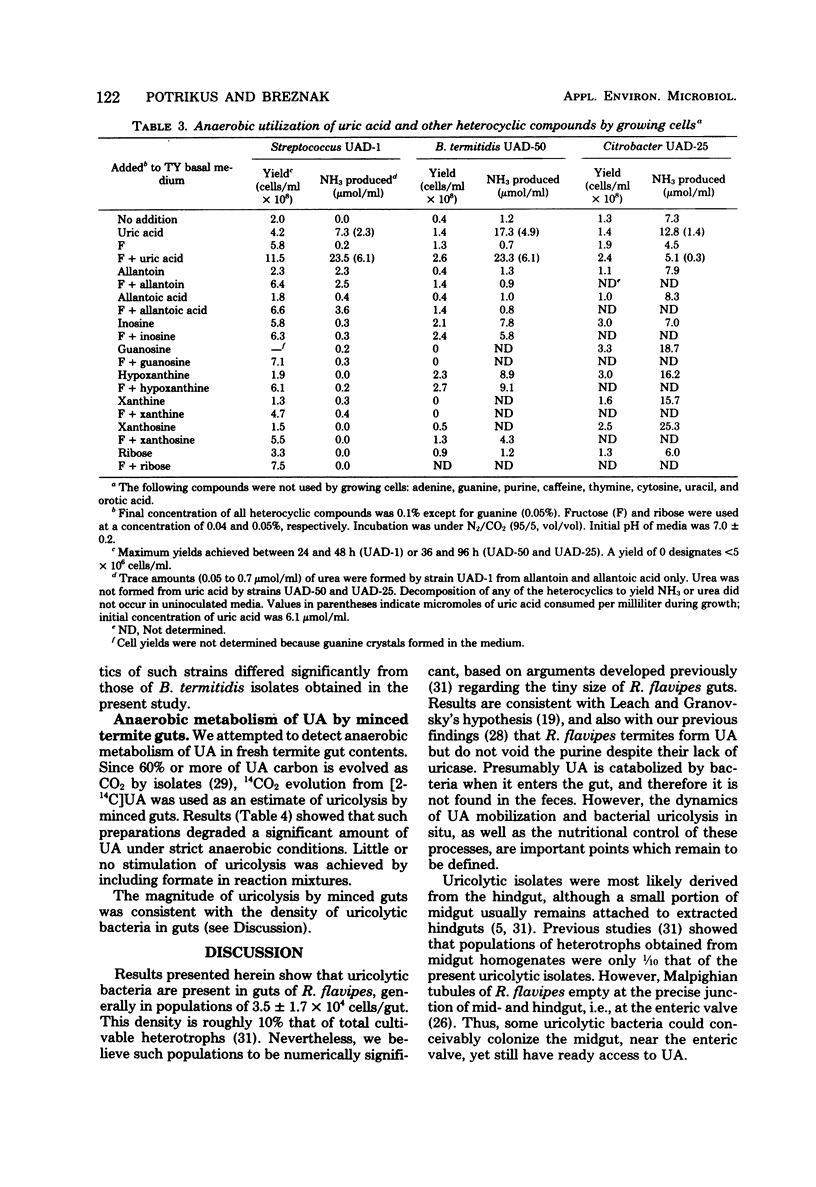
Abstract
Uricolytic bacteria were present in guts of Reticulitermes flavipes in populations up to 6 × 104 cells per gut. Of 82 strains isolated under strict anaerobic conditions, most were group N Streptococcus sp., Bacteroides termitidis, and Citrobacter sp. All isolates used uric acid (UA) as an energy source anaerobically, but not aerobically, and NH3 was the major nitrogenous product of uricolysis. However, none of the isolates had an absolute requirement for UA. Utilization of heterocyclic compounds other than UA was limited. Fresh termite gut contents also degraded UA anaerobically, as measured by 14CO2 evolution from [2-14C]UA. The magnitude of anaerobic uricolysis [0.67 pmol of UA catabolized/(gut × h)] was entirely consistent with the population density of uricolytic bacteria in situ. Uricolytic gut bacteria may convert UA in situ to products usable by termites for carbon, nitrogen, energy, or all three. This possibility is consistent with the fact that R. flavipes termites from UA, but they do not void the purine in excreta despite the lack of uricase in their tissues.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Impey C. S. The occurence and properties of uric acid decomposing anaerobic bacteria in the avian caecum. J Appl Bacteriol. 1974 Sep;37(3):393–409. doi: 10.1111/j.1365-2672.1974.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Breznak J. A., Pankratz H. S. In situ morphology of the gut microbiota of wood-eating termites [Reticulitermes flavipes (Kollar) and Coptotermes formosanus Shiraki]. Appl Environ Microbiol. 1977 Feb;33(2):406–426. doi: 10.1128/aem.33.2.406-426.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznak J. A. Symbiotic relationships between termites and their intestinal microbiota. Symp Soc Exp Biol. 1975;(29):559–580. [PubMed] [Google Scholar]
- Donnellan J. F., Kilby B. A. Uric acid metabolism by symbiotic bacteria from the fat body of Periplaneta americana. Comp Biochem Physiol. 1967 Jul;22(1):235–252. doi: 10.1016/0010-406x(67)90184-3. [DOI] [PubMed] [Google Scholar]
- Furano A. V. A very rapid method for washing large numbers of precipitates of proteins and nucleic acids. Anal Biochem. 1971 Oct;43(2):639–640. doi: 10.1016/0003-2697(71)90300-9. [DOI] [PubMed] [Google Scholar]
- Holligan P. M., Gooday G. W. Symbiosis in Convoluta roscoffenis. Symp Soc Exp Biol. 1975;(29):205–227. [PubMed] [Google Scholar]
- Jeon K. W. Simple method for staining and preserving epoxy resin-embedded animal tissue sections for light microscopy. Life Sci. 1965 Oct;4(19):1839–1841. doi: 10.1016/0024-3205(65)90064-0. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach J. G., Granovsky A. A. NITROGEN IN THE NUTRITION OF TERMITES. Science. 1938 Jan 21;87(2247):66–67. doi: 10.1126/science.87.2247.66-a. [DOI] [PubMed] [Google Scholar]
- Mead G. C. Anaerobic utilization of uric acid by some group D streptococci. J Gen Microbiol. 1974 Jun;82(2):421–423. doi: 10.1099/00221287-82-2-421. [DOI] [PubMed] [Google Scholar]
- Potrikus C. J., Breznak J. A. Anaerobic degradation of uric Acid by gut bacteria of termites. Appl Environ Microbiol. 1980 Jul;40(1):125–132. doi: 10.1128/aem.40.1.125-132.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrikus C. J., Breznak J. A. Nitrogen-fixing Enterobacter agglomerans isolated from guts of wood-eating termites. Appl Environ Microbiol. 1977 Feb;33(2):392–399. doi: 10.1128/aem.33.2.392-399.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Schultz J. E., Breznak J. A. Cross-Feeding of Lactate Between Streptococcus lactis and Bacteroides sp. Isolated from Termite Hindguts. Appl Environ Microbiol. 1979 Jun;37(6):1206–1210. doi: 10.1128/aem.37.6.1206-1210.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J. E., Breznak J. A. Heterotrophic bacteria present in hindguts of wood-eating termites [Reticulitermes flavipes (Kollar)]. Appl Environ Microbiol. 1978 May;35(5):930–936. doi: 10.1128/aem.35.5.930-936.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


