carcinoma A549 and prostate cancer cells PC-3 in special RPMI-1640 media (10% FBS) devoid of cystine and methionine (fig. 1). Addition of methionine did not restore growth whereas cystine addition did restore normal growth. This result indicates that cystine is required for the normal growth of cancer cells. The complete growth inhibition of cells was not observed when the cystine and methionine were not present, as reported by others [27], which could be due to the use of non-dialyzed serum which contains the amino acids. FaDu tumor cells were further used to determine the effect of anticancer agent irinotecan on cystine transporter xc–. To investigate whether the xc– specific inhibitor MSG inhibits the growth of FaDu cells, different concentrations of MSG were used. FaDu cells required high concentration of MSG to inhibit the growth. Recently Olm et al. showed that 60 mM MSG did not affect the cell growth of different human lung carcinoma cells [36]. FaDu cell growth was significantly inhibited by MSG (150–180 mM) and BME treatment prevented this inhibition effect (fig. 1b, c). BME alone did not affect the growth.
Fig. 1.
External cystine requirement for the growth of tumor cells. a The percent growth inhibition of cancer cells after 5 days of growth in special RPMI-1640 medium (10% FBS) with and without amino acids. For the complete medium, L-glutamine (2.05 mM) and L-methionine (100 μM) were added at their normal concentration present in the regular RPMI-1640 medium. L-cystine (84 μM) at its physiological concentration was added to the medium. + and − symbols indicate presence or absence of the amino acids in the medium. ∗ p < 0.001, ∗∗ p ≤ 0.003. b Representative photomicrographs of FaDu cells treated with various concentration of MSG with and without BME in special RPMI-1640. Cells were treated for 24 h, rinsed with sterile PBS and fresh medium was added. Cells were further incubated for 72 h and pictures were captured with 100 × magnification using inverted microscope. c Percent growth inhibition of FaDu cells treated with different concentrations (100, 150 and 180 μM) of MSG alone and in combination with BME. Special RPMI-1640 medium was used. ∗ p ≤ 0.005, ∗∗ p ≤ 0.001.
Treatment with Irinotecan Inhibited Tumor Growth and Reduced the Concentration of GSH
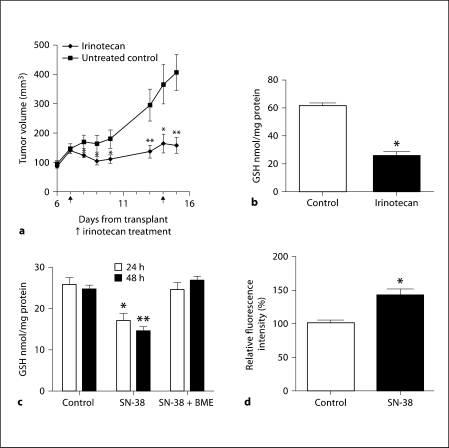
Since irinotecan exerts antitumor activity in vivo in various solid tumor malignancies, we investigated the effect of irinotecan at its maximum tolerated dose (100 mg/kg) with clinically relevant schedule (twice weekly) on the growth inhibition of FaDu xenografts and GSH concentration. The data presented in figure 2a demonstrate the significant tumor growth inhibition by irinotecan. The tumor burdens were significantly different in the untreated versus irinotecan-treated groups for time points after 24 h after the first irinotecan treatment. Linear regression analysis of the growth curves showed a significant inhibition (p ≤ 0.05) by irinotecan.
Fig. 2.
Irinotecan inhibits FaDu tumor growth with decreased GSH and increased ROS. a Effect of irinotecan on the growth of FaDu tumor xenografts. FaDu tumors were transplanted and allowed to grow for a week before being treated with the maximum tolerated dose of irinotecan (100 mg/kg/week i.v. × 2). The control and irinotecan-treated tumor volumes were measured for up to 15 days. Five different mice were used for each condition. ∗ p < 0.05, ∗∗ p < 0.009. b GSH concentrations in the FaDu tumor xenografts treated with irinotecan. Total GSH levels were determined in the tumor xenografts 24 h after the second dose of irinotecan. Five different tumor samples were used. ∗ p < 0.004. c Concentration of GSH in FaDu cells treated with SN-38. FaDu cells treated with SN-38 (0.1 μM) and GSH were analyzed after 24 and 48 h. The experiments were repeated three times with triplicate samples. BME was added to the culture medium to facilitate cystine transport via formation of mixed disulfide consisting of cysteine and BME. ∗ p < 0.02, ∗∗ p < 0.002. d Effect of SN-38 on ROS levels. FaDu cells treated with 0.1 μM SN-38 had a significantly increased accumulation of intracellular ROS compared to the untreated controls. The experiment was performed 3 times with triplicate samples. ∗ p < 0.01.
To document the association between the observed antitumor activity of irinotecan and its effect on GSH, tumor tissues were removed 24 h after the administration of the 2nd weekly dose of irinotecan and the GSH levels were analyzed. The data in figure 2b clearly demonstrate that significant reduction of GSH pools were observed at the time of tumor growth inhibition by irinotecan. The GSH levels were decreased by 58% in FaDu xenografts (fig. 2b). To investigate the effect of SN-38 on GSH, FaDu cells were treated with IC50 (0.1 μM) of SN-38, and intracellular levels of GSH were evaluated; the results are summarized in figure 2c. In agreement with the in vivo findings, the levels of GSH were inhibited by 31 and 41% at 24 and 48 h of SN-38, respectively. Addition of BME in the medium prevented the reduction of GSH levels by SN-38-treated cells, which is likely due to the BME-induced cystine uptake via formation of mixed disulfide consisting of cysteine and BME. This result suggest that the SN-38 effect is based on interference with the cellular uptake of cystine due to the downregulation of xCT protein, the catalytic light chain of cystine transporter system xc–. Since GSH is involved in the attenuation of ROS generated during oxidative metabolism, the effect of SN-38-induced GSH downregulation on ROS was investigated in FaDu cells. As shown in figure 2d, SN-38 treatment led to a 40% increase in ROS levels, indicating that there was ROS generation with SN-38 treatment in FaDu cells.
Irinotecan Increased DNA Double-Strand Breaks
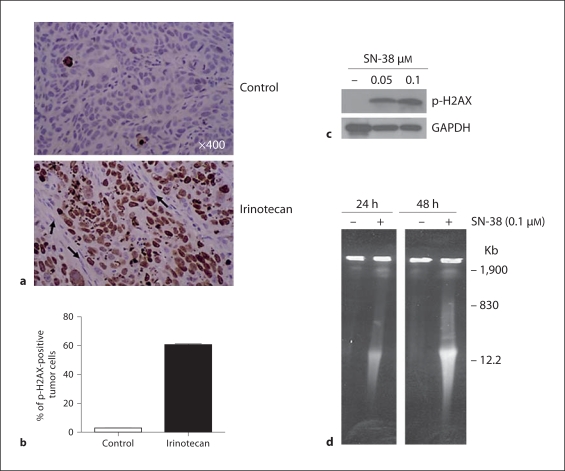
The effect of irinotecan on DNA damage was investigated. The results indicate that p-H2AX, a surrogate marker for DNA damage, increased significantly in FaDu xenografts (fig. 3a) 24 h after treatment with the therapeutic dose of irinotecan compared to the untreated controls. The percentage of p-H2AX-positive cells in the tumor xenografts was 61% in the irinotecan treatments compared to 2% in untreated controls (fig. 3b). A similar dose-dependent increase of p-H2AX protein was observed by Western blot in FaDu cells treated with SN-38 (fig. 3c). We investigated the DNA double-strand breaks in the FaDu cells treated with SN-38 with pulsed-field gel electrophoresis. As presented in figure 3d, the DNA double-strand breaks were detected in the FaDu cells treated with SN-38 in vitro.
Fig. 3.
Irinotecan treatment induced DNA damage in tumor cell nuclei of FaDu xenografts. a FaDu tumor xenografts were removed 24 h after the second dose of irinotecan, were fixed and processed for the immunohistochemical staining with anti-p-H2AX. Photomicrographs are representative of the whole tumor section. In the upper panel the control has only a few tumor cell nuclei positive for p-H2AX, whereas in the lower panel the irinotecan-treated tumor show high expression of p-H2AX protein in many tumor cell nuclei. Arrows show the absence of p-H2AX in the stromal cell nuclei. b H2AX-positive tumor cells were counted and the percentage was calculated and presented in the chart showing the increase with irinotecan treatment. c p-H2AX protein levels as detected by Western blot analysis increased significantly in FaDu cells treated with SN-38. GAPDH was used as loading control. d Double-strand DNA breaks at 24 and 48 h after SN-38 (0.1 μM) treatment as observed by pulsed-field gel electrophoresis in FaDu cells. A megabase DNA damage was observed, as shown at 1,900 kb size.
xCT Was Downregulated by Irinotecan in FaDu Tumor Xenografts and Cells
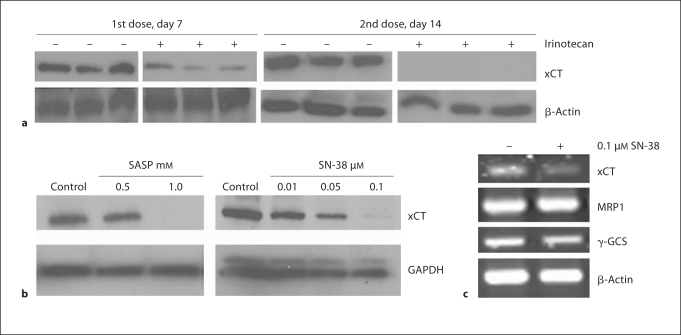
The availability of cystine is the rate-limiting component for the synthesis of GSH and system xc– is the exchanger for cystine-glutamate, a lack of key component of system xc– (xCT) would be expected to be affected by irinotecan. The effect of irinotecan on xCT expression levels were investigated in FaDu tumor xenografts. As shown in figure 4a, the effect of irinotecan on xCT protein expression is time dependent and maximum downregulation was found at 24 h after the second weekly treatment. The expression of xCT mRNA and protein were downregulated by SN-38, the active metabolite of irinotecan in FaDu cells (fig. 4b, c). Interestingly, the GSH synthesis regulator γ-GCS and its efflux enhancer MRP1 expression levels were not affected by SN-38 (fig. 4c), indicating that GSH downregulation could be due to the downregulation of xCT that compromises the cystine transporter xc– function.
Fig. 4.
Downregulation of xCT light chain of cystine transporter xc− in the FaDu tumor xenografts and cells treated with SN-38 active metabolite of irinotecan. a Western blot analysis demonstrated that xCT protein levels were depressed 24 h after the first dose of irinotecan, and further decreased to undetectable levels 24 h after the second dose. + and − symbols were used for the irinotecan-treated and untreated samples. Three different tumor samples were used for each condition. β-Actin was used as loading control. b Western blot analysis shows that SN-38 treatment downregulated xCT protein in FaDu cells. A known xCT inhibitor, sulfasalazine, was used to demonstrate the specific inhibition of xCT in FaDu cells. GAPDH was used as loading control. c RTPCR analysis shows that xCT mRNA levels were inhibited by SN-38 in FaDu cells. MRP1 and γ-GCS expression levels were not altered by SN-38. β-Actin served as loading control.
Discussion
The cystine/glutamate exchanger xc– is involved in the transport of cystine, and is a key regulator of cellular GSH levels mediating chemoresistance to cytotoxic agents [16,37]. Inhibition of the cystine transporter with the aim of depleting GSH has been suggested as a therapeutic potential in treatment of human lymphomas and leukemias [14,38,39], breast cancers [12,40] and gliomas [41,42]. High expression of xCT the catalytic partner of system xc– with increased GSH has been correlated to the cisplatin drug resistance in human ovarian cancer cells A2780DDP [43]. There has been considerable interest in xCT as a critical chemotherapeutic target. Sulfasalazine, an inhibitor of xCT, has been suggested for the treatment of prostate cancer [27]. Our results with several human cancer cell lines demonstrate growth inhibition when the cystine was depleted from the medium (fig. 1). Addition of cystine restored normal growth whereas the methionine, a known precursor of thiols, was not able to restore the normal growth. The cystine/cysteine cycle regulated by xc– has been reported as the key regulatory system for cell survival and cell death and also its over-expression itself protects against oxidative damage by not elevating GSH levels [26]. Cystine uptake mediated by system xc– is required for the growth of normal cells like mouse fibroblast [24], and is also a rate-limiting step in the synthesis of GSH. A decrease in GSH content has been reported to lead to the generation of oxidative stress and growth arrest of the cells [44].
Irinotecan, an anticancer agent, has been reported to be an inducer of ROS in gastric cancer cells [19], suggesting its effects on GSH. Irinotecan-mediated anti-tumor activities were extensively evaluated in our laboratory against tumor xenografts [29]. The present study was initiated to determine whether xCT is a target for irinotecan, and whether its inhibition correlated with therapeutic outcome using FaDu tumors as a representation of the cell lines tested. We determined the inhibition of tumor growth by irinotecan (fig. 2a) correlated with the downregulation of xCT (fig. 4). The xCT downregulation resulted in 58% reduction in GSH concentration in xenografts (fig. 2b). These findings indicate that downregulation of xCT could be one of the critical determinants of the in vivo response to irinotecan treatment. Consistent with this, inhibition of the cystine transporter by sulfasalazine in prostate tumors [27] and primary brain tumors [41] has been reported as the primary reason for the growth retardation supporting this contention. Apart from this, Lyons et al. [42] reported that chronic inhibition of xCT leads to smaller glioma tumors that are less invasive compared to controls, suggesting that xCT may play a role in invasion and metastasis besides tumor growth.
The invasive function of xCT appears to be due to the secretion of neurotoxin MSG that leads to cell death and provides extra space in the skull so that glioma cells replicate. This could be a different mechanism by which cells can metastasize. In addition to the observed downregulation of GSH by irinotecan in tumor xenografts, we also found a significant decrease in GSH concentration in the SN-38-treated FaDu cells compared to control cells (fig. 2c). In the presence of BME the GSH levels were restored because the BME induced cystine uptake by forming mixed disulfide consisting of cysteine, and BME prevented the reduction of GSH levels by SN-38-treated cells, which is likely due to the cysteine transportation by another transporter, making it available for the GSH synthesis. The decrease in GSH concentration potentially increases the ROS in the cellular system. Consistent with this we found an increase in ROS levels in the SN-38-treated FaDu cells compared to control cells (fig. 2d). SN-38 has been reported as a ROS inducer in gastric cancer cells [19]. Topotecan, a derivative of camptothecin (irinotecan family) exerting the cytotoxic action through the inhibition of topo I, also can cause ROS production [20]. These results are consistent with the data generated with other chemotherapeutic agents such as doxorubicin, daunorubicin, mitoxantrone, bleomycine and cisplatin, which are known to generate ROS that promote oxidative stress [45,46,47,48,49,50,51].
The increased ROS due to a decrease in GSH could lead to the generation of redox imbalance that may trigger DNA damage. We found an increase in the p-H2AX, a surrogate marker for DNA damage, in the FaDu tumors (fig. 3a, b) treated with irinotecan and in FaDu cells treated with SN-38 (fig. 3c) indicating DNA damage. We also demonstrated double-strand breaks (fig. 3d) in FaDu cells treated with SN-38. The DNA damage mediated by ROS could be caused by the hydroxylation of guanine [52] and also by the formation of bulky DNA lesions [53].
We have previously reported that the combination of irinotecan with 5FU is highly synergistic resulting in a 100% cure in mice bearing FaDu xenografts where irinotecan was administered 24 h prior to 5FU [54]. Interestingly, when 5FU was administered 24 h prior to irinotecan with the same dose and schedule, the antitumor efficacy was significantly reduced. The cytotoxic effects of 5FU have been directly linked to the levels of GSH with a 50% decrease resulting in a 20–31% increase in cell kill in HT-29 cells [55]. The decrease of GSH by 5FU has been shown to be due to the inhibition of γ-GCS, an enzyme that uses cysteine to synthesize GSH [56], which is downstream from the xCT inhibition by irinotecan found in this study. Our results presented in figure 4c also demonstrate that γ-GCS expression has not been regulated by SN-38, the active metabolite of irinotecan. Irinotecan targeting xCT could inhibit the cystine transportation inside the cells, resulting in the unavailability of cysteine substrate to γ-GCS enzyme to synthesize GSH. The synergistic antitumor efficacy reported by us with the pretreatment with irinotecan followed by 5FU could be due to the upstream inhibition of xCT by irinotecan that reduces the availability of cysteine in the cells. This could be one of the reasons for the synergy of FaDu xenografts when treated with irinotecan prior to 5FU. Thus, drugs such as irinotecan that could modulate intracellular GSH may also regulate the cystine/cysteine cycle. The cystine/cysteine cycle itself is enough to protect the cells against oxidative damage [26]. Therapeutically targeting this cycle might be more effective in cancer cells when used in combination chemotherapy, due in part to increased intracellular ROS. We have demonstrated the SN-38 inhibitory effects on xCT mRNA (fig. 4c), indicating that SN-38 acts on the transcription of xCT mRNA leading to the downregulation of xCT protein (fig. 4b), which could lead to the inhibition of cystine transporter xc–function. We also found the xCT protein was downregulated in FaDu tumor xenografts (fig. 4a). We did not investigate the effects of irinotecan on the tumor growth promoting stromal cells, including fibroblast and dendritic cells, which secrete cysteine into the tumor microenvironment. Further studies are warranted to find out if irinotecan affects these cells.
In summary, the present investigation identified xCT as a new and potentially critical target of irinotecan in addition to its known target, topo I. Lowering GSH, a known marker for drug resistance, as a consequence of downregulation of xCT could be a critical mechanism associated with the observed therapeutic effect of irinotecan in the model system of FaDu head and neck tumor evaluated in the present study. These findings suggest that xCT could be a target for irinotecan.
Acknowledgment
We thank Dr. Richard Swank for reading the manuscript and Gerald Jahreis for his technical assistance. This work was supported by a Comprehensive Cancer Center Support Grant CA 16056 from the National Cancer Institute, Bethesda, Md., USA.
References
- 1.Palacin M, Nunes V, Font-Llitjos M, Jimenez-Vidal M, Fort J, Gasol E, Pineda M, Feliubadalo L, Chillaron J, Zorzano A. The genetics of heteromeric amino acid transporters. Physiology (Bethesda) 2005;20:112–124. doi: 10.1152/physiol.00051.2004. [DOI] [PubMed] [Google Scholar]
- 2.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 3.Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. Faseb J. 1999;13:1169–1183. [PubMed] [Google Scholar]
- 4.Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 5.Lo M, Wang YZ, Gout PW. The x(c)-cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol. 2008;215:593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- 6.Benderra Z, Trussardi A, Morjani H, Villa AM, Doglia SM, Manfait M. Regulation of cellular glutathione modulates nuclear accumulation of daunorubicin in human mcf7 cells overexpressing multidrug resistance associated protein. Eur J Cancer. 2000;36:428–434. doi: 10.1016/s0959-8049(99)00288-9. [DOI] [PubMed] [Google Scholar]
- 7.Vanhoefer U, Cao S, Minderman H, Toth K, Skenderis BS, 2nd, Slovak ML, Rustum YM. D,l-buthionine-(s,r)-sulfoximine potentiates in vivo the therapeutic efficacy of doxorubicin against multidrug resistance protein-expressing tumors. Clin Cancer Res. 1996;2:1961–1968. [PubMed] [Google Scholar]
- 8.Rudin CM, Yang Z, Schumaker LM, VanderWeele DJ, Newkirk K, Egorin MJ, Zuhowski EG, Cullen KJ. Inhibition of glutathione synthesis reverses bcl-2-mediated cisplatin resistance. Cancer Res. 2003;63:312–318. [PubMed] [Google Scholar]
- 9.Hamilton D, Batist G. Glutathione analogues in cancer treatment. Curr Oncol Rep. 2004;6:116–122. doi: 10.1007/s11912-004-0023-4. [DOI] [PubMed] [Google Scholar]
- 10.Zhang K, Mack P, Wong KP. Glutathione-related mechanisms in cellular resistance to anticancer drugs. Int J Oncol. 1998;12:871–882. doi: 10.3892/ijo.12.4.871. [DOI] [PubMed] [Google Scholar]
- 11.Fojo T, Bates S. Strategies for reversing drug resistance. Oncogene. 2003;22:7512–7523. doi: 10.1038/sj.onc.1206951. [DOI] [PubMed] [Google Scholar]
- 12.Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells. Anticancer Res. 2003;23:4571–4579. [PubMed] [Google Scholar]
- 13.Gout PW, Kang YJ, Buckley DJ, Bruchovsky N, Buckley AR. Increased cystine uptake capability associated with malignant progression of nb2 lymphoma cells. Leukemia. 1997;11:1329–1337. doi: 10.1038/sj.leu.2400739. [DOI] [PubMed] [Google Scholar]
- 14.Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)-cystine transporter: a new action for an old drug. Leukemia. 2001;15:1633–1640. doi: 10.1038/sj.leu.2402238. [DOI] [PubMed] [Google Scholar]
- 15.Dai Z, Huang Y, Sadee W, Blower P. Chemoinformatics analysis identifies cytotoxic compounds susceptible to chemoresistance mediated by glutathione and cystine/glutamate transport system xc. J Med Chem. 2007;50:1896–1906. doi: 10.1021/jm060960h. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Dai Z, Barbacioru C, Sadee W. Cystine-glutamate transporter slc7a11 in cancer chemosensitivity and chemoresistance. Cancer Res. 2005;65:7446–7454. doi: 10.1158/0008-5472.CAN-04-4267. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs C, Mitchell EP, Hoff PM. Irinotecan in the treatment of colorectal cancer. Cancer Treat Rev. 2006;32:491–503. doi: 10.1016/j.ctrv.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Fischer B, Arcaro A. Current status of clinical trials for small cell lung cancer. Rev Recent Clin Trials. 2008;3:40–61. doi: 10.2174/157488708783330503. [DOI] [PubMed] [Google Scholar]
- 19.Kishida O, Miyazaki Y, Murayama Y, Ogasa M, Miyazaki T, Yamamoto T, Watabe K, Tsutsui S, Kiyohara T, Shimomura I, Shinomura Y. Gefitinib (‘Iressa’, zd1839) inhibits SN38-triggered EGF signals and IL-8 production in gastric cancer cells. Cancer Chemother Pharmacol. 2005;55:393–403. doi: 10.1007/s00280-004-0904-0. [DOI] [PubMed] [Google Scholar]
- 20.Akbas SH, Timur M, Ozben T. The effect of quercetin on topotecan cytotoxicity in MCF-7 and MDA-MB 231 human breast cancer cells. J Surg Res. 2005;125:49–55. doi: 10.1016/j.jss.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Choi WY, Kim GY, Lee WH, Choi YH. Sanguinarine, a benzophenanthridine alkaloid, induces apoptosis in MDA-MB-231 human breast carcinoma cells through a reactive oxygen species-mediated mitochondrial pathway. Chemotherapy. 2008;54:279–287. doi: 10.1159/000149719. [DOI] [PubMed] [Google Scholar]
- 22.Yang KB, Zhao SG, Liu YH, Hu EX, Liu BX. Tetraethylammonium inhibits glioma cells via increasing production of intracellular reactive oxygen species. Chemotherapy. 2009;55:372–380. doi: 10.1159/000235730. [DOI] [PubMed] [Google Scholar]
- 23.Rangan SR. A new human cell line (FaDu) from a hypopharyngeal carcinoma. Cancer. 1972;29:117–121. doi: 10.1002/1097-0142(197201)29:1<117::aid-cncr2820290119>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 24.Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT. Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci USA. 2005;102:10964–10969. doi: 10.1073/pnas.0502856102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato H, Shiiya A, Kimata M, Maebara K, Tamba M, Sakakura Y, Makino N, Sugiyama F, Yagami K, Moriguchi T, Takahashi S, Bannai S. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem. 2005;280:37423–37429. doi: 10.1074/jbc.M506439200. [DOI] [PubMed] [Google Scholar]
- 26.Banjac A, Perisic T, Sato H, Seiler A, Bannai S, Weiss N, Kolle P, Tschoep K, Issels RD, Daniel PT, Conrad M, Bornkamm GW. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27:1618–1628. doi: 10.1038/sj.onc.1210796. [DOI] [PubMed] [Google Scholar]
- 27.Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate. 2007;67:162–171. doi: 10.1002/pros.20508. [DOI] [PubMed] [Google Scholar]
- 28.Azrak RG, Frank CL, Ling X, Slocum HK, Li F, Foster BA, Rustum YM. The mechanism of methylselenocysteine and docetaxel synergistic activity in prostate cancer cells. Mol Cancer Ther. 2006;5:2540–2548. doi: 10.1158/1535-7163.MCT-05-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao S, Durrani FA, Rustum YM. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin Cancer Res. 2004;10:2561–2569. doi: 10.1158/1078-0432.ccr-03-0268. [DOI] [PubMed] [Google Scholar]
- 30.Javle MM, Cao S, Durrani FA, Pendyala L, Lawrence DD, Smith PF, Creaven PJ, Noel DC, Iyer RV, Rustum YM. Celecoxib and mucosal protection: translation from an animal model to a phase I clinical trial of celecoxib, irinotecan, and 5-fluorouracil. Clin Cancer Res. 2007;13:965–971. doi: 10.1158/1078-0432.CCR-06-0551. [DOI] [PubMed] [Google Scholar]
- 31.Dun Y, Mysona B, Van Ells T, Amarnath L, Ola MS, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (xc-) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res. 2006;324:189–202. doi: 10.1007/s00441-005-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chawla RK, Lewis FW, Kutner MH, Bate DM, Roy RG, Rudman D. Plasma cysteine, cystine, and glutathione in cirrhosis. Gastroenterology. 1984;87:770–776. [PubMed] [Google Scholar]
- 33.Ishii T, Bannai S, Sugita Y. Mechanism of growth stimulation of l1210 cells by 2-mercaptoethanol in vitro: role of the mixed disulfide of 2-mercaptoethanol and cysteine. J Biol Chem. 1981;256:12387–12392. [PubMed] [Google Scholar]
- 34.Chintala S, Toth K, Cao S, Durrani FA, Vaughan MM, Jensen RL, Rustum YM. Se-methylselenocysteine sensitizes hypoxic tumor cells to irinotecan by targeting hypoxia-inducible factor 1alpha. Cancer Chemother Pharmacol. 2010 doi: 10.1007/s00280-009-1238-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Yin MB, Hapke G, Toth K, Rustum YM. Induction of biphasic DNA double strand breaks and activation of multiple repair protein complexes by DNA topoisomerase I drug 7-ethyl-10-hydroxy-camptothecin. Mol Pharmacol. 2002;61:742–748. doi: 10.1124/mol.61.4.742. [DOI] [PubMed] [Google Scholar]
- 36.Olm E, Fernandes AP, Hebert C, Rundlof AK, Larsen EH, Danielsson O, Bjornstedt M. Extracellular thiol-assisted selenium uptake dependent on the x(c)-cystine transporter explains the cancer-specific cytotoxicity of selenite. Proc Natl Acad Sci USA. 2009;106:11400–11405. doi: 10.1073/pnas.0902204106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu R, Blower PE, Pham A, Fang J, Dai A, Wise C, Green B, Teitel CH, Ning A, Ling W, Lyn-Cook BD, Kadlubar FF, Sadee W, Huang Y. Cystine-glutamate transporter SLC7A11 mediates resistance to geldanamycin but not to 17-AAG. Molecular Pharmacology. 2007;72:1637–1646. doi: 10.1124/mol.107.039644. [DOI] [PubMed] [Google Scholar]
- 38.Uren JR, Lazarus H. L-cyst(e)ine requirements of malignant cells and progress toward depletion therapy. Cancer Treat Rep. 1979;63:1073–1079. [PubMed] [Google Scholar]
- 39.Iglehart JK, York RM, Modest AP, Lazarus H, Livingston DM. Cystine requirement of continuous human lymphoid cell lines of normal and leukemic origin. Biol Chem. 1977;252:7184–7191. [PubMed] [Google Scholar]
- 40.Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Sulfasalazine-induced reduction of glutathione levels in breast cancer cells: enhancement of growth-inhibitory activity of doxorubicin. Chemotherapy. 2007;53:210–217. doi: 10.1159/000100812. [DOI] [PubMed] [Google Scholar]
- 41.Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci. 2005;25:7101–7110. doi: 10.1523/JNEUROSCI.5258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 2007;67:9463–9471. doi: 10.1158/0008-5472.CAN-07-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okuno S, Sato H, Kuriyama-Matsumura K, Tamba M, Wang H, Sohda S, Hamada H, Yoshikawa H, Kondo T, Bannai S. Role of cystine transport in intracellular glutathione level and cisplatin resistance in human ovarian cancer cell lines. Br J Cancer. 2003;88:951–956. doi: 10.1038/sj.bjc.6600786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwata S, Hori T, Sato N, Hirota K, Sasada T, Mitsui A, Hirakawa T, Yodoi J. Adult T cell leukemia (ATL)-derived factor/human thioredoxin prevents apoptosis of lymphoid cells induced by L-cystine and glutathione depletion: possible involvement of thiol-mediated redox regulation in apoptosis caused by pro-oxidant state. J Immunol. 1997;158:3108–3117. [PubMed] [Google Scholar]
- 45.Hagiwara SI, Ishii Y, Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med. 2000;162:225–231. doi: 10.1164/ajrccm.162.1.9903129. [DOI] [PubMed] [Google Scholar]
- 46.Baek SM, Kwon CH, Kim JH, Woo JS, Jung JS, Kim YK. Differential roles of hydrogen peroxide and hydroxyl radical in cisplatin-induced cell death in renal proximal tubular epithelial cells. J Lab Clin Med. 2003;142:178–186. doi: 10.1016/S0022-2143(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 47.Baliga R, Ueda N, Walker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Drug Metab Rev. 1999;31:971–997. doi: 10.1081/dmr-100101947. [DOI] [PubMed] [Google Scholar]
- 48.Mansat-de Mas V, Bezombes C, Quillet-Mary A, Bettaieb A, D'Orgeix AD, Laurent G, Jaffrezou JP. Implication of radical oxygen species in ceramide generation, c-jun N-terminal kinase activation and apoptosis induced by daunorubicin. Mol Pharmacol. 1999;56:867–874. doi: 10.1124/mol.56.5.867. [DOI] [PubMed] [Google Scholar]
- 49.Minotti G, Ronchi R, Salvatorelli E, Menna P, Cairo G. Doxorubicin irreversibly inactivates iron regulatory proteins 1 and 2 in cardiomyocytes: evidence for distinct metabolic pathways and implications for iron-mediated cardiotoxicity of antitumor therapy. Cancer Res. 2001;61:8422–8428. [PubMed] [Google Scholar]
- 50.Novak RF, Kharasch ED. Mitoxantrone: propensity for free radical formation and lipid peroxidation: implications for cardiotoxicity. Invest New Drugs. 1985;3:95–99. doi: 10.1007/BF00174155. [DOI] [PubMed] [Google Scholar]
- 51.Russo A, Mitchell JB, McPherson S, Friedman N. Alteration of bleomycin cytotoxicity by glutathione depletion or elevation. Int J Radiat Oncol Biol Phys. 1984;10:1675–1678. doi: 10.1016/0360-3016(84)90526-1. [DOI] [PubMed] [Google Scholar]
- 52.Goetz ME, Luch A. Reactive species: a cell damaging rout assisting to chemical carcinogens. Cancer letters. 2008;266:73–83. doi: 10.1016/j.canlet.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y. Bulky DNA lesions induced by reactive oxygen species. Chem Res Toxicol. 2008;21:276–281. doi: 10.1021/tx700411g. [DOI] [PubMed] [Google Scholar]
- 54.Azrak RG, Cao S, Slocum HK, Toth K, Durrani FA, Yin MB, Pendyala L, Zhang W, McLeod HL, Rustum YM. Therapeutic synergy between irinotecan and 5-fluorouracil against human tumor xenografts. Clin Cancer Res. 2004;10:1121–1129. doi: 10.1158/1078-0432.ccr-0913-3. [DOI] [PubMed] [Google Scholar]
- 55.Chen MF, Chen LT, Boyce HW., Jr 5-fluorouracil cytotoxicity in human colon HT-29 cells with moderately increased or decreased cellular glutathione level. Anticancer Res. 1995;15:163–167. [PubMed] [Google Scholar]
- 56.Fujishima H, Nakano S, Masumoto N, Esaki T, Tatsumoto T, Kondo T, Niho Y. Inhibition by 5-fluorouracil of ERCC1 and gamma-glutamylcysteine synthetase messenger RNA expression in a cisplatin-resistant HST-1 human squamous carcinoma cell line. Oncol Res. 1997;9:167–172. [PubMed] [Google Scholar]