Abstract
Previously, we reported that human p53 is functionally inactivated by S-glutathionylation at Cys-141 during oxidative and DNA-damaging treatments. Here, we describe the presence of thiolated p53 and dynamic nature of this modification in human tissues using unique and specific polyclonal antibodies raised against a 12-residue p53 peptide bearing a mixed disulfide at Cys-141. The affinity- purified antibodies (glut-p53) were sequence-specific in that they recognized the antigenic peptide but not the unthiolated peptide or a scrambled glutathionylated peptide in ELISAs. On immunoblots, the purified antibodies did not react with native p53 or recombinant p53 (rp53), but readily detected the glutathionylated or cysteinylated or ethanethiol-treated rp53 only under non-reducing conditions. Untreated HCT116 cells showed low levels of glut-p53 which increased markedly after H2O2, diamide, cisplatin, and doxorubicin treatments. Glut-p53 levels decreased sharply after passing cells to oxidant-free media, suggesting efficient dethiolation. The mutant p53 present in HT29 and T47D human cancer cells was also recognized. In vitro, the glut-p53 was rapidly degraded by rabbit reticulocyte lysates. Human prostate and prostate cancer tissues showed abundant presence of glut-p53 in luminal epithelium, a site well-known to generate ROS. Melanoma and colon cancer samples were also positive for glut-p53. Availability of the thiolation-specific antibodies should enhance our knowledge of p53 regulation in redox-perturbed states found in various diseases including cancer.
Introduction
The p53 tumor suppressor protein is a versatile transcription factor whose functional inactivation triggers oncogenic events and facilitates the emergence of unstable genomes in 50% of all human cancers. It regulates multiple biological processes including genotoxic responses, cell cycle control, DNA repair, differentiation, and apoptosis through transcriptional activation of a large number of target genes to counteract a variety of endogenous and exogenous cues [1-3]. Diverse cellular events such as endogenous and therapy-induced damage to DNA, hypoxia, stresses associated with metabolism, DNA replication and redox imbalance govern the activation and stabilization of p53 protein through a variety of posttranslational modifications, such as site-specific phosphorylations, acetylation, and sumoylation [4-6]. In contrast, it is well known that the transcription factor activity of p53 is highly susceptible to oxidative inactivation during physiological and pathological stresses that generate reactive oxygen species (ROS) and nitrogen species (RON), as well as their byproducts, H2O2, and hydroxyl radicals [7, 8]. Abundant evidence such as the need for a reductant in p53-DNA binding reactions [7, 9], attenuation of p53-dependent reporter gene expression by oxidants and the reactivation of oxidized p53 by Ref-1 and thioredoxin [10-12] in cells, indicate that p53 is a redox-dependent transcription factor, similar to NF-κB and AP-1. Consistent with these observations, we and other investigators have shown that human p53 harbors several redox-sensitive cysteines in the DNA-binding domain [7, 13, 14].
While most of p53's posttranslational modifications stabilize and/or facilitate the p53 protein to enable it to become a fully competent transcription factor, we recently showed that the tumor suppressor is also negatively regulated via glutathionylation when human cells are subjected to DNA damage, as well as, oxidative and nitrosative stresses [14]. S-Glutathionylation, (often called S-thiolation collectively referring to small molecular weight thiols) is a reversible posttranslational modification involving the formation of mixed disulfides between anionic cysteines present on protein surfaces and glutathione (GSH or GSSG) [15, 16]. These reactive thiol groups are highly sensitive to attack by ROS (and RNS) and step-wise oxidations to thiyl radical (S*), sulphenic acid (SOH), sulphinic acid (SO2H), and the terminal sulphonic acids (SO3H). The oxidized cysteines, SO2H and SO3H generally lead to irrevocable loss of biological activities for most proteins. However, all oxidized forms of cysteines except the SO2H and SO3H can be stabilized through glutathionylation within the protein environment and recycled back to the thiol state either through enzymatic (glutaredoxin / thioredoxin /disulfide isomerase -catalyzed) or non-enzymatic dethiolation [15-17]. Thus, glutathionylation of reactive cysteines in metabolic enzymes, kinases, phosphatases, and transcription factors has emerged as a central mechanism by which changes in the intracellular redox state may be transduced into functional responses. Very much like phosphorylation, this modification can modulate enzyme activities, alter transcription profiles, and modify protein-protein interactions, and regulate adaptive cell signaling [16].
In a previous study, we demonstrated that human p53 protein is a substrate for glutathionylation and that this modification perturbs p53 structure and inhibits binding to DNA. Both oxidants and DNA damaging agents were shown to significantly increase the levels of glutathione-conjugated p53 in cancer cells [14]. Cysteines 124, 141 and 182 were identified as targets for this modification; of these Cys-141 was identified by two mass spectrometry procedures to be the most reactive and to constitute a major site for glutathionylation [14]. Oxidation of cysteine-141 that contributes to p53 inactivation has been confirmed in another study; in this case, the oxidative damage to DNA was shown to be relayed to the distantly bound p53 through a charge transfer [18]. p53 function may be negated frequently by the chronic oxidative stress prevalent in human cancers and some normal tissues. As such, the extent of p53 glutathionylation can serve as a valuable biomarker in a variety of diseases including cancer. Therefore, to easily quantitate the dynamics of p53 glutathionylation, we generated polyclonal antibodies (p53-glut) that uniquely recognize the Cys 141-glutathionylated state of p53. This report describes the generation and characterization of p53-glut antibodies and its efficacy in visualizing the glutathionylated p53 and its dynamics in cancer cell lines, prostate cancer, and other malignancies.
Materials and Methods
Cell lines and reagents
Human cancer cell lines HCT116, HT29 and T47D were obtained from ATCC and maintained in Dulbecco's minimum essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics. Most reagents, of the highest available grade, were procured from Sigma Chemicals unless otherwise mentioned. The anticancer drugs cisplatin, doxorubicin and camptothecin were obtained from LKT laboratories (St. Paul, MN).
Antibody production
A 12 residue p53 peptide having the sequence 136QLAKTCPVQLWV147 that harbors the major glutathionylation site Cys-141 was synthesized, and a mixed disulfide with glutathione was induced through on-resin air oxidation [19]. The thiolated peptide was purified, the presence of glutathione linkage was verified by mass spectrometry, and coupled with keyhole limpet hemocyanin [20]. The peptide-hemocyanin conjugate was mixed with incomplete Fruend's adjuvant for the initial injection and with complete Fruend's adjuvant for booster injections. After the initial subcutaneous injection with the peptide into 5 rabbits, two booster injections were administered. Antisera were collected after the second booster dose and the initial assessment for the presence of p53-glut specific antibodies was performed. Finally, blood from rabbits was collected by cardiac puncture according to the guidelines set by the Institutional Animal Use and Care Committee.
Affinity-purification of antiserum
The glutathionylated antigen peptide and the non-glutathionylated p53 peptide were coupled to the HighTrap NHS-activated HP resin (GE Healthcare, Piscataway, NJ) through the amino groups at neutral pH according to manufacturer's instructions. The antiserum from the rabbit 11388 was first circulated through the non-glutathionylated affinity column for 5 h and the unbound fraction was collected. This flow-through was then applied to the glutathionylated peptide column followed by extensive washing with PBS containing 0.05% Tween-20. The bound antibodies specific to glut-p53 were eluted with 0.1 M glycine-HCl (pH 2.6) and fractions were collected in tubes containing 2 M Tris-HCl buffer (pH 8.5) for neutralization [20]. The antibody fractions were freeze dried and dissolved in PBS at 0.3 mg/ml.
In vitro glutathionylation / cysteinylation of recombinant p53 protein
Recombinant p53 (rp53) protein was expressed in E. coli and purified essentially as described previously [14]. For in vitro glutathionylation or cysteinylation, rp53 (1μg) was incubated with 10 mM reduced glutathione (GSH), or 10 mM cysteine, in phosphate buffer (pH 7.5) at 37°C for 30 min. Protein samples were passed through Biogel-P6 spin columns to remove excess of thiols [14]. For demonstrating the reversibility of the reactions, some samples were incubated with 10 mM dithiothreitol (DTT) for 10 min. All samples were electrophoresed on 12% non-reducing SDS-gels and proteins were transferred to PVDF membranes. The blots were blocked with 5% non-fat dry milk in Tris-buffered saline containing 0.5% Tween 20 (TTBS), followed by an overnight incubation with the primary antibody (1:200 dilution). The blots were subsequently incubated with anti rabbit IgG-HRP-conjugated secondary antibody (1:5000 dilution) and developed using enhanced chemiluminiscence (ECL reagent Pierce).
Assay for determination of glutathionylation of p53 in cells
Exponentially growing HCT116 cells were exposed to oxidizing agents viz., H2O2 (0.4 mM), diamide (DA, 0.6 mM) or tert-butyl hydroperoxide (TBH, 0.6 mM) for the specified times. After the incubation, the control and oxidant-treated cells were washed with phosphate buffered saline (PBS), trypsinized and sonicated in 30 mM Tris-HCl (pH 7.8) containing 1 mM PMSF and 3 mM benzamidine. Equal protein amounts (50 μg) were electrophoresed on 12% non-reducing SDS gels. One set of samples were mixed with 5 mM DTT for 10 min before electrophoresis. The proteins were transferred onto PVDF membranes followed by blocking with 5% non-fat dry milk, incubation with affinity-purified anti-glut p53 antibodies (1:200 dilution) and anti-rabbit IgG-HRP and ECL for specific detection of thiolated p53.
ELISA
The presence of p53-glut antibodies in the rabbit antisera and their specificity was determined using an ELISA kit (Bethyl Labs) according to the manufacturer's instructions. For confirming the sequence specificity of antibodies, a scrambled peptide with the sequence KVPQVLWTLCAQ (compared to QLAKTCPVQLWV sequence of p53) was synthesized (Biosynthesis Inc, Lewisville, TX) and glutathionylated as described previously [19]. Next, the microtiter plates were coated with 1-25 ng glutathionyalted antigenic peptide or non-glutathionylated peptide with the same sequence as the antigen or glutathionylated scrambled peptide overnight at 4°C. Affinity-purified p53-glut antibodies (1:1000 dilution) were added and plates were incubated at 4°C for 6 h. Plates were washed and incubated with the HRP-conjugated anti rabbit secondary antibody for 1 h at room temperature. The color was developed using 3,3′, 5,5″-tetramethylbenzidine (TMB) as the substrate, and the absorbance determined at 450 nm using a microplate reader (Molecular Devices Corp.).
Immunofluorescence analysis of Glut-p53 expression
HCT116, HT29, and T47D cells were cultured on sterile cover-slips and treated with the oxidizing agents as described previously. Untreated and treated cells were fixed with 4% paraformaldehyde for 20 min and washed three times with PBS. Next, they were blocked using 5% bovine serum albumin (BSA) containing 0.1% Triton X-100 for 2 h. Cells were stained with the p53-glut antibody (1:500 dilution) overnight at 4 °C, washed thrice with PBS and incubated with Alexa Flour 594-conjugated goat anti-rabbit IgG (Invitrogen) for 1h. Cells were counterstained with Hoechst to observe nuclei, washed and mounted on slides. Cells were viewed under a fluorescence microscope (Olympus IX 81) and, the images acquired.
Immunohistochemistry
Human cancer tissue microarrays (prostate cancer and adjacent normal tissue, gastric cancer, colon cancer, and melanoma) mounted on glass slides were obtained from Millipore, Imgenex and Biomax Companies. The slides were processed by standard immunohistochemical procedures [20, 21]. Briefly, they were de-paraffinized at 62°C followed by two changes in xylene bath and rehydrations in 100%-75% ethanol and PBS. Antigen retrieval was performed by 20-min incubation in 10 mM citrate buffer (pH 6.0) at 95°C followed by inactivation of endogenous peroxidases with 3% H2O2. The sections were then blocked in 5% BSA for 2 h and overlaid with the p53-glut antibody (1:500 dilution) and incubated overnight at 4°C. They were washed with PBS and incubated with HRP-conjugated anti-rabbit antibody (1:2,000 dilution) for 1 h. The washed slides were subjected to diaminobenzidine (DAB) - peroxidase staining, counter stained with hematoxylin. All sections were examined by light microscopy to qualitatively assess the presence or absence of immunostaining, its intensity of positivity, strength, and its distribution. Immunostaining intensity in non-neoplastic and neoplastic tissues was visualized in all areas of the slides, the scores were averaged and presented as undetectable (-), weak (++), moderate (+++), and intense (++++).
In vitro degradation of glutathionylated p53 protein
Glutathionylated rp53 was prepared as described earlier and used as the substrate. Proteolysis assays in the presence of rabbit reiculocyte lysates (35 μl final volume) were performed in Tris-HCl (pH 8.0) containing 0.5 mM dithithreitol, 4 mM MgCl2, 1 mM ATP, 1 μg ubiquitin, 1 μg rp53 or 1μg glutathionated rp53. Reactions were started by the addition 10 μl rabbit reticulocyte lysate (Promega) and incubation at 37°C for 10-40 min. Samples were then subjected to SDS-PAGE and western blotting using anti-p53 (DO-1) antibodies. The p53 protein bands were quantitated by densitometry.
Statistical analysis
All experiments including the western blotting and immunohistochemical analyses were performed at least four times independently. Results were assessed by Student's t test. Significance was defined as P<0.05.
Results
Production of polyclonal antibodies and their initial characterization
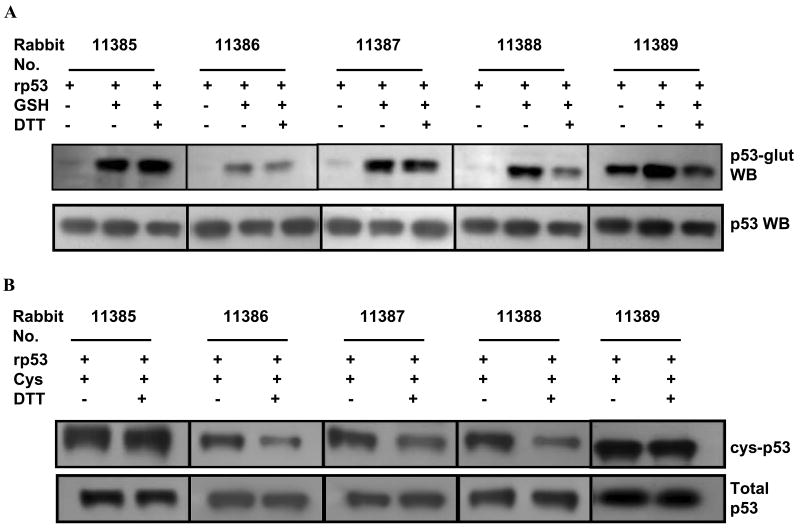
Our recent study using mass spectrometry showed Cys-141 to be a major and preferred site of glutathionylation for human p53 [14]. To quantify the extent of and probe the biological effects of this inhibitory posttranslational modification, we raised polyclonal antibodies using a p53 peptide (amino acids 136-147) in which Cys141 was bound to glutathione through a disulfide bond. The modified peptide was immunized in 5 rabbits. Sera were collected after two booster injections and subjected to initial characterization for p53-glut specific antibodies. For this, the recombinant p53 protein was glutathionylated, gel-filtered to remove the residual thiols and used as the target. In these assays, rp53, glutathionylated rp53, glutathionylated rp53 incubated with 5 mM DTT were electrophoresed on non-reducing SDS-polyacrylamide gels and western blotted using whole rabbit sera. The top panel of Fig. 1A shows a representative blot. The sera from most rabbits except 11389 recognized the unmodified denatured rp53 protein feebly. However, they all detected the glutathione-conjugated p53, albeit to different extents. When glutathionylated p53 samples were exposed to DTT (prior to SDS-PAGE) for reducing and reversing the mixed disulfide linkages between GSH and cysteines on the p53 protein surface [14], the band intensities were consistently diminished with the sera from rabbits 11386 and 11388. Reprobing the membranes with a monoclonal antinbody (DO-1) that recognizes the wild-type human p53 showed the presence of p53 protein at equivalent levels in all lanes (Fig. 1A, bottom panel). These data demonstrate that (i) the rabbits generated a mixture of antibodies recognizing different epitopes of the peptide antigen, namely, glutathionylated p53 and denatured and/or reduced p53 protein, and (ii) the anti-glut p53 antibodies were present at sufficient levels in the sera from the rabbits 11386 and 11388.
FIG. 1. Initial screening for anti p53-glut antibodies in the serum of 5 immunized rabbits.
(A) Top panel- Recombinant p53 (rp53) protein was glutathionylated in vitro and the free GSH in the reactions removed by gel-filtration. One μg each of rp53, glutathionylated rp53 or glutathionylated rp53 treated with 10 mM DTT were subjected to non-reducing SDS-PAGE and the resulting blots probed with whole sera from rabbits at 1:1000 dilution. The bottom panel shows equivalent levels of rp53 present in all lanes after reprobing the membrane with monoclonal anti-p53 (DO-1) antibody. (B) Recognition of in vitro cysteinylated rp53 by different rabbit antisera. Cysteinylated rp53 prepared as above was electrophoresed with or without 10 mM DTT exposure and subjected to western blot analysis. Cys, – cysteine.
Recognition of cysteinylated p53 by the antisera
Although glutathione, being the most abundant thiol, is a major component in forming mixed disulfide linkages with the protein-bound reactive cysteines (S-glutathionylation), cysteine and homocysteine are also capable of generating such disulfides (S-cysteinylation and S-homocysteinylation) [15]. These variants of S-thiolation can trigger signaling events much similar to glutathionylation under oxidative stress [15, 22]. We reasoned that p53-glut antibodies may also recognize cysteinylation of the tumor suppressor due to the innate cysteine involvement of glutathione peptide in disulfide bonding. Therefore, rp53 was cysteinylated, gel-filtered to remove unreacted cysteine and subjected to western blot analysis using whole sera against the p53-glut peptide. Figure 1B shows that similar to glutathionylation, the polyclonal antibodies also detected the cysteinylation of p53. The cysteinylation was also reversible by DTT (lanes 4, 6 and 8), and rabbits 11386 and 11388 had specific antibodies that recognized this posttranslational modification. Collectively, the results shown in Figures 1AB indicate that small amounts of antibodies directed to the mixed disulfide at Cys-141 of the p53 protein were indeed generated, despite the presence of antibodies targeted to the p53 peptide that predictably lost the GSH-linkage in vivo.
ELISA using affinity-purified antibodies
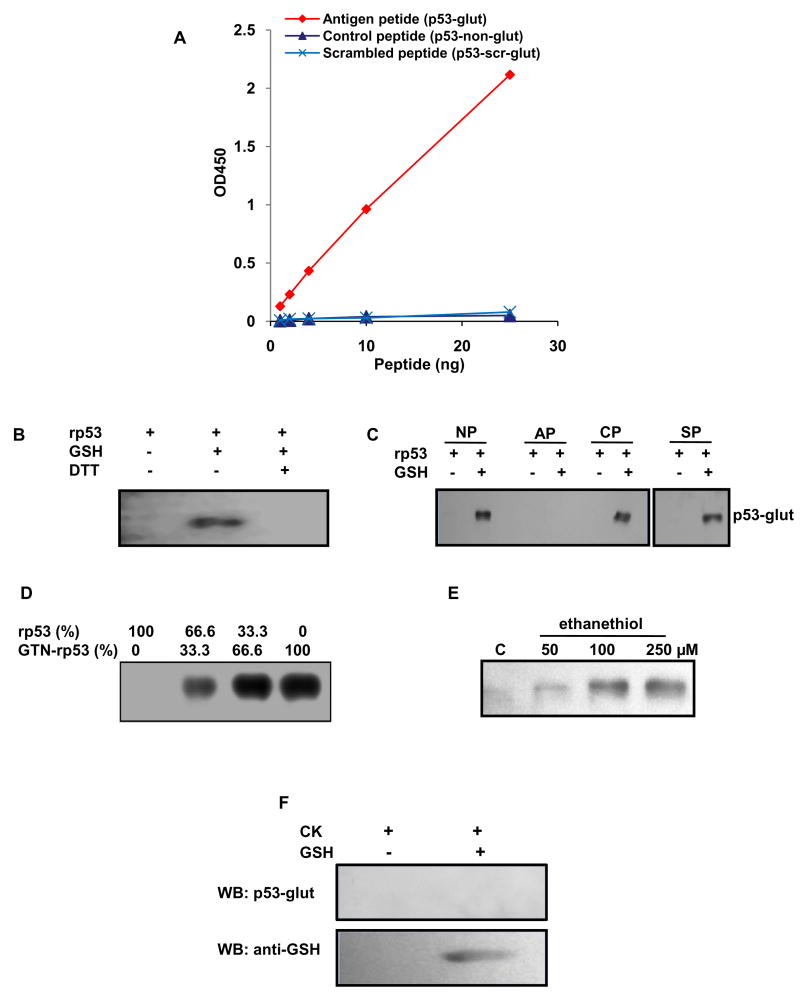
Antiserum from rabbit 11388, which had the highest amount of the desired species, was affinity-purified step-wise, first collecting antibodies that did not bind to the non-glutathionylated peptide, and then binding them with the antigenic peptide column as described in Methods. The specificity of the purified antibodies was tested by quantitating their ability to bind the antigenic (glutathionylated p53) or control (unglutathionylated p53) or glutathionylated scrambled peptides in a sandwich ELISA format. Figure 2A shows the dose-dependent binding of the antigen peptide to the antibodies and the complete failure of the non-glutathionylated and thiolated scrambled counterpart to bind the antibodies in these assays. Exposure of the antigen-coated plates to 5 mM DTT for 20 min eliminated the ELISA signal. The minimum detectable concentration for the antigen was determined to be 1 ng/well and the detection range for OD450 was linear to 25 ng/well (Fig. 2A). The data shows that affinity-purified p53-glut antibodies were sequence-specific and sensitive to bind small amounts of the antigen.
FIG. 2. ELISA and immunoblot analyses using purified anti p53-glut antibodies.
(A) The glutathionylated antigenic, non-glutathionylated control and glutathionylated scrambled peptides were coated in increasing amounts (1 ng/well to 25ng/well) in microtiter plates and ELISA was performed with purified p53-glut antibodies. The absorbance was read at 450 nm. The graph shows dose-dependent binding of affinity purified p53-glut antibodies to the antigen peptide (filled ◊) but not to the non-glutathionylated control peptide (filled Δ) or glutathionylated scrambled peptide (×). (B) Western blot showing specific detection of glut-rp53; unmodified rp53 and glutathionylated rp53 exposed to DTT were not detected. (C) Western blot showing the abrogation of glut-rp53 detection on pre-treating the p53-glut antibody with the glutathionylated antigenic peptide. The antibody detected glut-rp53 under conditions of no pre-treatment (NP) or pre-treatment with non-glutathionylated control peptide (CP) and glutathionylated scrambled peptide (SP). Antibodies were mixed with 10 μg of peptides in a volume of 1ml for 1h at room temperature. (D) Western blot showing increased band intensities after mixing glut-p53 at increasing ratios with non-glutathionylated rp53. (E) Western blot showing the thiolation of rp53 by ethanethiol. (F) Specificity of p53-glut antibodies. One μg rabbit creatine kinase (CK) was glutathionylated in vitro and electrophoresed in duplicate with the unmodified protein. The blots were probed with p53-glut antibodies or anti-GSH monoclonal antibody. The results show that p53-glut antibodies did not recognize the mixed disulfide in CK.
Determination of specificity of purified antibodies
Western blot analyses of modified rp53 were performed to confirm the specificity and utility of the purified antibodies. As shown in Fig. 2B, the affinity-purified IgG detected the glutathionylation of rp53 under non-reducing conditions, but not after DTT exposure. The unmodified protein was not recognized. Further, competition experiments were performed by mixing the purified IgG with the antigenic (glutathionylated) or non-glutathionylated 12-residue and scrambled glutathionylated peptides and using the resulting samples as primary antibody sources for immunoblotting. Figure 2C shows that antibodies depleted with the antigen peptide failed to interact with the glutathionylated rp53 while the unmodified control peptide or glutathionylated scrambled peptide failed to eliminate the detection of same target. Next, to assess the quantitative detection of the antigen, glutathionylated and unmodified rp53 were mixed in different ratios followed by Western blotting. Figure 2D shows that the band intensities increased proportionately with the increased input of modified p53; this relationship was linear in different experiments as verified by densitometry (not shown), thereby suggesting the usefulness of antibodies for quantitative analysis of glut-p53 in biological systems. Because inactivating human p53 through glutathionylation may have therapeutic relevance, we explored the ability of a small- molecule ethanethiol (C2H5SH) to thiolate the rp53 protein. The western blot shown in Fig. 2E reveals that p53-glut antibodies detected the chemical-induced thiolation of p53 at Cys-141.
It is necessary to establish that the antibodies are specific to the thiolation in the p53 protein and does not recognize the mixed disulfide bonds in global proteins. We performed two types of experiments to prove this fact. First, we electrophoresed extracts from HCT116 cells (control and diamide-treated) and probed the resulting blot with purified anti-p53 glut antibodies. The western blot shown in Fig.S1 did not reveal protein species other than a 53 kDa band corresponding to p53 (also see Fig. 3C). Secondly, we used the rabbit muscle creatine kinase (CK, Sigma Chemicals), which is an established substrate for glutathionylation [23] for proving the specificity of p53-glut antibodies. In this experiment, purified creatine kinase protein was glutathionylated in vitro [14] and western blotted separately using the p53-glut antibodies or a monoclonal antibody to GSH (Virogen) that is capable of detecting glutathionylation in any protein [24]. The results shown in Fig. 2F reveal that the p53-glut antibodies did not recognize GSH-treated CK while the anti-GSH antibody did, thus, confirming the non-reactivity of our antibodies with bulk protein thiolation.
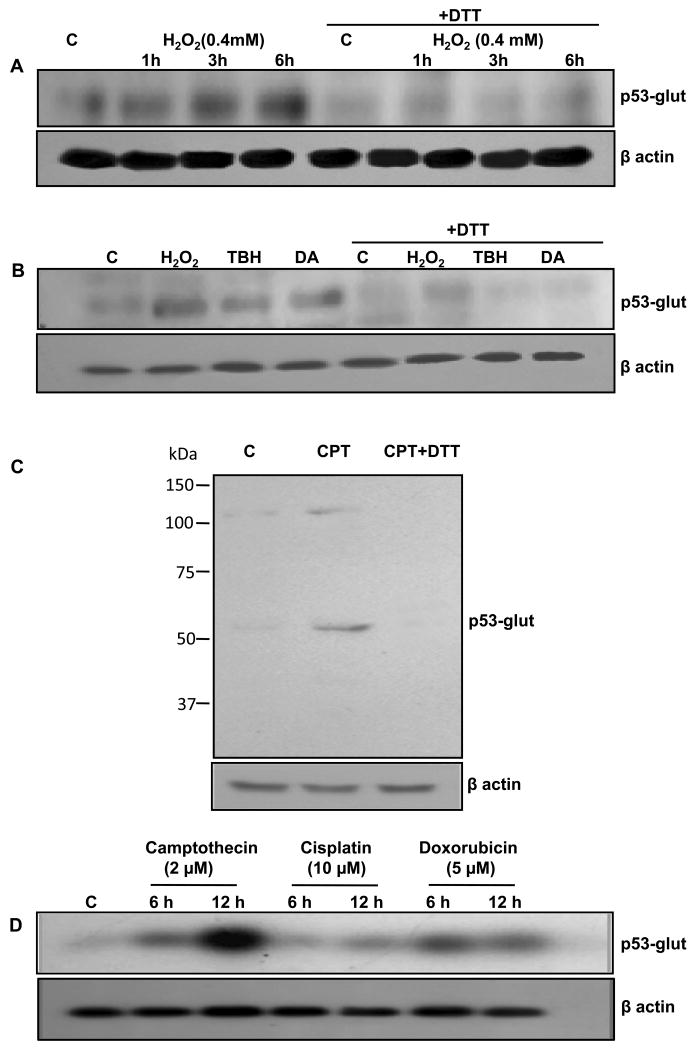
FIG. 3. Detection of glutathionylated p53 protein in human tumor cells using anti p53-glut antibodies.
Induction of glut-p53 protein was determined in HCT116 cells after 0.4 mM H2O2 treatment for different times (A), after 20 min incubation with H2O2 (0.4 mM), TBH (0.6 mM) and diamide (0.6 mM) (B), with camptothecin (2 μM, 5h) (C), and with camptothecin (2 μM), cisplatin (10 μM), and doxorubicin (5 μM) for 6 and 12 h (C). In the last four lanes of (A) and (B) and last lane of (C) the protein samples were treated with DTT before SDS-PAGE to reverse p53 glutathionylation. C, untreated control; CPT, camptothecin.
Detection of glutathionylated p53 protein in human tumor cells
Using immunoprecipitation/western blot analysis, we demonstrated previously that acute oxidative stress and DNA damage increase glut-p53 protein levels in human tumor cells [14]. Therefore, direct western blot analysis using p53-glut antibodies was performed to confirm these data. HCT116 cells were exposed to moderate concentrations of H2O2, TBH and DA for up to 20 min. The cells were also exposed to DNA-damaging chemotherapeutic agents, camptothecin and doxorubicin (inducers of topoisomerase I- and II- mediated strand breaks, respectively) and cisplatin (crosslinking) for 6-12 h. A total of 40 μg protein in the cell lysates were electrophoresed under non-reducing conditions and subjected to western blot analysis. As shown in Fig. 3 AB, a short-term redox imbalance induced by all oxidants increased the amount of glut- p53. As expected, exposing the protein samples to DTT before SDS-PAGE diminished the intensity of immunoreactive bands demonstrating the reversibility of the modification and specificity of the antibodies. A full-length western blot from camptothecin-treated cells that was developed for a slightly longer time (3 min) is shown in Fig. 3C. On this blot, we observed an extra band around 107 kDa; both the 53 and 107 kDa bands were eliminated when the samples were treated with DTT and electrophoresed (lane 3 in Fig. 3C), clearly suggesting that the upper band was a dimer linked through a disulfide bridge. Furthermore, the genomic damage induced by the anticancer drugs also enhanced glut-p53 levels in a time-dependent manner with camptothecin being superior to doxorubicin and cisplatin (Fig. 3D). Total p53 levels assessed by western blot analyses using the DO1 monoclonal antibody approximately paralleled the raises in glutathionylated p53 protein (not shown); similar observations have been made in other experiments, implying that as cellular p53 levels increase, small proportions of the protein may be thiolated perhaps as an acute defense mechanism (14). Taken together, these results indicate that different types of cellular stresses can induce Cys-141 thiolation in human p53 protein.
Glutathionylated p53 is prone to ATP-dependent degradation
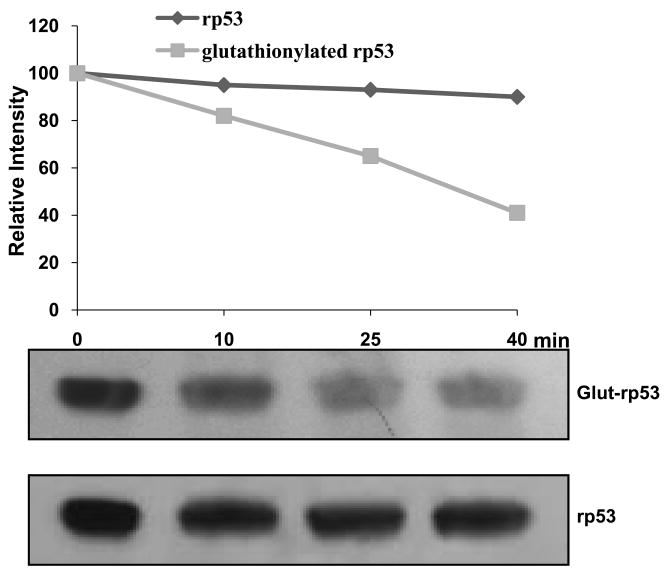
To explore if the glut-p53 is perceived by human cells as an inactivated protein and eliminated, we compared the in vitro degradation of rp53 and glut-rp53 in rabbit reticulocyte lysates, which are well known to support proteasomal and ubiquitination-dependent protein degradation [25, 26]. rp53 and glutathionylated rp53 proteins were incubated with reticulocyte lysates in the presence of Mg2+ and ATP at 37°C. The reaction mixtures were immunoblotted and probed with D0-1 monoclonal antibodies for p53 which recognize the full-length (modified and unmodified) p53 protein [27]. Figure 4 shows that while rp53 remained largely undegraded during the 10-40 min incubation, glut-p53 disappeared progressively. The results suggest that the thiolated p53 may be an unstable protein with susceptibility for fragmentation or elimination through ubiquitination-dependent proteolysis.
FIG. 4. Glutathionylated p53 is selectively degraded in ATP-dependent reactions.
Unmodified rp53 or purified glutathionylated rp53 proteins were incubated in the presence of ATP, Mg++, and rabbit reticulocyte lysates as decribed in Methods. Samples were western blotted and probed with DO-1 anti-p53 monoclonal antibodies (lower panel). The band intensities were quantitated and the relative values are shown (upper panel).
Immunoflourescence localization of glutathionylated p53 in human cancer cell lines
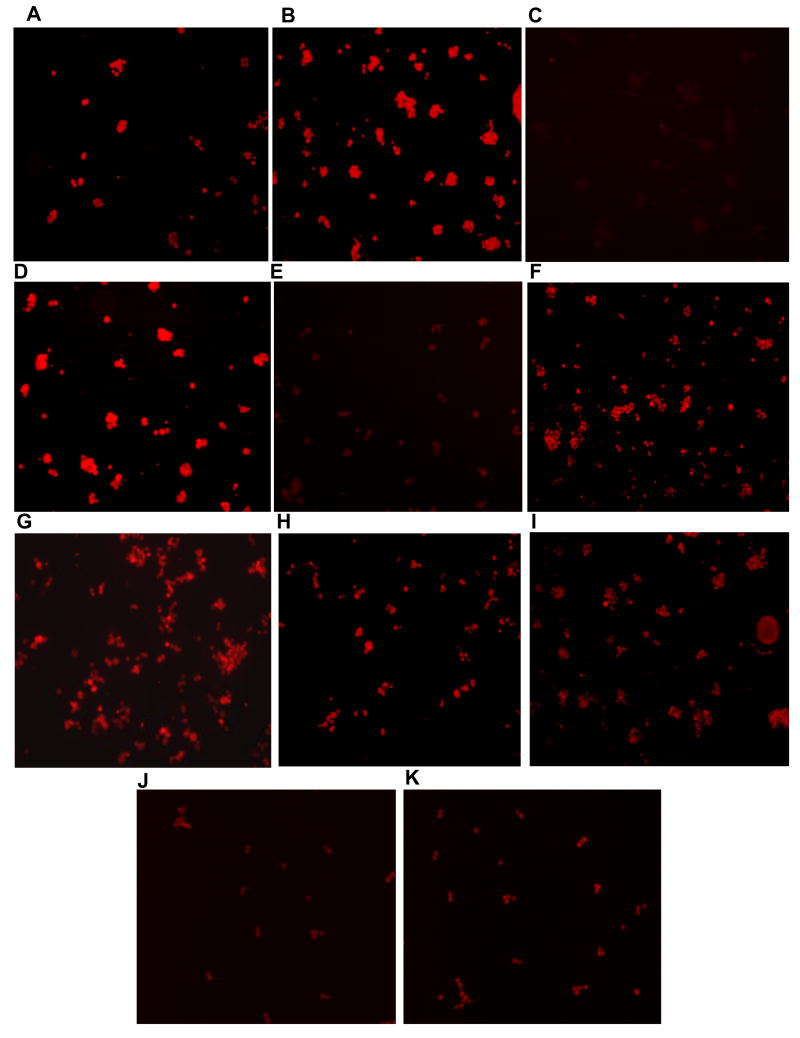
To determine the immunocytochemical applications of the new antibodies and to study the dynamics of p53 Cys141 thiolation in human tumor cells, the colon cancer cells HCT116 and HT29, and T47D breast cancer cells were grown on cover slips, and treated with oxidizing and DNA-damaging agents as described above. The cells were fixed, treated with purified glut-p53 antibodies, and visualized using anti-rabbit IgG labeled with Alexa 594. As shown in the Fig. 5A, glut-p53 was present in untreated HCT116 cells at low but detectable levels, suggesting that normal levels of cellular stress can induce this modification. Induction of acute oxidative stress by diamide and H2O2 markedly increased glut-p53 staining (Fig. 5B, and D). When diamide-treated cells were exposed to 10 mM DTT prior to incubation with glut-p53 antibodies, a significant reduction in the fluorescence signal was observed (Fig. 5C). Postincubation of H2O2-treated cells for 2 hours in oxidant-free media (DMEM with 10% serum) also resulted in diminished glut-p53 staining (Fig. 5E) suggesting of protein dethiolation and the dynamic nature of the modification. The majority of the staining was confined to the nuclei, as evident by the overlap with the nuclear Hoechst staining (not shown). The photomicrographs in Fig. 5F and 5G show the markedly enhanced levels of glut-p53 after doxorubicin and cisplatin treatments respectively. Staining of HCT116 cells for total p53 protein using the DO1 monoclonal antibody was also performed after these treatments. Consistent with the activation of p53 known after various stresses, an increased immunocytochemical staining was seen after the exposures above (Fig. S2). Further, it was of interest to test whether the mutant p53 present in HT29 and T47D cells [28] also respond to oxidative stress. The p53 mutations facilitate the accumulation of p53 protein to higher steady-state levels in these cell lines. Interestingly, the untreated HT29 cells harbored higher levels of glut-p53 which did not change significantly after diamide exposure (Fig. 5HI). Similar results were obtained with the mutant p53 in T47D cells (Fig. 5JK). The inability of diamide to induce Cys-141 thiolation of mutant p53 is surprising, and may relate to the conformational changes in the protein, and/or accessibility of the redox-sensitive cysteines. The diminished staining of glut-p53 after DTT exposure (Fig. 5C) is consistent with the reversibility of the modification and confirms the antibody specificity. Similar to our data, DTT and 2-mercaptoethanol have been shown to attenuate protein glutathionylation signals in immunohistochemical, ELISA and western blot analyses [29-31], however, less than complete reversal of staining has been reported in several publications [29-33]. Overall, these results demonstrate the usefulness of antibodies for immunohistochemical analyses and suggest that both wild-type and mutant p53 are susceptible to Cys-141 glutathionylation.
FIG. 5. Immunofluorescence staining of glutathionylated p53 in HCT116, HT29, and T47D cells upon treatment with oxidizing and DNA damaging agents.
Immunofluorescence showing the presence of p53-glut in untreated HCT116 cells (A); HCT116 cells treated with 0.6 mM diamide for 30 min (B); HCT116 cells treated as in (B) followed by 10 mM DTT treatment for 30 min (C); HCT116 cells treated with 0.4 mM H2O2 for 30 min (D); HCT116 cells treated as in (D) and postincubated in H2O2 free medium for 2h (E); HCT116 cells treated with 5 μM doxorubicin for 5h (F), and HCT116 cells treated with 10 μM cisplatin for 5h (G). Immunofluorescence showing the presence of p53-glut in untreated HT29 cells (H); HT29 cells treated with 0.6 mM diamide for 30 min (I); untreated T47D cells (J), and T47D cells treated with 0.6 mM diamide for 30 min (K). After the respective treatments, cells were fixed using 4% paraformaldehyde and incubated with p53-glut antibody (1:500 dilution) followed by goat anti rabbit Alexa Flour 594 secondary antibody. The cells were photographed using an Olympus IX 81 microscope. Similar patterns of staining were observed in three independent experiments.
Immunohistochemistry of glutathionylated p53 in human prostate cancer and other cancer tissue microarrays
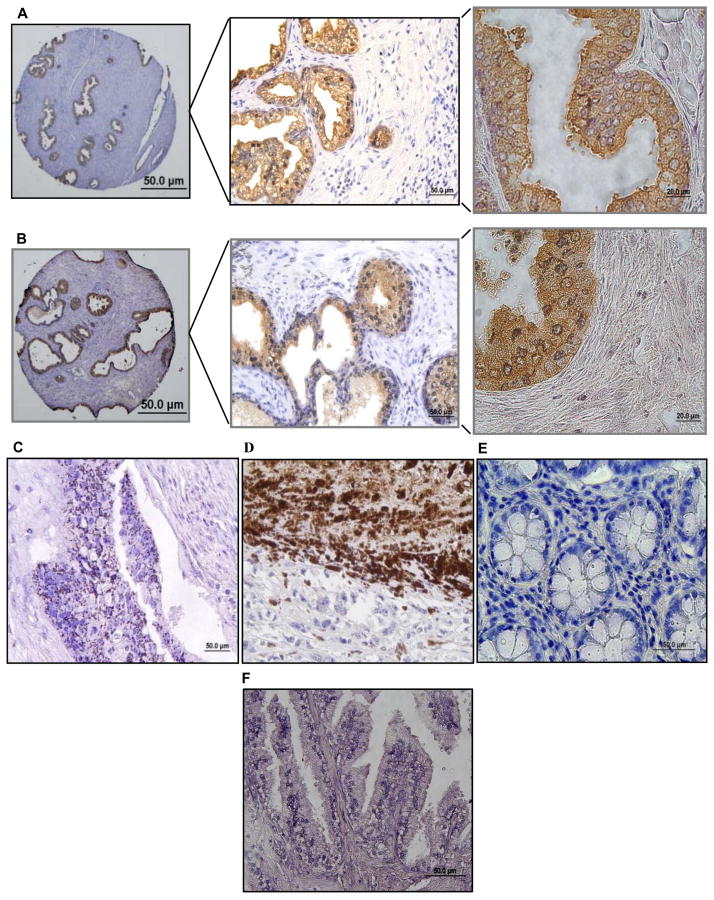
In view of the increased and sustained oxidative stress known to exist in various human cancers [34, 35], we investigated the presence of glut-p53 in tissue microarrays from apparently normal prostate adjacent to cancer, prostate adenocarcinomas, gastric cancer, and melanoma tissues. The paraffin-embedded tissue sections were processed by routine procedures, immunostained with the affinity-purified p53-glut antibodies and visualized by the brownish stain resulting from the 3-aminobenzidine reaction with HRP. We examined 6 prostate tissues located near cancer, 15 prostate adenocarcinomas (Gleason's scores 1 to 4), 2 gastric cancers, 3 melanomas and 4 colon cancer tissues. Figure 6 shows the representative staining pattern and distribution of glut-p53 in these tissues. Interestingly, among the tissues tested, all apparently normal prostate samples (possibly hyperplastic) present adjacent to the cancers were strongly positive for glut-p53 with moderate (+++) to intense levels of staining (Fig. 6A). In these tissues, the staining was mostly cytoplasmic, and restricted to the luminar epithelium, with very little staining in the secretory cells (stained with hematoxylin). The pattern of staining in prostate adenocarcinomas was similar with the epithelial lining of malignant glands showing the presence of glut-p53. However, the staining was diffuse, with nuclear to cytoplasmic distribution. Interestingly, the collective p53-glut staining in prostate cancers was significantly lower (+ to +++ score range), when compared to corresponding normal tissue, both within a single specimen and among different specimens (Fig. 6B). Glut-p53 staining was eliminated when a prostate cancer section was treated with DTT before antibody exposure (Fig. 6F), again verifying the specificity. The p53-glut staining in colon cancer samples was punctuate and diffuse (Fig. 6C); however, melanoma samples showed the most intense staining (nuclear + cytoplasmic) of all tissues examined (Fig. 6D). In contrast, the two gastric cancer arrays were negative for glut-p53 (Fig. 6E), which is surprising given the acidic and ROS-inducing conditions prevalent in this tissue. However, we stress the need for analyzing a large number of tumor samples before drawing conclusions. These data provide strong evidence for the presence of glut- p53 in human normal and malignant tissues, and suggest that inherent oxidative stress and accompanying pathophysiological conditions can generate thiolated p53 and functionally inactivate the tumor suppressor protein.
FIG. 6. Immunohistochemistry of glutathionylated p53 in human prostatic tissue and other cancer microarrays using p53-glut antibodies.
Paraffin-embedded sections were processed for glut-p53 detection as described in Methods. Representative staining in each cancer or apparently normal tissue adjacent to cancer is shown. In panels A and B, the prostate gland sections were photographed at 20×, 40× and 60× magnifications to visualize the overall and detailed staining distribution in different structures. A, Apparently normal prostatic tissue adjacent to a cancer. p53-glut staining was restricted to the glandular epithelium and was not seen in stroma stained with hematoxylin. B, Prostate adenocarcinoma (Gleason Score 3). The staining pattern and distribution in different hyperplastic and malignant adenocarcinomas were similar to that seen in normal prostate. C, Particulate staining seen in a colon cancer section. D, Intense glut-p53 staining observed in a melanoma. E, Gastric cancer showing no staining for glut-p53. F, Lack of staining in a prostate adenocarcinoma treated with 10 mM DTT for 1h before exposing to glut-p53 antibodies.
Discussion
ROS and RNS- generated by cells as products or by-products can function either as signaling molecules or as cellular toxins. Both ROS and p53 participate in multiple cellular processes such as signaling, cell cycle regulation, gene expression and apoptosis; therefore, there is a frequent interplay among them. However, the transcription factor activity of human p53 is inhibited by oxidizing and nitrosylating agents and its biological functions promoted by a reduced milieu both in vitro and in vivo [7-10, 36]. Previously, we showed that oxidative and genotoxic stresses inhibit human p53 protein through glutathionylaton of Cys 141 in the DNA-binding domain and also curtail the oligomerization [14].
Increased production of ROS and RNS resulting from oncogenic transformation, accelerated metabolism and/or mitochondrial dysfunction is a characteristic of almost all human cancers [37]. The heightened and chronic oxidative stress levels present in cancers can frequently cause sequential oxidative inactivation of p53 sulfhydryls (SOH, SO2H and the terminal SO3H) or induce glutathionylation of the same cysteines. Detection and quantitation of this posttranslational modification is cumbersome, as it requires immunoprecipitation of p53 and subsequent probing of western blots with anti-glutathione antibodies [14]. However, such methods are not applicable for cultured cells or fixed tissue sections. Here, we report successful generation and characterization of polyclonal antibodies raised against a peptide bearing the Cys141-glutathionylated peptide of human p53. This is also the first report of glutathionylation-specific antibodies for any protein and is analogous to phospho-specific and acetylation-specific antibodies described for various signaling proteins in the literature.
Modification of cellular proteins by glutathionylation in response to the recurring episodes of oxidative stress both under physiologic and pathologic conditions is an area of intense research. Disulfide linkages are introduced between the ionized cysteines on protein surfaces and glutathione (or GSSG in thiol-exchange reaction) when cells perceive even slight changes in the redox balance. Whether these redox changes occur in the overall intracellular context, nuclear or organelle microenvironments, human cells appear to respond in a unified manner by glutathionylating regulatory and signaling proteins including protein kinases, phosphatases and transcription factors [15, 16, 38-40]. In most cases, the cysteines targeted for glutathionylation perform essential functions, either structural or catalytic, possess lower pKa values due to the neighboring basic amino acids, and are poised for oxidation or nitrosylation by ROS and RNS respectively. Thus, the same cysteines are likely to be targeted for both glutathionylation and nitrosylation events. Further, the two modifications are closely related and interconvertible. For example, S-nitrosoglutathione can interact with protein-SH groups to induce glutathionylation and nitrosylated protein sulfhydryls (PSNO) can react with GSH to form mixed disulfides [41]. With most substrates, glutathionylation inhibits protein functions and it is widely accepted as a protective mechanism against the irreversible oxidation of SH-groups in redox-sensitive proteins, often at the expense of temporary loss of their activities [15, 16].
Human p53 encodes ten cysteine residues, present as two clusters, both within the DNA binding domain of the protein. One cluster contains three cysteines (residues 176, 238, and 242) responsible for the coordination of a zinc ion. Zinc binding is essential for the stabilization of p53 in the folded form. The other cluster of cysteines (residues 124, 135, 141, 277) is located near the loop-sheet-helix region of p53 that makes contact with the consensus DNA sequences [13, 14]. Of these, we found Cys-141 was found to be the preferred site for glutathionylation using two mass spectrometry procedures. Furthermore, Cys-141 has been shown to be a site prone to oxidation in p53 bound to DNA [18]. Not all rabbits were efficient in generating the desired antibodies; it appeared that the immunized peptide lost the GSH-linkage and antibodies were produced to the deglutathionylated peptide as well. The antibodies also recognized the cysteinylated p53 but not any other glutathionylated proteins suggesting that the mixed disulfide in the p53 peptide was the target epitope. The specificity of anti-glut-p53 antibodies was verified by ELISA (Fig. 2A), DTT-induced dethiolation and elimination of signal on western blots (Figs. 2B, 3A, 3B) and immunoflourescence (Fig. 5C), dose-dependent response observed for the glutathionylated p53 in western blots in mixing experiments (Fig. 2C), and neutralization of antibodies by the antigenic peptide, but not the unthiolated p53 peptide (Fig. 2E). It appeared that drug-induced glutathionylation can also generate intermolecular crosslinking of p53 (Fig. 3C) as has been observed for the oligopeptidase EP24.15 (42); such an event occurs through a thiol-disulfide exchange between thiolated and unthiolated protein molecules.
We observed that glut-p53 was present in non-stressed cells albeit at low levels, but it increased significantly after oxidative and DNA damaging treatments (Fig. 3). When oxidant-treated cells were returned to oxidant-free medium, glut-p53 decreased to undetectable levels, suggesting that the posttranslational modification is transient and easily reversible. These observations support the idea that thiolation and dethiolation of p53 are rapid and dynamic events. Significantly, two cellular mechanisms, namely, protein degradation and dethiolation appear to determine the steady-state glut-p53 levels in human cells. Our experiments indicated that glut-p53 may be unstable in human cells (Fig. 4). Other data has shown that p53 can specifically associate with glutaredoxin-1 in HCT116 cells (unpublished). Glutaredoxins are the most thoroughly characterized dethiolating enzymes, and this list now includes sulfiredoxin, thioredoxin and ref-1 [17, 43]. Therefore, the fate of glut-p53 may be determined in a short window of time during which it can be dethiolated and returned to the active p53 pool or degraded in ATP-dependent reactions.
One of the most significant aspects of this study was the demonstration of glut-p53 in human cancer tissues (Figs. 6 B-D). The immunohistochemical analyses provided first evidence for the presence of p53 glutathionylated at Cys141, particularly in apparently normal/hyperplastic prostate, prostate adenocarcinoma and melanoma tissues. A vast amount of epidemiological, experimental and clinical evidence suggests that oxidative stress and aging contribute greatly to the etiology and pathogenesis of the prostate cancer [44]. Elevated and constant oxidative stress has been linked to the emergence of proliferative inflammatory atrophy (PIA) and prostatic hyperplasia [45]. Continued ROS production coupled with aberrant androgen signaling, inefficient antioxidant systems, and the consequent accumulation of DNA damage have been tightly linked with the generation of pre-malignant intraepithelial neoplastic (PIN) lesions. Additionally, the expression of a major antioxidant enzyme, glutathione transferase-pi (GSTP1) is silenced through promoter methylation very early in prostate carcinogenesis with virtually all prostate cancers lacking GSTP1 expression [46]. Also, the androgen-stimulated polyamine oxidation leading to the production of H2O2 is a contributing factor in prostatic oxidative stress [47]. Many of these oxidative conditions alone or in combination may explain the presence of abundant glut-p53 in apparently prostatic tissues. Further, the pattern of glut-p53 distribution was similar to that of GST-pi reported in normal glandular prostatic epithelium [48]. The net amount of glut-p53 was lower in prostate cancers compared to the corresponding normal and hyperplastic tissues, implying the possible terminal oxidation of p53, which is not recognized by our antibodies. Therefore, glutathionylation or terminal oxidation of cysteines in p53 protein may represent a hitherto unrecognized mechanism for inactivating the tumor suppressor in human cancers. Although p53 mutations have been reported to be less frequent in prostate cancers [49], it is possible that thiolation of Cys-141 and other reactive cysteines may curtail p53 function and accelerate prostate carcinogenesis.
Why do cells respond by glutathionylating a part of their p53 pool under adverse conditions including DNA damage and redox imbalance? Truly, this negative regulation is unexpected, given the well-defined roles p53 performs in maintaining the genomic stability. While the exact reasons are unclear, it is tempting to hypothesize that p53 inactivation through a disulfide relay may represent an acute cellular defense strategy for terminating the apoptotic stimuli generated by various stresses. Further, it may facilitate gene expression to suit cellular adaptation and survival. Irrespective of this notion, our studies establish p53 glutathionylation as a physiologically relevant modification. The availability of specific antibodies for glut-p53 should enhance our understanding of redox control of the tumor suppressor in human cancers and p53 response to anticancer therapies, and other disease settings. For example, a recent report described the presence of glut-p53 in the brain tissues from Alzheimer's patients and its predominantly monomeric / dimeric state [50], clearly in agreement with our previous findings [14].
Supplementary Material
Western blot showing the induction of glut-p53 protein in HCT116 cells after treatment with diamide (DA, 0.5 mM for 30 min). The lower panel shows the p53 levels as detected by DO1 monoclonal antibody for p53 in HCT116 cells treated as above.
Immunofluorescence showing the p53 protein levels in untreated HCT116 cells (A), cells treated with 0.6 mM diamide for 30 min (B), 0.4 mM H2O2 for 30 min (C), 5 μM doxorubicin for 5h (D), and 10 μM cisplatin for 5h (E). After the respective treatments, cells were fixed using 4% paraformaldehyde and incubated with p53 (DO1) monoclonal antibody (1:200 dilution) followed by goat anti mouse Alexa Flour 488 secondary antibody. The cells were photographed using an Olympus IX 81 microscope. Independent experiments revealed similar patterns of staining.
Acknowledgments
This study was supported by Grants from the National Institutes of Health (RO1 CA097343 and RO3 CA125872) and Laura W. Bush Institute for Women's Health, Permian Basin, TX to K.S.S.
Abbreviations
- Glut-p53 or p53-glut
glutathionylated p53
- rp53
recombinant p53
- DA
diamide
- TBH
tert-butyl hydroperoxide
- WB
Western blot
- IP
immunoprecipitation
- DTT
dithiothreitol
- Cys
cysteine
- CK
creatine kinase
Footnotes
Disclosure Statement: No competing financial interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lane DP. Cancer: p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 2.Vousden KH. p53: death star. Cell. 2000;103:691–694. doi: 10.1016/s0092-8674(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 3.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 4.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 5.Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 6.Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 7.Hainaut P, Milner J. Redox modulation of p53 conformation and sequence-specific DNA binding in vitro. Cancer Res. 1993;53:4469–4473. [PubMed] [Google Scholar]
- 8.Verhaegh GW, Richard MJ, Hainaut P. Regulation of p53 by metal ions and by antioxidants: Dithiocarbamate down-regulates p53 DNA-binding activity by increasing the intracellular level of copper. Mol Cell Biol. 1997;17:5699–5706. doi: 10.1128/mcb.17.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delphin C, Cahen P, Lawrence JJ, Baudier J. Characterization of baculovirus recombinant wild-type p53. Dimerization of p53 is required for high-affinity DNA binding and cysteine oxidation inhibits p53 DNA binding. Eur J Biochem. 1994;223:683–692. doi: 10.1111/j.1432-1033.1994.tb19041.x. [DOI] [PubMed] [Google Scholar]
- 10.Merwin JR, Mustacich DJ, Pearson GD, Merril GF. Reporter gene transactivation by human p53 is inhibited in thioredoxin reductase null yeast by a mechanism associated with thioredoxin oxidation and independent of changes in the redox state of glutathione. Carcinogenesis. 2002;23:1609–1615. doi: 10.1093/carcin/23.10.1609. [DOI] [PubMed] [Google Scholar]
- 11.Jayaraman L, Murthy KG, Zhu C, Curran T, Xanthoudakis S, Prives C. Identification of redox/repair protein Ref-1 as a potent activator of p53. Genes Dev. 1997;11:558–570. doi: 10.1101/gad.11.5.558. [DOI] [PubMed] [Google Scholar]
- 12.Ueno M, Masutani H, Arai RJ, Yamauchi A, Hirota K, Sakai T, Inamoto T, Yamaoka Y, Yodoi J, Nikaido T. Thioredoxin-dependent redox regulation of p53-mediated p21 activation. J Biol Chem. 1999;274:35809–35815. doi: 10.1074/jbc.274.50.35809. [DOI] [PubMed] [Google Scholar]
- 13.Rainwater R, Parks D, Anderson ME, Tegtmeyer P, Mann K. Role of cysteine residues in regulation of p53 function. Mol Cell Biol. 1995;15:3892–3903. doi: 10.1128/mcb.15.7.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velu CS, Niture SK, Doneanu CE, Pattabiraman N, Srivenugopal KS. Human p53 is inhibited by glutathionylation of cysteines present in the proximal DNA-binding domain during oxidative stress. Biochemistry. 2007;46:7765–7780. doi: 10.1021/bi700425y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- 16.Ghezzi P, Bonetto V, Fratelli M. Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation. Antioxid Redox Signal. 2005;7:964–972. doi: 10.1089/ars.2005.7.964. [DOI] [PubMed] [Google Scholar]
- 17.Gallogly MM, Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol. 2007;7:381–391. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Augustyn KE, Merino EJ, Barton JK. A role for DNA-mediated charge transport in regulating p53: Oxidation of the DNA-bound protein from a distance. Proc Natl Acad Sci U S A. 2007;104:18907–18912. doi: 10.1073/pnas.0709326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Annis I, Barany G. Curr Protocols in Protein Sc. Unit 18.6. Chapter 18. New York: John Wiley and Sons, Inc; 2001. Disulfide bond formation in peptides. [DOI] [PubMed] [Google Scholar]
- 20.Harlow E, Lane DP, editors. Antibodies: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 21.Elias JM. Immunohistochemical methods. In: Elias JM, editor. Immunohistopathology. A Practical Approach to Diagnosis. Illinois: ASCP Press; 2003. pp. 1–110. [Google Scholar]
- 22.Thomas JA, Mallis R, Sies H. Protein S-thiolation, S-nitrosylation, and irreversible sulfhydryl oxidation: roles in redox regulation. In: Gitler C, Danon A, editors. Cellular Implications of Redox Signaling. London: Imperial College Press; 2003. pp. 141–174. [Google Scholar]
- 23.Reddy S, Jones AD, Cross CE, Wong PSY, van der Vliet A. Inactivation of creatine kinase by S-glutathionylation of the active-site cysteine residue. Biochem J. 2000;347:821–827. [PMC free article] [PubMed] [Google Scholar]
- 24.Brennan JP, Wait R, Begum S, Bell JR, Dunn MJ, Eaton P. Detection and mapping of widespread intermolecular protein disulfide formation during cardiac oxidative stress using proteomics with diagonal electrophoresis. J Biol Chem. 2004;279:41352–41360. doi: 10.1074/jbc.M403827200. [DOI] [PubMed] [Google Scholar]
- 25.Hough R, Rechsteiner M. Effects of temperature on the degradation of proteins in rabbit reticulocyte lysates and after injection into HeLa cells. Proc Natl Acad Sci U S A. 1984;81:90–94. doi: 10.1073/pnas.81.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett RG, Hamel FG, Duckworth WC. Insulin inhibits the ubiquitin-dependent degrading activity of the 26S proteasome. Endocrinology. 2000;141:2508–2517. doi: 10.1210/endo.141.7.7575. [DOI] [PubMed] [Google Scholar]
- 27.Vojtesek B, Bartek J, Midgley CA, Lane DP. An immunological analysis of the human nuclear phosphoprotein p53: New monoclonal antibodies and epitope mapping using recombinant p53. J Immunol Methods. 1992;151:237–244. doi: 10.1016/0022-1759(92)90122-a. [DOI] [PubMed] [Google Scholar]
- 28.Bossi G, Lapi E, Strano S, Rinaldo C, Blandino G, Sacchi A. Mutant p53 gain of function: reduction of tumor malignancy of human cancer cell lines through abrogation of mutant p53 expression. Oncogene. 2006;25:304–309. doi: 10.1038/sj.onc.1209026. [DOI] [PubMed] [Google Scholar]
- 29.Jarry A, Charrier L, Bou-Hanna C, Devilder MC, Crussaire V, Denis MG, Vallette G, Laboisse CL. Position in cell cycle controls the sensitivity of colon cancer cells to nitric oxide-dependent programmed cell death. Cancer Res. 2004;64:4227–4234. doi: 10.1158/0008-5472.CAN-04-0254. [DOI] [PubMed] [Google Scholar]
- 30.Johansson M, Lundberg M. Glutathionylation of beta-actin via a cysteinyl sulfenic acid intermediary. BMC Biochem. 2007;8:26. doi: 10.1186/1471-2091-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craghill J, Cronshaw AD, Harding JJ. The identification of a reaction site of glutathione mixed-disulphide formation on γ S-crystallin in human lens. Biochem J. 2004;379:595–600. doi: 10.1042/BJ20031367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brennan JP, Miller JIA, Fuller W, Wait R, Begum S, Dunn MJ, Eaton P. The utility of N,N-Biotinyl glutathione disulfide in the study of protein S-glutathionylation. Mol Cell Proteomics. 2006;5:215–225. doi: 10.1074/mcp.M500212-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Passarelli C, Di Venere A, Piroddi N, Pastore A, Scellini B, Tesi C, Petrini S, Sale P, Bertini E, Poggesi C. Susceptibility of isolated myofibrils to in vitro glutathionylation: potential relevance to muscle functions. Cytoskeleton. 2010;67:81–89. doi: 10.1002/cm.20425. [DOI] [PubMed] [Google Scholar]
- 34.Benz CC, Yau C. Ageing, oxidative stress and cancer: paradigms in parallax. Nat Rev Cancer. 2008;8:875–879. doi: 10.1038/nrc2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trachootham D, Zhang H, Zhang W, Feng L, Du M, Zhou Y, Zhao C, Pelicano H, Plunkett W, Wierda WG, Keating MJ, Huang P. Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood. 2008;112:1912–1922. doi: 10.1182/blood-2008-04-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu B, Chen Y, St Clair DK. ROS and p53: A versatile partnership. Free Radic Biol Med. 2008;44:1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim GJ, Chandrasekaran K, Morgan WF. Mitochondrial dysfunction, persistently elevated levels of reactive oxygen species and radiation-induced genomic instability: a review. Mutagenesis. 2006;21:361–367. doi: 10.1093/mutage/gel048. [DOI] [PubMed] [Google Scholar]
- 38.Humphires KM, Juliano C, Taylor SS. Regulation of cAMP-dependant protein kinase activity by glutathionylation. J Biol Chem. 2002;277:43505–43511. doi: 10.1074/jbc.M207088200. [DOI] [PubMed] [Google Scholar]
- 39.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 40.Li S, Whorton AR. Regulation of protein phosphatase 1B in intact cells by S-nitrosothiols. Arch Biochem Biophys. 2003;410:269–279. doi: 10.1016/s0003-9861(02)00696-3. [DOI] [PubMed] [Google Scholar]
- 41.Giustarini D, Milzani Aldini G, Carini M, Rossi R, Dalle-Donne I. S-nitrosation versus S-glutathionylation of protein sulfhydryl groups by S-nitrosoglutathione. Antioxid Redox Signal. 2005;7:930–939. doi: 10.1089/ars.2005.7.930. [DOI] [PubMed] [Google Scholar]
- 42.Demasi M, Piassa Fiho GM, Castro LM, Ferreira JC, Rioli V, Ferro ES. Oligomerization of the cysteinyl-rich oligopeptidase EP24.15 is triggered by S-glutathionylation. Free Radic Biol Med. 2008;44:1180–1190. doi: 10.1016/j.freeradbiomed.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Findlay VJ, Townsend DM, Morris TE, Fraser JP, He L, Tew KD. A novel role for sulfiredoxin in the reversal of glutathionylation. Cancer Res. 2006;66:6800–6806. doi: 10.1158/0008-5472.CAN-06-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minelli A, Bellezza I, Conte C, Culig Z. Oxidative stress-related aging: A role for prostate cancer. Biochem Biophys Acta. 2009;1795:83–91. doi: 10.1016/j.bbcan.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Khandrika L, Kumar B, Koul S, Maroni P, Koul H. Oxidative stress in prostate cancer. Cancer Lett. 2009;282:125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee WH, Morion RA, Epstein JI, Brooks JD, Campbell PA, Bova GS, Hsieh WS, Issacs WB, Nelson WG. Cytidine methylation of regulatory sequences near the pi-class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc Natl Acad Sci U S A. 1994;91:11733–11737. doi: 10.1073/pnas.91.24.11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basu HS, Thompson TA, Church DR, Clower CC, Mehraein-Ghomi F, Amlong CA, Martin CT, Woster PM, Lindstrom MJ, Wilding G. A small molecule polyamine oxidase inhibitor blocks androgen-induced oxidative stress and delays prostate cancer progression in the transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2009;69:7689–7695. doi: 10.1158/0008-5472.CAN-08-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cookson M, Reuter V, Linkov I, Fair W. Glutathione S-transefase Pi (GST-pi) class expression by Immunohistochemistry in benign and malignant prostate tissue. J Urol. 1997;157:673–676. [PubMed] [Google Scholar]
- 49.Effert PJ, McCoy RH, Walther PJ, Liu ET. p53 gene alterations in human prostate carcinoma. J Urol. 1993;150:257–261. doi: 10.1016/s0022-5347(17)35458-7. [DOI] [PubMed] [Google Scholar]
- 50.Di Domenico F, Cenini G, Sultana R, Perluigi M, Uberti D, Memo M, Butterfield DA. Glutathionylation of pro-apoptotic protein p53 in Alzheimer's disease brain: Implications for AD pathogenesis. Neurochem Res. 2009;34:727–733. doi: 10.1007/s11064-009-9924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western blot showing the induction of glut-p53 protein in HCT116 cells after treatment with diamide (DA, 0.5 mM for 30 min). The lower panel shows the p53 levels as detected by DO1 monoclonal antibody for p53 in HCT116 cells treated as above.
Immunofluorescence showing the p53 protein levels in untreated HCT116 cells (A), cells treated with 0.6 mM diamide for 30 min (B), 0.4 mM H2O2 for 30 min (C), 5 μM doxorubicin for 5h (D), and 10 μM cisplatin for 5h (E). After the respective treatments, cells were fixed using 4% paraformaldehyde and incubated with p53 (DO1) monoclonal antibody (1:200 dilution) followed by goat anti mouse Alexa Flour 488 secondary antibody. The cells were photographed using an Olympus IX 81 microscope. Independent experiments revealed similar patterns of staining.