Abstract
Background
Glucose modulates β-cell mass and function through an initial depolarization and Ca2+ influx, which then triggers a number of growth regulating signaling pathways. One of the most important downstream effectors in Ca2+ signaling is the calcium/Calmodulin activated serine threonine phosphatase, calcineurin. Recent evidence suggests that calcineurin/NFAT is essential for β-cell proliferation, and that in its absence loss of β-cells results in diabetes. We hypothesized that in contrast, activation of calcineurin might result in expansion of β-cell mass and resistance to diabetes.
Methodology/Principal Findings
To determine the role of activation of calcineurin signaling in the regulation of pancreatic β-cell mass and proliferation, we created mice that expressed a constitutively active form of calcineurin under the insulin gene promoter (caCnRIP). To our surprise, these mice exhibited glucose intolerance. In vitro studies demonstrated that while the second phase of Insulin secretion is enhanced, the overall insulin secretory response was conserved. Islet morphometric studies demonstrated decreased β-cell mass suggesting that this was a major component responsible for altered Insulin secretion and glucose intolerance in caCnRIP mice. The reduced β-cell mass was accompanied by decreased proliferation and enhanced apoptosis.
Conclusions
Our studies identify calcineurin as an important factor in controlling glucose homeostasis and indicate that chronic depolarization leading to increased calcineurin activity may contribute, along with other genetic and environmental factors, to β-cell dysfunction and diabetes.
Introduction
The normal response of pancreatic islet β-cells to various conditions associated with Insulin resistance is to increase the mass of Insulin producing cells. Plasma glucose concentration is an important factor in this response and mediates increases in glucose-induced islet β-cell growth and proliferation [1], [2], [3], [4]. In contrast, chronic elevation in plasma glucose, so called glucotoxicity, can have deleterious effects on β-cell function and survival [5], [6], [7], [8], [9], [10], [11], [12], [13]. On the other hand, glucose starvation negatively affects β-cell survival [13], [14], [15]. The explanation for the different responses to glucose levels is unclear but changes in intracellular Ca2+ concentrations play an important role. The idea that chronically elevated intracellular Ca2+ concentrations due to high glucose can result in deleterious effects on β-cell proliferation, survival and/or function is consistent with the Ca2+ set-point hypothesis described in the neuronal literature [16]. This concept states that very low or high intracellular Ca2+ levels are incompatible with survival and that between these extremes, Ca2+ concentrations have protective and physiological effects on neuronal function.
Increase in intracellular Ca2+ by glucose and depolarizing agents activates several intracellular pathways including, Ca2+/Calmodulin kinases (CaMK) and extracellular signal-regulated protein kinases (ERK1 and ERK2) and calcineurin among others [17], [18], [19], [20], [21]. Calcineurin is the only serine/threonine protein phosphatase under the direct control of intracellular Ca2+ and plays a critical role in coupling Ca2+ signals to cellular responses [22]. Therefore, calcineurin is a major candidate to mediate signals activated by glucose-induced depolarization and Ca2+ influx. Calcineurin is a heterodimer containing a catalytic/Calmodulin-binding subunit, calcineurin A, tightly bound to a calcineurin phosphatase regulatory Ca2+-binding subunit, calcineurin b1 (Cnb1) [22]. Calcineurin is an important regulator of multiple biological functions, but very few studies have investigated its role in pancreatic β-cells. Elegant experiments by Heit, et. al. demonstrated a role for this signaling pathway in regulation of β-cell growth and function [23]. These studies showed that mice with conditional deletion of Cnb1 in β-cells developed diabetes as a result of decreased β-cell mass, proliferation and insulin content [23]. This phenotype was associated with decreases in critical genes necessary for β-cell development and function including, ins1, ins2, glut2, mafA, pdx1, beta2 and cyclin D2. Interestingly, the metabolic phenotype and altered gene expression were restored by conditional expression of active NFATc1 in cnb1-deficient β-cells [23]. Nuclear factor of activated T cells (NF-AT) is one of the most recognized calcineurin targets. Moreover, experiments with calcineurin inhibitors FK506 and cyclosporin A (CsA) have provided further insights into the role of calcineurin in metabolism and β-cell function. CsA and FK506 inhibit calcineurin activity by binding to regulatory proteins of the enzyme, Cyclophilin A and FKBP-12 respectively [24]. Administration of CsA and FK506 to rodents [25] or humans [26], [27] induces hyperglycemia and hypoinsulinemia. Complementary in vitro experiments in vitro using insulinoma cells and human islets have demonstrated that CsA and Fk506 reduce Insulin biosynthesis and secretion [28], [29] [30]. While these studies demonstrated that calcineurin deficiency resulted in β-cell failure and diabetes, it is unclear whether increased glucose-induced Ca2+ influx and subsequent calcineurin activation will mimic the hypertrophic effects of chronic depolarization on β-cell function and mass.
The experiments reported herein explored the role of sustained activation of calcineurin activity in regulation of pancreatic β-cell mass and function. To achieve this, we generated transgenic mice overexpressing a constitutively active calcineurin mutant in β-cells under the control of the rat insulin promoter. These mice developed hyperglycemia and hypoinsulinemia as a result of decreased β-cell mass and Insulin secretion. The changes in β-cell mass resulted from decreased proliferation and augmented apoptosis. The current work demonstrated that sustained calcineurin hyperactivity negatively impacts β-cell growth and function. These studies imply that calcineurin could mediate some of the glucotoxic effects induced by chronic hyperglycemia in type 2 diabetes.
Methods
Generation of transgenic mice
The constitutively active calcineurin used for these experiments lacks the regulatory domain of calcineurin A (CnMut) [31], [32]. The calcineurin mutant was provided by Gerald R. Crabtree (Stanford University School of Medicine) and was generated by introducing a stop codon at nucleotide 1259 as described [31]. This sequence was inserted at the EcoRI site in a RIP-I/β-Globin expression vector. This chimeric gene (caCnRIP) was excised by enzymatic digestion, purified, and microinjected into fertilized eggs of C57Bl6 × CBA mice according to standard technique. Three transgenic founders (#167, #138 and #139) expressing the caCnRIP chimeric gene were generated in a C57Bl6 × CBA genetic background. Founders were backcrossed to C57BL6J mice. Experiments were performed on comparable mixed background. Two lines exhibited a similar phenotype. The studies described herein were performed on animals derived from the #138 line. All procedures were approved by the Washington University Animal Studies Committee.
MIN6 cell culture and adenoviral infection
MIN6 cells were maintained in DMEM (Gibco) as previously described [33]. The cells were transduced either with a control GFP or a constitutively active calcineurin adenovirus overnight at an MOI of 19. The transduced cells were maintained in the media for 48 hours before harvesting.
Immunoblotting
For western blot analysis, blots of isolated pancreatic islet lysates and MIN6 cells were probed with antibodies against calcineurin A (BD Biosciences), phospho Akt S473 (Cell Signaling) and tubulin (Sigma). Protein obtained from islets (50 g; ∼100 islets) were used for each experiment. Briefly, islet lysates were separated by electrophoresis on polyacrylamide gels and transferred to nitrocellulose or PVDF membranes (Bio-Rad). After blocking overnight, membranes were incubated for 24 hours with primary antibodies at the dilutions recommended by the manufacturer. Immunoblotting experiments were performed at least three times in duplicate.
Immunostaining, islet morphometry and analysis of proliferation and apoptosis
Pancreata obtained from 12-week-old mice were used for morphometry and immunohistochemistry. Immunostaining for insulin, glucagon, somatostatin and pancreatic polypeptide cells was performed as described [34], [35]. The β-cell mass was calculated by point counting morphometry from 5 insulin stained sections (5 µm) separated by 200 µm using the NIH ImageJ software (v1.43n freely available at http://rsb.info.nih. gov/ij/index.html [36] as described [34], [35]. Pancreata from neonates were obtained during the first 12 hours of life. Proliferation was assessed in insulin and Ki67 (Novocastra, Burlingame, CA) stained sections as previously described [34]. Apoptosis was determined in pancreatic sections using cleaved Caspase 3 (Cell Signaling) and Insulin staining as described [35]. At least 1000 insulin stained cells were counted for each animal.
Assessment of glucose metabolism and insulin secretion
Fasting blood samples were obtained after overnight fasting from the tail vein. All the metabolic studies were performed in male mice. Glucose was measured on whole blood using AccuChek II glucometer (Roche Diagnostics, Indianapolis). Plasma insulin levels were determined on 5 µl aliquots by using a Rat Insulin ELISA kit (Crystal Chem, Chicago, Illinois). Glucose tolerance tests were performed in 12-hour fasted animals by injecting glucose (2 mg/g) intraperitoneally as described [34].
Islet isolation and in vitro insulin secretion
Islet isolation was accomplished by collagenase digestion and differential centrifugation through Ficoll gradients using a modification of procedures described previously for rat islets [34]. After isolation, islets were hand picked and lysed in lysis buffer (Cell Signaling, Beverly, Massachusetts). Insulin secretion in vitro was assessed by static incubation of islets. After overnight culture in RPMI media containing 5 mM glucose, islets of similar size from caCnRIP mice and wild-type mice were handpicked and pre-cultured for an hour in Krebs-Ringer medium containing 2 mM glucose. Groups of five islets in triplicate were incubated in Krebs-Ringer medium containing either 2 mM glucose, 20 mM glucose, or 30 mM KCl, and incubated at 37°C. After 1-hour incubation, medium was collected and stored at –20°C, after which insulin was measured by RIA. Islet perifusion experiments were carried out as described [37]. Briefly, groups of 80 were suspended in Bio-Gel P2 beads and perifused at 1 mL/min using a temperature-controlled multi-chamber perifusion system (Cellex Biosciences, Minneapolis, MN). Net hormone release responses of perifused cell columns to treatments were quantified by integrating the baseline-subtracted area under the curve during the treatment period. Each time point was subtracted from the prepulse mean, defined as the average of the three time points before the treatment period.
Statistical analysis
All values are expressed as mean ± SEM. Paired Student's t test was used for all comparisons. Differences were considered statistically significant at p<0.05.
Results
Generation of mice expressing a constitutively active form of calcineurin in β-cells
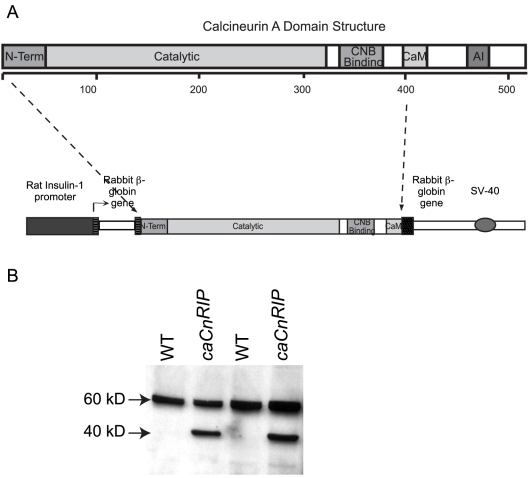
The constitutively active calcineurin mutant used for these studies lacks the regulatory domain of calcineurin A (caCn) and exhibits Ca2+-independent constitutive phosphatase activity in vitro and in vivo [31], [32], [38]. This was achieved by deleting the carboxy terminal sequence including a fraction of the Calmodulin binding domain and the auto inhibitory domain of calcineurin A as described [31], [32], [38]. This sequence was inserted downstream of the rat insulin I promoter sequence (caCnRIP, Figure 1A). Two lines with a similar expression levels and phenotypes were obtained. The studies described herein were performed on animals derived from one of these lines. Expression of the transgene in islet lysates from WT and transgenic mice demonstrated expression of the mutant protein (40 Kd band) only in caCnRIP mice (Figure 1B). No alterations in weight were observed in caCnRIP mice suggesting that there were no major abnormalities in appetite control by expression of the transgene in the hypothalamus (data not shown).
Figure 1. Transgene construct and expression in islet lysates from WT and caCnRIP mice.
A. Domain structure of calcineurin A. The mutant form including the first 1259 bp (400 amino acids) was subcloned into a vector containing the rat insulin promoter. B. Immunoblotting for calcineurin using islet lysates from WT and caCnRIP mice. The endogenous calcineurin A band migrates at 60 kD and the caCn mutant at 40 kD. The western is representative of two experiments performed in duplicate.
caCnRIP mice exhibit hyperglycemia
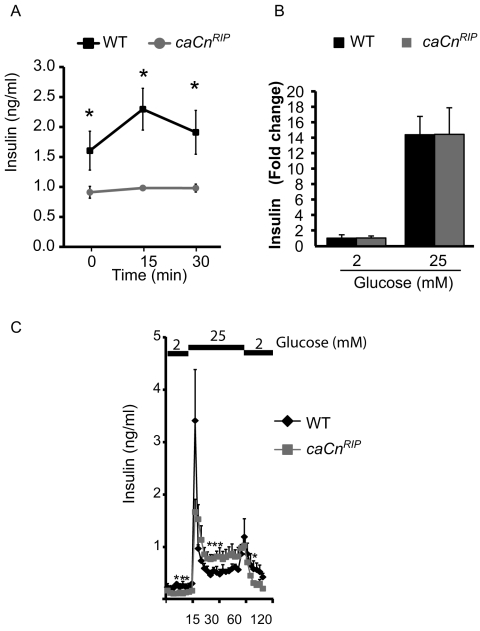
To determine the effects of constitutively active calcineurin in islet β-cells on glucose metabolism, we examined random glucose and insulin levels in 8–12 week-old mice. Random glucose levels were higher in caCnRIP mice (Figure 2A). caCnRIP mice exhibited concomitant hypoinsulinemia (Figure 2B). Intraperitoneal glucose tolerance testing demonstrated that caCnRIP mice displayed higher glucose levels after 30 and 60 minutes after glucose injection (Figure 2C). Similar glucose intolerance was observed in 12 week old mice (Figure 2C). The glucose intolerance was also observed in caCnRIP females (Figure S1).
Figure 2. RIP-CnMut mice have higher fed serum blood glucose compared to control littermates.
Serum glucose (A) and Insulin (B) concentrations in non-fasting 2–3 month old caCnRIP mice (n = 5) and control littermates (n = 8). C. Intraperitoneal glucose tolerance tests in 8 and 12-week old caCnRIP mice (n = 7) and control littermate (n = 4) males as indicated (left and right panels; n = 4). Results are expressed as mean ± SEM. * p<0.05.
Overexpression of constitutively active calcineurin in islets induces the second phase of insulin secretion
To begin to elucidate the mechanisms responsible for impaired glucose tolerance in caCnRIP mice, we assessed insulin secretion in vivo and in vitro. Insulin levels in caCnRIP mice were reduced relative to those in WT mice after overnight fasting and did not increase after glucose injection (Figure 3A). Insulin levels were lower in caCnRIP islets cultured in 2 mM glucose for 60 min (p<0.05, data not shown). Glucose stimulated insulin secretion in isolated islets was similar in caCnRIP mice (Figure 3B). Since calcineurin signaling has been reported to modulate the different phases of Insulin secretion [39], we performed islet perifusion experiments. Basal Insulin secretion at 2 mM glucose before and after glucose stimulation was decreased in caCnRIP islets (Figure 3C). No significant difference was observed in the first phase of Insulin secretion (Figure 3C). Interestingly, the second phase of insulin secretion was enhanced in caCnRIP islets (Figure 3C). However, the area under the curve for glucose stimulated Insulin secretion was comparable (p>0.05, data not shown).
Figure 3. Transgenic mice expressing calcineurin showed altered insulin secretion.
A. Glucose stimulated insulin secretion in vivo assessed by intraperitoneal glucose injection. B. Static Insulin secretion in isolated islets from 8–12 week old WT and caCnRIP mice. C. Insulin secretion assessment by islet perifusion experiments with low (2 mM) and high (25 mM) glucose in islets from WT and caCnRIP mice (n = 3). Data expressed as mean ± SEM. *p<0.01.
Pancreas morphometry on WT and caCnRIP mice
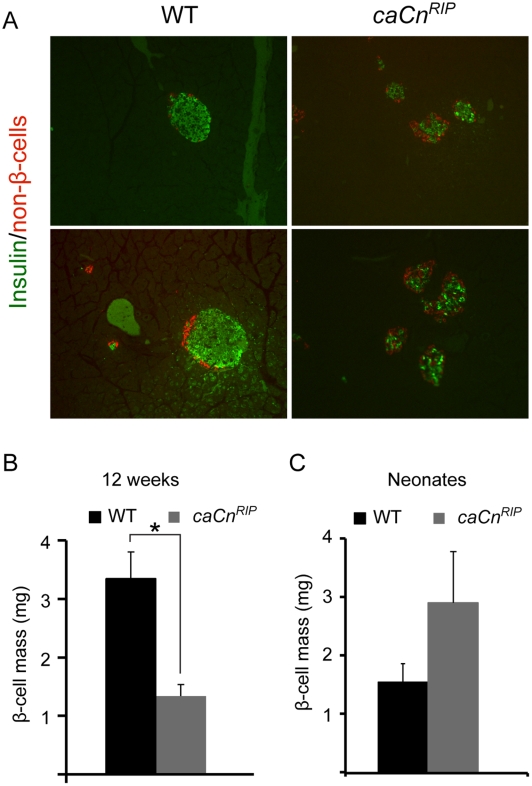
Morphometric analysis was then performed to determine the cause of altered glucose tolerance and Insulin secretion in caCnRIP mice. Staining for Insulin and a cocktail of antibodies for non β-cells revealed that islets from caCnRIP mice showed decreased size and irregular shape (Figure 4A). Analysis of β-cell mass in 12-week-old mice indicated that caCnRIP mice exhibited more than a 50% reduction in β-cell mass (Figure 4B). To determine whether this reduced mass was a developmental defect or acquired post-natally, the β-cell mass in WT and caCnRIP neonates was examined and found to be not significantly different (Figure 4C). These studies demonstrate that caCnRIP mice are born with normal β-cell mass and develop decrease in mass during the first 12 weeks of life.
Figure 4. Pancreas morphometry on WT and caCnRIP mice.
A. Immunostaining for Insulin and non β-cells in pancreas from WT and caCnRIP mice. Images presented were obtained at different magnifications (Upper panel 10x and lower panel 40x). Assessment of β-cell mass using point-counting morphometry in WT and caCnRIP mice at 12 weeks of age (B) and neonates (C). Results are mean ± SEM (n = 4). * p<0.05.
Decrease in β-cell mass in caCnRIP mice results from decreased proliferation and increased apoptosis
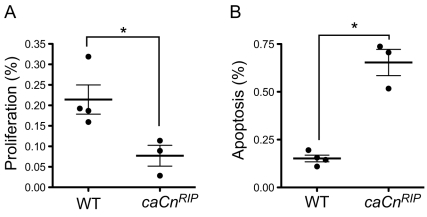
We next examined whether the decrease in β-cell mass in caCnRIP mice was the result of decreased proliferation or increased apoptosis. Analysis of proliferation performed by Ki67 staining demonstrated that caCnRIP mice exhibited decreased proliferation (Figure 5A). caCnRIP mice also displayed a concomitant increase in apoptosis revealed by cleaved-caspase 3 staining (Figure 5B), indicating that calcineurin affects both proliferation and apoptosis.
Figure 5. Assessment of proliferation and apoptosis.
A. Proliferation assessed by BrdU staining in 2–3 month old wild type and mutant mice. B. Apoptosis determined by cleaved Caspase 3 staining in wild type and caCnRIP mice. Data is express as mean + SEM. *p<0.05.
Discussion
The current studies extend our knowledge of the role of calcineurin in pancreatic islet β-cells by examining the effects of chronic activation of calcineurin on β-cell mass and function. These experiments demonstrate that long-term activation of calcineurin induces impaired glucose tolerance by alterations in β-cell mass. We also show that activation of calcineurin signaling negatively affects proliferation and survival of β-cells. These morphological alterations resemble in part the phenotype of β-cells exposed to chronic hyperglycemia and suggest that chronic activation of calcineurin could be an important component of the glucotoxic effect of hyperglycemia in type 2 diabetes and possibly also explain the failure of these agents to control diabetes after long-term therapy with this medication.
The expression of the calcineurin mutant in β-cells resulted in major disturbances in plasma glucose levels. A small fraction of the animals developed frank diabetes making it difficult to maintain the line. The abnormalities in glucose were associated with decreased Insulin levels. Transgenic mice exhibited severe impairment in glucose-induced Insulin secretion in vivo. The severe defect in Insulin secretion in the context of 50% of normal β-cell mass suggested the possibility that caCnRIP mice might exhibit some degree of impaired Insulin secretion. However, in vitro Insulin secretion in response to glucose was similar in static incubation and perifusion experiments (Figure 3). Islet perifusion experiments showed that islets from caCnRIP mice exhibited a robust first phase of Insulin secretion implying that the readily releasable pool was not significantly altered. Interestingly, we observed a significant increase in the second phase of insulin secretion suggesting that calcineurin may modulate events associated with insulin granule trafficking [39]. The changes in second phase of Insulin secretion could be explained in part by dephosphorylation of Kinesin Heavy chain (KHC) on β-granules. Phosphorylation of KHC inhibits the binding of granules to microtubules and prevents the transport towards the cell membrane [39], [40]. In contrast, inhibition of calcineurin-mediated KHC dephosphorylation using inhibitors and adenoviruses inhibits second phase of insulin secretion [39]. In summary the discrepancies in insulin secretion in vitro and in vivo are difficult to reconcile but it is possible that the stress of the isolation procedure and the selection of islets for perifusion are biased to favor the availability and collection of healthier islets and these islets are not completely representative of the integrated response obtained in in vivo experiments. It is also probable that activation of calcineurin in neurons could contribute to the regulation of in vivo insulin secretion in this model. However, we believe that this is less likely due to lack of evidence of central expression of the promoter used for these experiments.
Decreased β-cell mass was an important component responsible for the hyperglycemic phenotype in caCnRIP mice. The diminished β-cell mass was caused by reduced proliferation and increased apoptosis. The mechanisms involved in the regulation of β-cell cycle by calcineurin are partially understood. Heit et al. showed that transgenic activation of NFATc1 in β-cells induces proliferation by inducing Cyclin D and Cdk4 levels [23]. This suggests that the decreased proliferation observed by activation of calcineurin is mediated in an NFATc1-independent manner. It is important to note that NFAT transcription factors are not the only calcineurin-downstream substrates and other calcineurin-regulated proteins such as Map Kinase Phosphatase 1 (MKP1) [41], Cdk4 [42], [43], PKA, NO synthase and the co-activator TORC could also be involved [44], [45], [46]. To this end, we have demonstrated that glucose and KCl-induced depolarization induces MKP1 expression in a calcineurin-dependent manner (data not shown). Therefore, increased MKP1 can inhibit Mitogen-Activated Protein Kinase (MAPK) activation and subsequent cell cycle progression. In summary, the current findings are consistent with a negative effect of calcineurin on cell cycle progression by activation of downstream signaling targets other than NFATc1. The loss of the transgenic line prevents us from pursuing some of these avenues.
The decreased β-cell mass in caCnRIP mice could also be explained in part by augmented apoptosis. The role of calcineurin in apoptosis has been extensively examined in neurons and lymphoid tissues, among others [16], [47], [48], [49]. In β-cells, inhibition of calcineurin is protective against apoptosis induced by proinflammatory cytokines and dexamethasone [47], [50], [51]. The mechanisms involved in apoptosis observed in caCnRIP mice could be multifactorial. As demonstrated in other systems, including β-cells, calcineurin-mediated dephosphorylation and activation of the pro-apoptotic Bcl-2 family protein Bad is a major component of apoptosis induced by elevated Ca2+/Calcienurin signaling [49], [50], [51]. Recent experiments showed that calcineurin decreased Akt signaling by dephoshorylation of S473 [52]. However, AktS473 phosphorylation was not altered in MIN6 cells expressing a constitutively active calcineurin suggesting that this mechanism is not likely to play a role (Figure S2). In summary, our studies suggest that the genetic activation of calcineurin signaling reduces β-cell mass by induction of apoptosis. It is unclear at this point if these calcineurin effects are mediated by NFAT.
In summary, the present work shows that chronic activation of calcineurin signaling regulates survival and proliferation of β-cells. These studies together with those obtained in mice with deletion of Cnb1 in β-cells [23] suggest that calcineurin signaling is a major component of the effects induced by glucose depolarization/Ca2+ influx. Similar pattern of responses derived from apoptotic responses to intracellular Ca2+ concentration in neurons have led to the development of the Ca2+ set-point hypothesis [16]. The results of the present study suggest that persistent activation of calcineurin signaling could be an important component responsible for the responses to chronic depolarization.
Supporting Information
A Random blood glucose levels in 8-week-old females (n = 4). B. Glucose tolerance in 8-week-old females. Intraperitoneal glucose tolerance tests was performed in 8 and 12-week old caCnRIP mice and littermates females (B) (n = 4).
(0.40 MB EPS)
Western blots on MIN6 cells transduced with a control (GFPAdv) or constitutively active calcineurin (caCnAdv) adenovirus. Immunoblotting for Calcineurin (A) or phospho Akt (473) (B) and tubulin. Expression levels were normalized to tubulin and quantified on the right. Results are mean ± SEM (n = 4). * p<0.05.
(0.71 MB EPS)
Acknowledgments
We would like to acknowledge the support of the Radioimmunoassay, Mouse Genetic core and β-cell Morphology Cores of The Washington University Diabetes Research & Training Center (NIH grant P60-DK020579-31). We also thank the Morphology core from Washington University Digestive Diseases Research Core Center (DDRCC) for histology sections (5P30 DK052574). We would like to acknowledge John Williams and Grzegorz Tadeusz Gurda for providing adenoviruses.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Institutes of Health grants R01DK073716-01 (E.B.M.), R37DK16746 (M.A.P.) and a Career Development Award from the American Diabetes Association (E.B.M.). J.D.J is supported by grants from the Juvenile Diabetes Research Foundation and the Canadian Institutes for Health Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- 2.Bernard C, Berthault MF, Saulnier C, Ktorza A. Neogenesis vs. apoptosis As main components of pancreatic beta cell ass changes in glucose-infused normal and mildly diabetic adult rats. FASEB J. 1999;13:1195–1205. doi: 10.1096/fasebj.13.10.1195. [DOI] [PubMed] [Google Scholar]
- 3.Paris M, Bernard-Kargar C, Berthault MF, Bouwens L, Ktorza A. Specific and combined effects of insulin and glucose on functional pancreatic beta-cell mass in vivo in adult rats. Endocrinology. 2003;144:2717–2727. doi: 10.1210/en.2002-221112. [DOI] [PubMed] [Google Scholar]
- 4.Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, et al. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes. 2007;56:1792–1801. doi: 10.2337/db06-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unger RH, Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia. 1985;28:119–121. doi: 10.1007/BF00273856. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser N, Corcos AP, Sarel I, Cerasi E. Monolayer culture of adult rat pancreatic islets on extracellular matrix: modulation of B-cell function by chronic exposure to high glucose. Endocrinology. 1991;129:2067–2076. doi: 10.1210/endo-129-4-2067. [DOI] [PubMed] [Google Scholar]
- 7.Leahy JL, Cooper HE, Deal DA, Weir GC. Chronic hyperglycemia is associated with impaired glucose influence on insulin secretion. A study in normal rats using chronic in vivo glucose infusions. J Clin Invest. 1986;77:908–915. doi: 10.1172/JCI112389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertson RP. Type II diabetes, glucose “non-sense,” and islet desensitization. Diabetes. 1989;38:1501–1505. doi: 10.2337/diab.38.12.1501. [DOI] [PubMed] [Google Scholar]
- 9.Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13:610–630. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- 10.Marshak S, Leibowitz G, Bertuzzi F, Socci C, Kaiser N, et al. Impaired beta-cell functions induced by chronic exposure of cultured human pancreatic islets to high glucose. Diabetes. 1999;48:1230–1236. doi: 10.2337/diabetes.48.6.1230. [DOI] [PubMed] [Google Scholar]
- 11.Maedler K, Spinas GA, Lehmann R, Sergeev P, Weber M, et al. Glucose induces beta-cell apoptosis via upregulation of the Fas receptor in human islets. Diabetes. 2001;50:1683–1690. doi: 10.2337/diabetes.50.8.1683. [DOI] [PubMed] [Google Scholar]
- 12.Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, et al. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50:1290–1301. doi: 10.2337/diabetes.50.6.1290. [DOI] [PubMed] [Google Scholar]
- 13.Efanova IB, Zaitsev SV, Zhivotovsky B, Kohler M, Efendic S, et al. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells. A process dependent on intracellular Ca2+ concentration. J Biol Chem. 1998;273:33501–33507. doi: 10.1074/jbc.273.50.33501. [DOI] [PubMed] [Google Scholar]
- 14.Hoorens A, Van de Casteele M, Kloppel G, Pipeleers D. Glucose promotes survival of rat pancreatic beta cells by activating synthesis of proteins which suppress a constitutive apoptotic program. J Clin Invest. 1996;98:1568–1574. doi: 10.1172/JCI118950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan S, Bernal-Mizrachi E, Ohsugi M, Permutt MA. Glucose promotes pancreatic islet beta-cell survival through a PI 3-kinase/Akt-signaling pathway. Am J Physiol Endocrinol Metab. 2002;283:E784–793. doi: 10.1152/ajpendo.00177.2002. [DOI] [PubMed] [Google Scholar]
- 16.Franklin JL, Johnson EM., Jr Elevated intracellular calcium blocks programmed neuronal death. Ann N Y Acad Sci. 1994;747:195–204. doi: 10.1111/j.1749-6632.1994.tb44410.x. [DOI] [PubMed] [Google Scholar]
- 17.Cousin SP, Hugl SR, Myers MG, Jr, White MF, Reifel-Miller A, et al. Stimulation of pancreatic beta-cell proliferation by growth hormone is glucose-dependent: signal transduction via janus kinase 2 (JAK2)/signal transducer and activator of transcription 5 (STAT5) with no crosstalk to insulin receptor substrate-mediated mitogenic signalling. Biochem J. 1999;344 Pt 3:649–658. [PMC free article] [PubMed] [Google Scholar]
- 18.Khoo S, Cobb MH. Activation of mitogen-activating protein kinase by glucose is not required for insulin secretion. Proc Natl Acad Sci U S A. 1997;94:5599–5604. doi: 10.1073/pnas.94.11.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persaud SJ, Wheeler-Jones CP, Jones PM. The mitogen-activated protein kinase pathway in rat islets of Langerhans: studies on the regulation of insulin secretion. Biochem J. 1996;313(Pt 1):119–124. doi: 10.1042/bj3130119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briaud I, Lingohr MK, Dickson LM, Wrede CE, Rhodes CJ. Differential activation mechanisms of Erk-1/2 and p70(S6K) by glucose in pancreatic beta-cells. Diabetes. 2003;52:974–983. doi: 10.2337/diabetes.52.4.974. [DOI] [PubMed] [Google Scholar]
- 21.Hugl SR, White MF, Rhodes CJ. Insulin-like growth factor I (IGF-I)-stimulated pancreatic beta-cell growth is glucose-dependent. Synergistic activation of insulin receptor substrate-mediated signal transduction pathways by glucose and IGF-I in INS-1 cells. J Biol Chem. 1998;273:17771–17779. doi: 10.1074/jbc.273.28.17771. [DOI] [PubMed] [Google Scholar]
- 22.Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 23.Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, et al. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443:345–349. doi: 10.1038/nature05097. [DOI] [PubMed] [Google Scholar]
- 24.Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352:803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- 25.Herold KC, Nagamatsu S, Buse JB, Kulsakdinun P, Steiner DF. Inhibition of glucose-stimulated insulin release from beta TC3 cells and rodent islets by an analog of FK506. Transplantation. 1993;55:186–192. doi: 10.1097/00007890-199301000-00035. [DOI] [PubMed] [Google Scholar]
- 26.Duijnhoven EM, Boots JM, Christiaans MH, Wolffenbuttel BH, Van Hooff JP. Influence of tacrolimus on glucose metabolism before and after renal transplantation: a prospective study. J Am Soc Nephrol. 2001;12:583–588. doi: 10.1681/ASN.V123583. [DOI] [PubMed] [Google Scholar]
- 27.Redmon JB, Olson LK, Armstrong MB, Greene MJ, Robertson RP. Effects of tacrolimus (FK506) on human insulin gene expression, insulin mRNA levels, and insulin secretion in HIT-T15 cells. J Clin Invest. 1996;98:2786–2793. doi: 10.1172/JCI119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrence MC, Bhatt HS, Watterson JM, Easom RA. Regulation of insulin gene transcription by a Ca(2+)-responsive pathway involving calcineurin and nuclear factor of activated T cells. Mol Endocrinol. 2001;15:1758–1767. doi: 10.1210/mend.15.10.0702. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence MC, Bhatt HS, Easom RA. NFAT regulates insulin gene promoter activity in response to synergistic pathways induced by glucose and glucagon-like peptide-1. Diabetes. 2002;51:691–698. doi: 10.2337/diabetes.51.3.691. [DOI] [PubMed] [Google Scholar]
- 30.Johnson JD, Ao Z, Ao P, Li H, Dai LJ, et al. Different effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human islets. Cell Transplant. 2009;18:833–845. doi: 10.3727/096368909X471198. [DOI] [PubMed] [Google Scholar]
- 31.Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 32.O'Keefe SJ, Tamura J, Kincaid RL, Tocci MJ, O'Neill EA. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992;357:692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- 33.Bernal-Mizrachi E, Wice B, Inoue H, Permutt MA. Activation of serum response factor in the depolarization induction of Egr-1 transcription in pancreatic islet beta-cells. J Biol Chem. 2000;275:25681–25689. doi: 10.1074/jbc.M003424200. [DOI] [PubMed] [Google Scholar]
- 34.Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest. 2001;108:1631–1638. doi: 10.1172/JCI13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernal-Mizrachi E, Fatrai S, Johnson JD, Ohsugi M, Otani K, et al. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Invest. 2004;114:928–936. doi: 10.1172/JCI20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girish V, Vijayalakshmi A. Affordable image analysis using NIH Image/ImageJ. Indian J Cancer. 2004;41:47. [PubMed] [Google Scholar]
- 37.Bernal-Mizrachi E, Cras-Meneur C, Ohsugi M, Permutt MA. Gene expression profiling in islet biology and diabetes research. Diabetes Metab Res Rev. 2003;19:32–42. doi: 10.1002/dmrr.331. [DOI] [PubMed] [Google Scholar]
- 38.Kincaid RL, Martensen TM, Vaughan M. Modulation of calcineurin phosphotyrosyl protein phosphatase activity by calmodulin and protease treatment. Biochem Biophys Res Commun. 1986;140:320–328. doi: 10.1016/0006-291x(86)91093-4. [DOI] [PubMed] [Google Scholar]
- 39.Donelan MJ, Morfini G, Julyan R, Sommers S, Hays L, et al. Ca2+-dependent dephosphorylation of kinesin heavy chain on beta-granules in pancreatic beta-cells. Implications for regulated beta-granule transport and insulin exocytosis. J Biol Chem. 2002;277:24232–24242. doi: 10.1074/jbc.M203345200. [DOI] [PubMed] [Google Scholar]
- 40.Meng YX, Wilson GW, Avery MC, Varden CH, Balczon R. Suppression of the expression of a pancreatic beta-cell form of the kinesin heavy chain by antisense oligonucleotides inhibits insulin secretion from primary cultures of mouse beta-cells. Endocrinology. 1997;138:1979–1987. doi: 10.1210/endo.138.5.5139. [DOI] [PubMed] [Google Scholar]
- 41.Lim HW, New L, Han J, Molkentin JD. Calcineurin enhances MAPK phosphatase-1 expression and p38 MAPK inactivation in cardiac myocytes. J Biol Chem. 2001;276:15913–15919. doi: 10.1074/jbc.M100452200. [DOI] [PubMed] [Google Scholar]
- 42.Baksh S, Widlund HR, Frazer-Abel AA, Du J, Fosmire S, et al. NFATc2-mediated repression of cyclin-dependent kinase 4 expression. Mol Cell. 2002;10:1071–1081. doi: 10.1016/s1097-2765(02)00701-3. [DOI] [PubMed] [Google Scholar]
- 43.Baksh S, DeCaprio JA, Burakoff SJ. Calcineurin regulation of the mammalian G0/G1 checkpoint element, cyclin dependent kinase 4. Oncogene. 2000;19:2820–2827. doi: 10.1038/sj.onc.1203585. [DOI] [PubMed] [Google Scholar]
- 44.Blumenthal DK, Takio K, Hansen RS, Krebs EG. Dephosphorylation of cAMP-dependent protein kinase regulatory subunit (type II) by calmodulin-dependent protein phosphatase. Determinants of substrate specificity. J Biol Chem. 1986;261:8140–8145. [PubMed] [Google Scholar]
- 45.Dawson VL, Dawson TM, Uhl GR, Snyder SH. Human immunodeficiency virus type 1 coat protein neurotoxicity mediated by nitric oxide in primary cortical cultures. Proc Natl Acad Sci U S A. 1993;90:3256–3259. doi: 10.1073/pnas.90.8.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Chang I, Cho N, Kim S, Kim JY, Kim E, et al. Role of calcium in pancreatic islet cell death by IFN-gamma/TNF-alpha. J Immunol. 2004;172:7008–7014. doi: 10.4049/jimmunol.172.11.7008. [DOI] [PubMed] [Google Scholar]
- 48.Miki T, Tashiro F, Iwanaga T, Nagashima K, Yoshitomi H, et al. Abnormalities of pancreatic islets by targeted expression of a dominant-negative KATP channel. Proc Natl Acad Sci U S A. 1997;94:11969–11973. doi: 10.1073/pnas.94.22.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 50.Grunnet LG, Aikin R, Tonnesen MF, Paraskevas S, Blaabjerg L, et al. Proinflammatory cytokines activate the intrinsic apoptotic pathway in beta-cells. Diabetes. 2009;58:1807–1815. doi: 10.2337/db08-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ranta F, Avram D, Berchtold S, Dufer M, Drews G, et al. Dexamethasone induces cell death in insulin-secreting cells, an effect reversed by exendin-4. Diabetes. 2006;55:1380–1390. doi: 10.2337/db05-1220. [DOI] [PubMed] [Google Scholar]
- 52.Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, et al. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci U S A. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A Random blood glucose levels in 8-week-old females (n = 4). B. Glucose tolerance in 8-week-old females. Intraperitoneal glucose tolerance tests was performed in 8 and 12-week old caCnRIP mice and littermates females (B) (n = 4).
(0.40 MB EPS)
Western blots on MIN6 cells transduced with a control (GFPAdv) or constitutively active calcineurin (caCnAdv) adenovirus. Immunoblotting for Calcineurin (A) or phospho Akt (473) (B) and tubulin. Expression levels were normalized to tubulin and quantified on the right. Results are mean ± SEM (n = 4). * p<0.05.
(0.71 MB EPS)