Abstract
The ω-3 polyunsaturated fatty acid eicosapentaenoic acid (EPA), in the free fatty acid (FFA) form, has been demonstrated to reduce adenoma number and size in patients with familial adenomatous polyposis. However, the mechanistic basis of the antineoplastic activity of EPA in the colorectum remains unclear. We tested the hypothesis that EPA-FFA negatively modulates synthesis of and signaling by prostaglandin (PG) E2 in human colorectal cancer (CRC) cells. EPA-FFA induced apoptosis of cyclooxygenase (COX)-2-positive human HCA-7 CRC cells in vitro. EPA-FFA in cell culture medium was incorporated rapidly into phospholipid membranes of HCA-7 human CRC cells and acted as a substrate for COX-2, leading to reduced synthesis of PGE2 and generation of PGE3. Alone, PGE3 bound and activated the PGE2 EP4 receptor but with reduced affinity and efficacy compared with its “natural” ligand PGE2. However, in the presence of PGE2, PGE3 acted as an antagonist of EP4 receptor-dependent 3′,5′ cyclic adenosine monophosphate induction in naturally EP4 receptor-positive LoVo human CRC cells and of resistance to apoptosis in HT-29-EP4 human CRC cells overexpressing the EP4 receptor. We conclude that EPA-FFA drives a COX-2-dependent “PGE2-to-PGE3 switch” in human CRC cells and that PGE3 acts as a partial agonist at the PGE2 EP4 receptor.
Introduction
Cyclooxygenase (COX)-derived prostaglandin (PG) E2 signaling is believed to play a critical role during colorectal carcinogenesis [1]. In vitro experiments have demonstrated that PGE2 promotes colorectal cancer (CRC) cell proliferation and invasion, as well as resistance to apoptosis [2]. PGE2 also has proangiogenic properties and may downregulate the host antitumor immune response [2]. In vivo, PGE2 has been demonstrated to drive intestinal tumorigenesis in the ApcMin/+ mouse model of familial adenomatous polyposis (FAP) [3] and in a rat model of carcinogen-induced CRC [4].
Epidemiological evidence and preclinical data suggest that omega (ω)-3 polyunsaturated fatty acids (PUFAs), which are found in large quantities in fish such as salmon and mackerel, have anti-CRC activity [5]. The mechanism(s) by which the main ω-3 PUFAs in dietary fish oil, namely 20:5ω3 eicosapentaenoic acid (EPA) and 22:6ω3 docosa-hexaenoic acid (DHA), have antineoplastic activity remains unclear [6]. One valid hypothesis is that the anti-CRC activity of EPA is explained by negative modulation of COX-PGE2 signaling.
In “western” diets, the predominant substrate for both COX isoforms (“constitutive“ COX-1 and “inducible” COX-2) is the ω-6 PUFA 20:4ω6 arachidonic acid (AA), from which two-series PGs such as PGE2 are synthesized [7]. However, EPA can incorporate into the phospholipid bilayer, displace AA, and acts as an alternative substrate for the COX enzymes [7]. EPA turnover in vitro (measured as Vmax) by COX-1 and COX-2 is 10% and 35%, respectively, of that for AA [7]. Importantly, COX converts EPA into PGH3, rather than PGH2, leading to production of equivalent three-series PGs such as PGE3 [7]. It has been demonstrated that EPA administration to COX-2-positive A549 human lung cancer cells and BxPC-3 human pancreatic cancer cells leads to synthesis of PGE3 at the expense of PGE2 [8,9]. More recently, ω-3 fish oil administration to rats was shown to be associated with the appearance of PGE3 and a reduction in PGE2 levels in colorectal mucosa [10], confirming that a “PGE2-to-PGE3 switch” can occur in vivo.
Although a reduction in tissue levels of protumorigenic PGE2 alone could explain the anticancer properties of EPA, it is possible that PGE3 per se could contribute to the antitumorigenic activity of EPA. Consistent with this concept, Yang et al. [8] have demonstrated that exogenous PGE3 increased apoptosis of A549 human lung cancer cells. However, the mechanistic basis of the antiproliferative activity of PGE3 was not explored in that study.
PGE2 signals through a family of four G protein-coupled receptors termed EP1 to EP4 (reviewed in Sugimoto and Narumiya [11]). At late stages of colorectal carcinogenesis (primary CRC growth and metastasis), preclinical evidence suggests a predominant role for the EP4 receptor in the protumorigenic activity of PGE2 [12]. EP4 receptor expression is increased in mouse and human CRCs compared with normal colorectal mucosa [13,14]. Moreover, PGE2-EP4 receptor signaling promotes tumorigenic behavior (proliferation, resistance to apoptosis, motility, and invasion) of human colorectal adenoma and CRC cells in vitro [13,14], whereas pharmacological antagonism of PGE2-EP4 receptor signaling has been demonstrated to inhibit transplantable CRC cell tumor growth and liver metastasis in Balb/c mice [15]. Funahashi et al. [9] recently concluded that EPA had antiproliferative activity against BxPC-3 human pancreatic cancer cells through a mechanism involving the EP4 receptor on the basis that EPA activity was abrogated by the selective EP4 receptor antagonist ONO-AE3-208.
We have recently reported that EPA, in the free fatty acid (FFA) form (which is better absorbed from the human small intestine than EPA in the ethyl ester or triglyceride form [16]), 2 g daily for 6 months reduces rectal polyp number and size in a randomized controlled trial (RCT) of patients with FAP [17]. The aim of this study was to investigate the mechanistic basis of the antineoplastic activity of EPA-FFA in the colorectum by testing the hypotheses that EPA-FFA drives a switch from synthesis of PGE2 to PGE3 in human CRC cells and that PGE3 acts through inhibition of EP4 receptor signaling, thereby contributing to the apoptotic activity of EPA against human CRC cells.
Materials and Methods
Reagents and Antibodies
EPA-FFA and Miglyol 810 (mixed capric and capryllic acid medium-chain triglycerides, which were used as the placebo in the RCTof EPA in FAP patients [17]) were kindly provided by SLA Pharma (Watford, UK). EPA-FFA was extracted from 500 mg of enteric-coated ALFA capsules using a sterile needle and diluted 1:100 in 95% (vol./vol.) ethanol immediately before use. A working solution of EPA was always freshly prepared from a new capsule to avoid auto-oxidation. AA (Sigma-Aldrich, Poole, UK) was dissolved in 95% (vol./vol.) ethanol as a 200-mM stock solution and stored at -20°C. PGE2 (20 mM stock solution in dimethyl sulfoxide [DMSO]) was also obtained from Sigma-Aldrich. PGE3 (10 mM stock solution in DMSO) was obtained from Cayman Chemical Co (Ann Arbor, MI). Working solutions of PGE3 were always freshly prepared from frozen stock that was then discarded to avoid freeze-thaw degradation. All other EP receptor agonists and antagonists were used as described previously [14]. SC-236 was a kind gift from Pfizer, Inc (Groton, CT). Methoxyamine HCl was obtained from Sigma-Aldrich, and all high-performance liquid chromatography-grade solvents were purchased from Fisher Scientific (Loughborough, UK).
Human embryonic kidney 293 cell EP4 and EP2 receptor membranes and [3H]-PGE2 (185.6 Ci/mmol) were obtained from PerkinElmer LAS Ltd (Bucks, UK).
Polyclonal rabbit antibody against the human EP4 receptor (N-terminal) was obtained from Cayman Chemical Co. Polyclonal goat anti-COX-2 antibody (C-19) was obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA), and mouse monoclonal anti-chicken β-actin antibody was obtained from Sigma-Aldrich. IR Alexa Fluor 488-and Alexa Fluor 594-conjugated secondary antibodies were obtained from Molecular Probes (Invitrogen, Paisley, UK). IRDye 700- and IRDye 800-conjugated secondary antibodies were obtained from Tebu-Bio Ltd (Peterborough, UK).
Cell Culture
Human CRC cell lines were all obtained from ECACC (Porton Down, UK) except mouse colon 26 (MC-26) cells, which were obtained from the National Cancer Institute (Frederick, MD). All cells were cultured in RPMI 1640 medium containing Glutamax supplemented with 10% (vol./vol.) heat-inactivated fetal bovine serum (all Invitrogen), except LoVo cells, which were cultured in Ham's F12 medium (Invitrogen) containing Glutamax supplemented with 10% (vol./vol.) heat-inactivated fetal bovine serum. All cells were cultured at 37°C in a humidified atmosphere containing 5% CO2. Cells were routinely subcultured using 0.25% (wt./vol.) trypsin (Invitrogen). Viable cells were counted using a hemocytometer in the presence of 0.04% (vol./vol.) trypan blue (Sigma-Aldrich).
Measurement of Apoptosis
Apoptosis was measured by counting 4% (wt./vol. in phosphate-buffered saline) paraformaldehyde-fixed, nonadherent HCA-7 and HT-29 human CRC cells as described and validated by us and other groups [14,18–25].
Immunoblot Analysis
Cells were lysed in ice-cold RIPA buffer (Sigma-Aldrich) and then passed through a QIAshredder homogenizer (Qiagen Ltd, Crawley, UK). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed using 15 µg of total protein, and protein was transferred to polyvinylidene fluoride membranes. Membranes were blocked in Odyssey Blocking Buffer (LI-COR Biosciences Ltd, Cambridge, UK) for 1 hour before incubating with anti-COX-2 antibody diluted 1:1000 in 1:1 Odyssey Blocking Buffer-Tween-Tris-buffered saline (TTBS) for 1 hour at 25°C. Membranes were washed 3 x 3 minutes in TTBS followed by incubation with IRDye 700-labeled secondary antibody (1:2500) diluted in 1:1 Odyssey Blocking buffer-TTBS. After 3 x 3 minutes washes with TTBS, membranes were incubated with anti-β-actin antibody (1:20,000), and IRDye 800-labeled secondary antibody (1:2500) was diluted in 1:1 Odyssey Blocking buffer-TTBS as above. Membranes were visualized using an Odyssey Infrared Imaging System and Odyssey v1.2 software (LI-COR).
Indirect Immunofluorescence
Immunofluorescence studies were performed with primary antibodies (anti-EP4, 1:25; anti-V5, 1:200) diluted in PBS containing 1% (wt./vol.) dried skimmed milk and 0.1% (vol./vol.) of Tween-20 as described [14], except that coverslips were mounted in Prolong Gold with 4′,6 diamidino-2-phenylindole (Invitrogen).
Assay of Intracellular 3′,5′ Cyclic Adenosine Monophosphate Content
A total of 1 x 106 CRC cells were incubated overnight in triplicate 35-mm wells. Cells were pretreated with 50 µM rolipram for 10 minutes before addition of 10 µM ONO-AE3-208 (ONO Pharmaceutical Co Ltd, Osaka, Japan) for 45 minutes before addition of EP receptor agonists. Intracellular 3′,5′ cyclic adenosine monophosphate (cAMP) content was assayed using a Biotrak enzyme immunoassay (GE Healthcare Amersham, Amersham, UK) using the nonacetylation protocol.
Measurement of PGE2 and PGE3 Levels in CRC Cell-Conditioned Medium
PGE2 and PGE3 levels were measured in CRC cell-conditioned medium by high-sensitivity immunoassay (R&D Systems Ltd, Abingdon, UK) or liquid chromatography-tandem mass spectrometry (LC/MS/MS) based on the method of Murphey et al. [26]. PGE2 and PGE3 standards were prepared by converting PGE2 and PGE3 to their O-methyloxime derivatives. Methoximated samples were diluted to a total volume of 10 ml with water adjusted to pH 3.0. The aqueous sample was applied to a C-18 Isolute SPE cartridge (Kinesis, St Neots, Cambridgeshire, UK), preconditioned with 5 ml of methanol and 5 ml of water (pH 3.0). The SPE cartridge was washed with 10 ml of water (pH 3.0) and 10 ml of heptane. PGs were then eluted from the cartridge with 10 ml of ethyl acetate, and any residual aqueous material was removed from the eluate by aspiration. The eluate was evaporated using a centrifugal evaporator, and the dried residue was resuspended in 25 µl of 95:5, 0.1 (vol./vol.) acetic acid-acetonitrile (mobile phase A).
LC was performed on an ACQUITY UPLC BEH C8 Column, 2.1 x 100 mm, 1.7 µm (Waters, Milford, MA) attached to an ACQUITY Ultra Performance LC (UPLC) System. Samples were separated by a gradient of 60% to 10% of mobile phase A in mobile phase B (50:50, 0.1 [vol./vol.] acetic acid-acetonitrile) for 4 minutes (total sample run time, 8 minutes) at a flow rate of 300 µl/min before delivery to a bench-top tandem quadrupole mass spectrometer (Quattro Premier XE; Waters) operating in multiple reaction monitoring mode. Capillary and cone voltages were 3.5 kV and 20 V, respectively, with a collision voltage of 16 V, with source and desolvation temperatures of 150°C and 300°C, respectively. Multiple reaction monitoring channels were established for PGE2 (m/z 380 → 267.9 and 380.0) and PGE3 (m/z 378 → 265.9, 266.9, and 378.0).
Measurement of Fatty Acid Phospholipid Membrane Content
Fatty acid content in cell membranes was measured by gas chromatography-mass spectrometry as described [17].
[3H]-PGE2 Binding Assay
EP4 receptor (20 µg/well) and EP2 receptor (10 µg/well) membrane preparations were incubated with [3H]-PGE2, with or without cold PGs, in 50 mM Tris pH 6.0 with 0.5% (wt./vol.) bovine serum albumin in 200-µl reactions in a 96-well polystyrene microplate (PerkinElmer) at 27°C for 1 hour. The samples were transferred to a GF/B filter (PerkinElmer) presoaked in PBS with 0.5% (wt./vol.) bovine serum albumin using a Tomtec cell harvester, and unbound [3H]-PGE2 was removed by washing eight times with ice-cold 50 mM Tris-HCl pH 7.4. The filter was left to dry overnight at room temperature before counting using a liquid scintillation counter (Wallac 1450 MicroBeta, PerkinElmer).
Statistical Analysis
The significance of differences associated with EPA and PG treatments was tested by one-way analysis of variance with least significant difference post hoc analysis. Significance was assumed if P < .05.
Results
EPA-FFA Induces Apoptosis of HCA-7 Human CRC Cells
We measured apoptosis of CRC cells by the established technique of nonadherent cell counting, which has been previously validated by fluorescence microscopy of 4′,6 diamidino-2-phenylindole-stained cells [14,18–25] and is recognized to correlate strongly with other apoptosis assays based on measurement of caspase-3/7 activity [25] and appearance of cell surface annexin V [14].
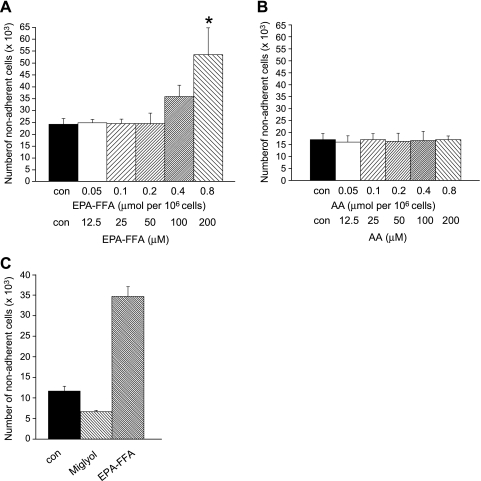
EPA-FFA promoted apoptosis of HCA-7 human CRC cells in a concentration-dependent manner (Figure 1A). EPA-FFA exposure equivalent to 0.8 µmol/106 cells, which represents addition of a 200-µM absolute concentration of EPA-FFA into aqueous cell culture medium, was associated with a significant increase in apoptosis of HCA-7 human CRC cells above control cells (Figure 1A). By contrast, an equivalent level of exposure to the ω-6 PUFA AA did not induce apoptosis of HCA-7 human CRC cells (Figure 1B). Addition of the same vol./vol. amount of capric/caprylic acid medium-chain triglycerides (Miglyol 810), which were used as the placebo in the recent RCT of EPA [17], to cell culture medium was also not associated with increased apoptosis of HCA-7 human CRC cells (Figure 1C).
Figure 1.
EPA-FFA induces apoptosis of HCA-7 human CRC cells. (A) Apoptosis of HCA-7 human CRC cells (measured by nonadherent cell counting; [14]) after treatment with increasing amounts of EPA-FFA (quoted as micromoles per 106 cells exposure and the equivalent working micromolar concentration below that) or the equivalent dilution (0.06% vol./vol.) of 95% (vol./vol.) ethanol carrier alone (con) to 0.8 µmol of EPA-FFA/106 cells (200 µM) for 24 hours after overnight incubation of 0.5 x 106 CRC in triplicate 35-mm wells. Columns and bars represent the mean and SEM of triplicate values, respectively. Data are representative of three independent experiments. *P < .01 for the comparison with con and EPA-FFA exposure less than 0.4 µmol/106 cells (100 µM). (B) Apoptotic HCA-7 human CRC cell counts after treatment with increasing amounts of AA (quoted as micromoles per 106 cells or the micromolar concentration) or the equivalent dilution (0.06% vol./vol.) of 95% (vol./vol.) ethanol carrier alone (con) to 0.8 µmol of AA/106 cells (200 µM) for 24 hours. Columns and bars represent the mean and SEM of triplicate values, respectively. (C) Comparison of the proapoptotic activity of EPA-FFA and Miglyol 810 (both 0.8 µmol/106 cells [200 µM] in 95% [vol./vol.] ethanol) on HCA-7 human CRC cells. Con represents the equivalent dilution (0.06% vol./vol.) of 95% (vol./vol.) ethanol alone. Columns and bars represent the mean and SEM of triplicate values, respectively.
EPA-FFA in Aqueous Cell Culture Medium Is Rapidly Incorporated into CRC Cell Membranes
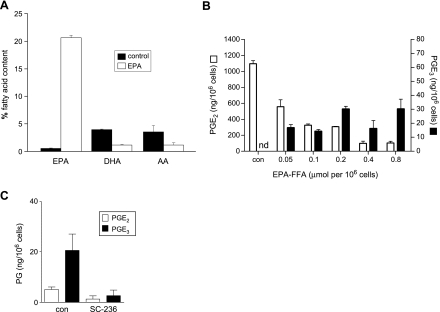
During the same 24-hour period, we demonstrated that EPA-FFA was incorporated into HCA-7 human CRC cell phospholipid membranes (Figure 2A). Exposure to EPA-FFA (0.8 µmol/106 cells) for 24 hours led to a large increase in EPA content so that EPA represented approximately 20% of the total PUFA content (Figure 2A). There was also a corresponding decrease in membrane AA content (Figure 2A). However, there was no evidence of EPA-DHA conversion in HCA-7 human CRC cells during the short-term exposure to EPA-FFA (Figure 2A).
Figure 2.
EPA-FFA incorporates into cell membranes and alters COX-2-dependent E-type PG synthesis in HCA-7 human CRC cells. (A) Changes in the fatty acid content of HCA-7 human CRC cell membranes after exposure to EPA-FFA (0.8 µmol/106 cells or 200 µM) or an equivalent dilution (0.06% [vol./vol.]) of 95% (vol./vol.) ethanol carrier alone (control) for 24 hours. Columns and bars represent the mean and SEM of triplicate values, respectively. (B) PGE2 and PGE3 levels in medium conditioned by HCA-7 human CRC cells in the absence or presence of EPA-FFA. PGE2 and PGE3 levels were measured by LC/MS/MS in medium conditioned by HCA-7 human CRC cells for 4 hours after 24 hours of prior exposure to EPA-FFA. Con represents the equivalent dilution (0.06% vol./vol.) of 95% (vol./vol.) ethanol alone. nd indicates not detected. Columns and bars represent the mean and SEM of triplicate values, respectively. Data are representative of three independent experiments. (C) The effect of SC-236 (1 µM) on EPA-FFA-dependent E-series PG synthesis by HCA-7 human CRC cells. SC-236 was added to the culture medium 90 minutes before EPA-FFA exposure (0.8 µmol/106 cells or 200 µM) for 24 hours. Fresh medium was conditioned for 4 hours as in panel B. The presence of SC-236 did not significantly change the viable cell number at the end of the experiment (data not shown). Columns and bars represent the mean and SEM of triplicate values, respectively.
EPA Incorporation Leads to Changes in E-type PG Synthesis by HCA-7 Human CRC Cells
HCA-7 human CRC cells constitutively express high levels of COX-2 protein and generate measurable levels of PGE2 in conditioned medium [18]. Therefore, we investigated whether the proapoptotic activity linked to EPA incorporation was associated with the simultaneous changes in E-type PG synthesis in this human CRC cell line using LC/MS/MS (Figure W1).
Exposure to EPA-FFA for 24 hours was associated with a dose-dependent decrease in PGE2 synthesis during a subsequent 4-hour period (Figure 2B), such that PGE2 synthesis was inhibited by greater than 90% by amounts of EPA-FFA that promoted apoptosis (0.4–0.8 µmol/106 cells). In the presence of culture medium containing only the ethanol carrier, PGE3 was not detected in HCA-7 human CRC cell-conditioned medium (Figure 2B). However, addition of EPA-FFA stimulated PGE3 synthesis by HCA-7 human CRC cells (Figure 2B). Levels of PGE3 in conditioned medium were an order of magnitude lower than the corresponding values for PGE2. Moreover, there was no observable concentration-response relationship for PGE3 synthesis. “Chronic” treatment of HCA-7 human CRC cells with EPA-FFA for 14 days was not associated with any further increase in PGE3 levels in the conditioned medium (data not shown), suggesting that the PGE3 levels measured in short-term experiments after a 24-hour EPA-FFA exposure represent maximal PGE3 synthesis. EPA-FFA-dependent PGE3 synthesis was also observed in MC-26 mouse CRC cells, which also constitutively express high levels of COX-2 (data not shown). Pretreatment of HCA-7 human CRC cells with the selective COX-2 inhibitor SC-236 reduced PGE3 synthesis by 87%, confirming that EPA-FFA-induced PGE3 synthesis is predominantly a COX-2-dependent process (Figure 2C).
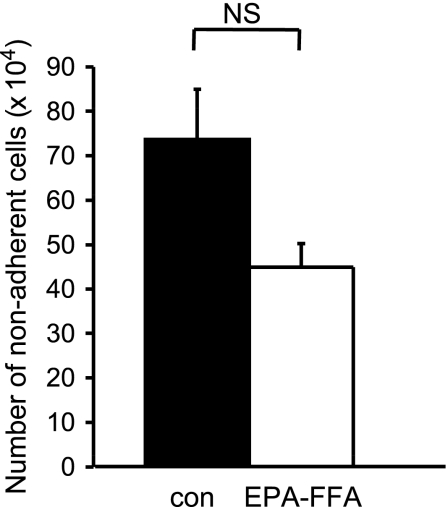
Consistent with a role for changes in E-type PG levels in the proapoptotic activity of EPA, HT-29-EP4 human CRC cells, which do not generate detectable amounts of PGE2 or PGE3 in conditioned medium [14], in either the absence or presence of EPA-FFA (data not shown), were resistant to EPA-FFA-induced apoptosis (Figure 3).
Figure 3.
HT-29-EP4 human CRC cells are resistant to EPA-FFA-induced apoptosis. Apoptosis of HT-29-EP4 human CRC cells measured by nonadherent cell counting [14] after treatment with 0.8 µmol per 106 cells (200 µM) EPA-FFA or 95% (vol./vol.) ethanol carrier alone (0.06% [vol./vol.]) for 24 hours after overnight incubation of 1 x 106 CRC in triplicate 35-mm wells. Columns and bars represent the mean and SEM of triplicate values, respectively. NS indicates not statistically significant (unpaired t test).
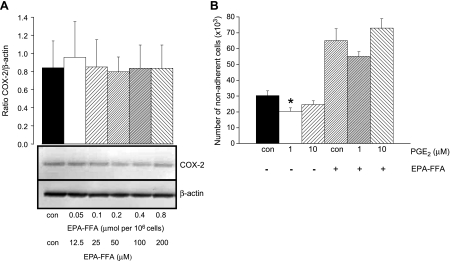
Proapoptotic Activity of EPA-FFA Is Not Explained by Decreased COX-2 Protein Expression or Reduced PGE2 Synthesis
It has previously been reported that EPA treatment decreases COX-2 gene expression in serum-starved HT-29 human CRC cells [27]. Therefore, reduced COX-2 protein levels could account for reduced PGE2 synthesis by HCA-7 human CRC cells treated with EPA-FFA. However, EPA exposure for 24 hours was not associated with changes in levels of COX-2 protein in HCA-7 human CRC cells (Figure 4A). Alternatively, reduced PGE2 synthesis could explain the proapoptotic activity of EPA-FFA. Therefore, we tested whether exogenous PGE2 “rescued” HCA-7 human CRC cells from EPA-FFA-induced apoptosis. In the absence of EPA-FFA, exogenously added PGE2 significantly reduced spontaneous apoptosis of HCA-7 human CRC cells (Figure 4B). Addition of 1 µM PGE2 partially “rescued” HCA-7 human CRC cells from the effect of EPA-FFA (a 15% decrease in apoptosis), but the presence of 10 µM PGE2 did not alter EPA-FFA-induced apoptosis (Figure 4B).
Figure 4.
Decreased COX-2 protein expression or decreased PGE2 synthesis alone does not explain the proapoptotic activity of EPA-FFA in human CRC cells. (A) Immunoblot analysis of COX-2 and β-actin protein levels in HCA-7 human CRC cells. Total protein cell lysates were obtained after treatment with 0.06% (vol./vol.) 95% (vol./vol.) ethanol alone (con) or differing amounts of EPA-FFA for 28 hours. Columns and bars represent the mean and SEM of fluorescence COX-2/β-actin ratios, respectively, in arbitrary units from two separate experiments. A representative immunoblot is included below. (B) Apoptosis of HCA-7 human CRC cells measured by nonadherent cell counting. Cells were cultured for 24 hours in the absence (0.06% [vol./vol.] dilution of 95% [vol./vol.] ethanol alone) or presence of 0.8 µmol per 106 cells (200 µM) EPA-FFA with or without PGE2. Columns and bars represent the mean and SEM of triplicate values, respectively. *P < .05 for the comparison with control cells.
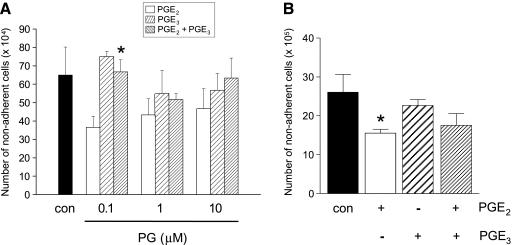
PGE3 Antagonizes the Protumorigenic Activity of PGE2 in HT-29-EP4 Human CRC Cells
An alternative hypothesis is that PGE3, alone or in combination with reduced levels of protumorigenic PGE2, underlies the proapoptotic activity of EPA-FFA. For initial experiments involving addition of exogenous PGE2 and/or PGE3, we used HT-29-EP4 human CRC cells [14], thereby avoiding the confounding effect of endogenous PGE2 production by CRC cells. As expected, PGE2 decreased spontaneous apoptosis of HT-29-EP4 cells compared with cells treated with ethanol carrier alone (Figure 5A), as described previously [14]. However, equimolar amounts of PGE3 alone did not significantly affect apoptosis of HT-29-EP4 cells (Figure 5A). Importantly, PGE3 abrogated the antiapoptotic activity of PGE2 when the two E-type PGs were present together at equimolar concentrations. This phenomenon was most prominent at a PG concentration of 0.1 µM (Figure 5A). Similar observations were made with HCA-7 human CRC cells, in which 1 µM PGE2, but not PGE3, had significant antiapoptotic activity (Figure 5B). In a similar manner to HT-29-EP4 cells, the combination of PGE2 and PGE3 did not have an additive effect on the propensity of HCA-7 human CRC cells to apoptosis (Figure 5B). However, PGE3 did not antagonize PGE2 activity in HCA-7 human CRC cells to the degree observed in HT-29-EP4 cells (Figure 5B), perhaps due to the high levels of PGE2 already produced endogenously by these cells.
Figure 5.
PGE3 antagonizes the protumorigenic activity of PGE2 in human CRC cells. (A) Apoptosis of HT-29-EP4 human CRC cells measured by nonadherent cell counting after treatment with PGE3 and PGE2, alone or in combination, for 24 hours. Columns and bars represent the mean and SEM of triplicate values for each condition, respectively. Con represents DMSO carrier (0.1% vol./vol.) alone. *P < .05 for the difference from cells treated with 0.1 µM PGE2 alone. (B) Apoptosis of HCA-7 human CRC cells measured by nonadherent cell counting after treatment with 1 µM PGE3 and PGE2, alone or in combination, for 24 hours. Columns and bars represent the mean and SEM of triplicate values for each condition, respectively. Con represents DMSO carrier (0.1% vol./vol.) alone. *P < .05 for the comparison with control cells.
PGE3 Directly Binds and Activates the EP4 Receptor on Human CRC Cells
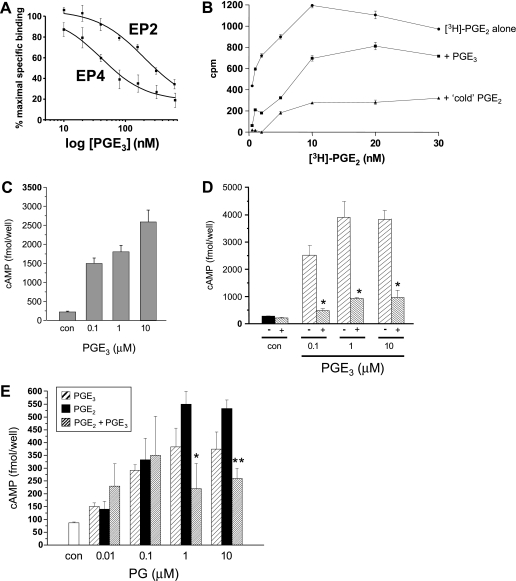
Given the close structural similarity between PGE2 and PGE3, we hypothesized that PGE3 antagonizes PGE2 activity by binding to PGE2 EP receptors. Evidence is strongest for a role for EP4 and EP2 receptors in PGE2 signaling during colorectal carcinogenesis [12–14,28,29]. Consistent with the concept that PGE3 abrogates PGE2 signaling through EP receptors, PGE3 competed with PGE2 (20 nM) for binding to purified human EP4 receptor (half maximal inhibitory concentration [IC50] = 48 nM) and, to a lesser extent, EP2 receptor (IC50 = 310 nM) membrane preparations in radioligand binding assays (Figure 6A).However, direct comparison of PGE3 and PGE2 in a radioligand competition assay using a purified human EP4 receptor preparation demonstrated that PGE3 bound the EP4 receptor with less affinity than the “natural” ligand PGE2 across a range of PGE2 concentrations (Figure 6B).
Figure 6.
PGE3 binds and acts as a partial agonist at the EP4 receptor in human CRC cells. (A) The effect of “cold” PGE3 on [3H]-PGE2 (20 nM) binding to purified EP4 and EP2 receptor membranes. Data represent the mean and SEM percentage specific binding (n = 3) compared with a “no PGE3” control. (B) Competition of “cold” PGE3 or PGE2 (20 nM) with [3H]-PGE2 binding to purified EP4 receptor membranes. Data represent the mean and SEM of triplicate measurements in counts per minute (cpm). (C) The effect of PGE3 on cAMP levels in HT-29-EP4 human CRC cells at 5 minutes. Cells were preincubated with the phosphodiesterase inhibitor rolipram. Columns and bars represent the mean and SEM of triplicate values for each condition, respectively. Con represents 0.1% (vol./vol.) of DMSO carrier alone. Levels of cAMP after stimulation with all three concentrations of PGE3 were significantly different from control cAMP levels (P < .001). (D) The effect of PGE3 alone on cAMP levels in LoVo human CRC cells at 5 minutes, in the absence (-) or presence (+) of an EP4 receptor antagonist (10 µM ONO-AE3-208 for 60 minutes). Columns and bars represent the mean and SEM of triplicate values for each condition, respectively. Con represents 0.1% (vol./vol.) of DMSO carrier alone. *P < .01 for the differences in cAMP levels in the absence and presence of ONO-AE3-208. (E) The effect of PGE3 and PGE2, alone or in combination, on cAMP levels in LoVo human CRC cells at 5 minutes. Columns and bars represent the mean and SEM of triplicate values for each condition. Con represents 0.1% (vol./vol.) of DMSO carrier alone. *P < .05 for the difference in cAMP levels between cells treated with PGE2 alone versus equimolar amounts of PGE2 and PGE3. **P < .01 for the difference in cAMP levels between cells treated with PGE2 alone versus equimolar amounts of PGE2 and PGE3.
Moreover, PGE3 treatment led to a concentration-dependent rise in cAMP levels in our HT-29-EP4 human CRC cell model of EP4 receptor overexpression [14], confirming that PGE3 can act as an EP4 receptor agonist (Figure 6C).We also tested the effect of PGE3 on LoVo human CRC cells, which do not synthesize PGE2 (detection limit 2 pg/ml per 105 cells; data not shown) and which naturally exhibit predominant PGE2-EP4 receptor signaling (Figure W2). PGE3 also induced a concentration-dependent rise in cAMP levels in EP4 receptor-positive LoVo human CRC cells (Figure 6D). Cyclic AMP induction by PGE3 was inhibited by preincubation with the selective EP4 receptor antagonist ONO-AE3-208 [14], thus confirming EP4 receptor agonist properties of PGE3 in a second “natural” human CRC cell line (Figure 6D).
PGE3 Is a Partial Agonist of the EP4 Receptor in Human CRC Cells
A direct comparison of EP4 receptor activation by PGE2 and PGE3 in LoVo human CRC cells demonstrated that, although PGE3 is an agonist at the EP4 receptor, PGE3 is less potent at activating the EP4 receptor than PGE2 (Figure 6E) with a 50% effective dose (ED50) of less than 100 nM, consistent with the lower binding affinity of PGE3 noted earlier (Figure 6B). In keeping with the effect of PGE3 on human CRC cell apoptosis (Figure 5), exposure of equimolar amounts of PGE2 and PGE3 to LoVo human CRC cells was not associated with an additive effect on cAMP elevation (Figure 6E). Instead, the combination of PGE3 and PGE2 was associated with a lower cAMP response than either ligand alone, suggesting a negative interaction between the three- and two-series PGs (Figure 6E). Similar observations were made in HT-29-EP4 human CRC cells (data not shown). Therefore, PGE3 is a partial agonist at the EP4 receptor such that PGE3 alone acts as an EP4 agonist, but in the presence of the natural ligand PGE2, it behaves as an EP4 receptor antagonist. The partial agonist activity of PGE3 on EP4 receptor-dependent cAMP elevation is consistent with a decrease in the antiapoptotic activity of PGE2 in the presence of PGE3 that was observed in EP4 receptor-positive HT-29-EP4 (Figure 5A).
Discussion
We report, for the first time, that the ω-3 PUFA EPA, in the FFA form, reduces COX-2-dependent PGE2 synthesis and simultaneously drives a “PGE2-to-PGE3 switch” in human CRC cells in vitro. We have also elucidated that PGE3 antagonizes protumorigenic signaling at the EP4 receptor by its natural ligand PGE2 in human CRC cells. The relative contributions of reduced PGE2 synthesis and PGE3 activity to the anti-CRC effects of EPA in vivo remain to be determined. We did not address potential COX-independent mechanisms of the antineoplastic activity of EPA [5,6] in our experiments, including modulation of lipid raft behavior and lipid peroxidation.
Although EPA has previously been demonstrated to induce apoptosis of human CRC cells [5,6], ours is the first report concerning the proapoptotic activity of EPA-FFA formulation that has been shown to have antineoplastic effects in humans with FAP [17]. Our data are consistent with the increase in apoptotic cell frequency in normal rectal mucosa, which was observed in a previous phase 2 trial of EPA-FFA in patients with a history of “sporadic” colorectal adenomas [30].
Although EPA can act as a substrate for COX-2 with a KM value similar to AA, enzymatic turnover values are approximately 35% those of AA [31]. Therefore, in the absence of any short-term change in COX-2 protein levels, reduced EPA substrate handling may explain the combination of a marked dose-dependent decrease in PGE2 synthesis with appearance of PGE3 at significantly lower levels than those observed for PGE2. However, other factors are also likely to contribute to “inefficient” E-type PG synthesis when EPA acts as a COX-2 substrate, which may include decreased PGE synthase activity on PGH3 compared with PGH2 [31]. Studies are currently underway in our laboratory that will address whether a similar magnitude “PGE2-to-PGE3 switch” occurs in EPA-FFA-treated mouse and human CRC tissue in vivo. It is interesting to note that in the one published study that has examined whether a tissue “PGE2-to-PGE3 switch” occurs in vivo, colonic mucosal PGE2 levels were reduced by approximately 75%, along with appearance of PGE3 (albeit at much lower concentrations), in rats administered a fish oil- and pectin-containing diet [10].
Another important observation is that EPA, in the FFA form, is incorporated efficiently into membrane phospholipids of human CRC cells when delivered into the aqueous cell culture medium. Our observations concur with those in murine epidermal cells [32], human breast cancer cells [33], and human endothelial cells [34], in which significant EPA incorporation occurred within 24 hours of addition to the cell culture medium in an ethanol carrier. These in vitro findings mirror the increase in the EPA content of rectal mucosa of FAP patients, who received EPA-FFA 2 g daily for 6 months [17]. Not unexpectedly, there was no evidence of ω-3 PUFA interconversion from EPA to the other main ω-3 PUFA DHA in short-term cell culture, which is likely explained by low elongase and Δ6-desaturase activities in intestinal epithelial cells necessary for EPA-DHA conversion [35].
An important component of our study was a functional screen of a panel of human CRC cells for dominant PGE2-EP4 receptor signaling based on the identification of PGE2-dependent cAMP induction that could be inhibited by a selective EP4 receptor antagonist, in combination with immunoreactive protein localization. Identification of dominant PGE2-EP4 receptor activity has significant advantages over previous semiquantitative reverse transcription-polymerase chain reaction measurements of EP receptor messenger RNA expression, which have uncertain functional significance [29,36]. Our functional screen identified LoVo and HCA-7 human CRC cells as exhibiting predominant EP4 receptor-dependent induction of cAMP by PGE2, for use in future in vitro studies of EP4 receptor function during colorectal carcinogenesis, alongside stably transfected HT-29-EP4 cells [14].
Previous studies using stably transfected human embryonic kidney 293 cells expressing human EP receptors have demonstrated that PGE3 acts as a partial agonist at EP1 to EP3 receptors, with the binding affinity and receptor activation potency of PGE3 being significantly less than PGE2 [31]. However, the 3.5-fold decrease in affinity and 6-fold decrease in potency (measured by cAMP induction) of PGE3 (ED50 = 3.5 nM) compared with PGE2 (ED50 = 0.58 nM) at the EP4 receptor in 293 cells failed to reach statistical significance in this study [30]. Our data confirm that PGE3 does indeed act as a partial agonist at the EP4 receptor in human CRC cells, which naturally express the EP4 receptor. PGE3 has been previously reported to act in an EP4 receptor-dependent manner in BxPC-3 human pancreatic cancer cells, although direct PGE3-EP4 receptor binding was not confirmed in this study [9].
Exogenous PGE3 has been reported to decrease proliferation of COX-2-positive human A549 lung cancer cells and BxPC-3 human pancreatic cancer cells [8,9] and decrease invasiveness of COX-2-positive 70W human melanoma cells [37]. However, treatment with PGE3 did not have any significant effect on the proliferation of NIH 3T3 fibroblasts [38] or nontransformed mouse mammary epithelial cells [39] and actually stimulated interleukin 6 production by unactivated mouse RAW264.7 macrophages, in a similar manner to PGE2 [38]. Partial agonist activity of PGE3 at EP receptors may explain these discrepant findings such that exogenously added PGE3 has antagonist properties on a background of ongoing PGE2-dependent signaling in COX-2-positive cells but has stimulatory activity when present alone with cells that do not synthesize PGE2.
Our novel insights into EPA-dependent alterations in E-type PG synthesis and EP receptor signaling in human CRC cells have important implications for future translational human studies of the anti-CRC activity of the ω-3 PUFA EPA. The role of COX-2 in EPA-dependent alterations in PG synthesis suggests that combination therapy of EPA with a COX-2 inhibitor could abrogate the antineoplastic activity of both agents. Consistent with this concept, the selective COX-2 inhibitor celecoxib and the nonselective COX inhibitor indomethacin have both been demonstrated to decrease the proapoptotic and antiproliferative effects of EPA, respectively [8,40]. Therefore, ω-3 PUFA and selective COX-2 inhibitor treatment should only be considered for evaluation as a combination anticancer therapy with caution. Measurement of erythrocyte and/or tissue ω-3 PUFA levels is established as a biomarker of ω-3 PUFA intake and bioavailability [41,42]. However, the degree of membrane ω-3 PUFA incorporation might not necessarily mirror bioactivity. We suggest that measurement of PGE3 (or its stable metabolite) levels in tissue, plasma, or urine should be investigated as a potential biomarker of COX-2-dependent EPA activity and therapeutic response in clinical studies.
Supplementary Material
Acknowledgments
The authors thank Sarah Perry for excellent technical assistance with cell culture and Amanda Race for technical support for LC/MS/MS measurements. Laure Bretsztajn contributed to this work as part of a University of Leeds LURE scholarship.
Abbreviations
- AA
arachidonic acid
- COX
cyclooxygenase
- CRC
colorectal cancer
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FAP
familial adenomatous polyposis
- FFA
free fatty acid
- PG
prostaglandin
- RCT
randomized controlled trial
- TTBS
Tween-Tris-buffered saline
Footnotes
This work was supported by Yorkshire Cancer Research, the Medical Research Council (UK), and an unrestricted scientific grant from SLA Pharma (UK), the manufacturers of the free fatty acid form of EPA used in this study.
This article refers to supplementary materials, which are designated by Figures W1 and W2 and are available online at www.neoplasia.com.
References
- 1.Eisinger AL, Prescott SM, Jones DA, Stafforini DM. The role of cyclooxygenase-2 and prostaglandins in colon cancer. Prostaglandins Other Lipid Mediat. 2007;82:147–154. doi: 10.1016/j.prostaglandins.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Wang D, DuBois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Wang H, Shi Q, Katkuri S, Walhi W, Desvergne B, Das SK, Dey SK, Dubois RN. Prostaglandin E2 promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor δ. Cancer Cell. 2004;6:285–295. doi: 10.1016/j.ccr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Kawamori T, Uchiya N, Sugimura T, Wakabayashi K. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003;24:985–990. doi: 10.1093/carcin/bgg033. [DOI] [PubMed] [Google Scholar]
- 5.Chapkin RS, McMurray DN, Lupton JR. Colon cancer, fatty acids and anti-inflammatory compounds. Curr Opin Gastroenterol. 2007;23:48–54. doi: 10.1097/MOG.0b013e32801145d7. [DOI] [PubMed] [Google Scholar]
- 6.Calviello G, Serini S, Piccioni E. n-3 Polyunsaturated fatty acids and the prevention of colorectal cancer: molecular mechanisms involved. Curr Med Chem. 2007;14:3059–3069. doi: 10.2174/092986707782793934. [DOI] [PubMed] [Google Scholar]
- 7.Smith WL. Cyclooxygenases, peroxide tone and the allure of fish oil. Curr Opin Cell Biol. 2005;17:174–182. doi: 10.1016/j.ceb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Yang P, Chan D, Felix E, Cartwright C, Menter DG, Madden T, Klein RD, Fischer SM, Newman RA. Formation and antiproliferative effect of prostaglandin E3 from eicosapentaenoic acid in human lung cancer cells. J Lipid Res. 2004;45:1030–1039. doi: 10.1194/jlr.M300455-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Funahashi H, Satake M, Hasan S, Sawai H, Newman RA, Reber HA, Hines OJ, Eibl G. Opposing effects of n-6 and n-3 polyunsaturated fatty acids on pancreatic cancer growth. Pancreas. 2008;36:353–362. doi: 10.1097/MPA.0b013e31815ccc44. [DOI] [PubMed] [Google Scholar]
- 10.Vanamala J, Glagolenko A, Yang P, Carroll RJ, Murphy ME, Newman RA, Ford JR, Braby LA, Chapkin RS, Turner ND, et al. Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPARδ/PGE2 and elevation of PGE3. Carcinogenesis. 2008;29:790–796. doi: 10.1093/carcin/bgm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 12.Hull MA, Ko SCW, Hawcroft G. Prostaglandin EP receptors: targets for treatment and prevention of colorectal cancer? Mol Cancer Ther. 2004;3:1031–1039. [PubMed] [Google Scholar]
- 13.Chell SD, Witherden IR, Dobson RR, Moorghen M, Herman AA, Qualtrough D, Williams AC, Paraskeva C. Increased EP4 receptor expression in colorectal cancer progression promotes cell growth and anchorage independence. Cancer Res. 2006;66:3106–3113. doi: 10.1158/0008-5472.CAN-05-3702. [DOI] [PubMed] [Google Scholar]
- 14.Hawcroft G, Ko CWS, Hull MA. Prostaglandin E2-EP4 receptor signalling promotes tumorigenic behaviour of HT-29 human colorectal cancer cells. Oncogene. 2007;26:3006–3019. doi: 10.1038/sj.onc.1210113. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Huang Y, Porta R, Yanagisawa K, Gonzalez A, Segi E, Johnson DH, Narumiya S, Carbone DP. Host and direct antitumor effects and profound reduction in tumor metastasis with selective EP4 receptor antagonism. Cancer Res. 2006;66:9665–9672. doi: 10.1158/0008-5472.CAN-06-1271. [DOI] [PubMed] [Google Scholar]
- 16.Lawson LD, Hughes BG. Human absorption of fish oil fatty acids as triacylglyercols, free acids or ethyl esters. Biochem Biophys Res Commun. 1988;152:328–335. doi: 10.1016/s0006-291x(88)80718-6. [DOI] [PubMed] [Google Scholar]
- 17.West NJ, Clark SK, Phillips RKS, Hutchinson JM, Leicester RJ, Belluzzi A, Hull MA. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010 doi: 10.1136/gut.2009.200642. E-pub ahead of print March. [DOI] [PubMed] [Google Scholar]
- 18.Fenwick SW, Toogood GJ, Lodge JPA, Hull MA. The effect of the selective cyclooxygenase-2 inhibitor rofecoxib on human colorectal cancer liver metastases. Gastroenterology. 2003;125:716–729. doi: 10.1016/s0016-5085(03)01061-8. [DOI] [PubMed] [Google Scholar]
- 19.Shiff SJ, Koutsos MI, Qiao L, Rigas B. Nonsteroidal anti-inflammatory drugs inhibit the proliferation of colon adenocarcinoma cells: effects on cell cycle and apoptosis. Exp Cell Res. 1996;222:179–188. doi: 10.1006/excr.1996.0023. [DOI] [PubMed] [Google Scholar]
- 20.Elder DJE, Halton DE, Hague A, Paraskeva C. Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drug: independence from COX-2 protein expression. Clin Cancer Res. 1997;3:1679–1683. [PubMed] [Google Scholar]
- 21.Piazza GA, Rahm AK, Finn TS, Li H, Stoumen AL, Pamukcu R, Ahnen DJ. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest and p53 induction. Cancer Res. 1997;57:2452–2459. [PubMed] [Google Scholar]
- 22.Qia L, Shiff SJ, Rigas B. Sulindac sulphide inhibits the proliferation of colon cancer cells: diminished expression of the proliferation markers PCNA and Ki-67. Cancer Lett. 1997;115:229–234. doi: 10.1016/s0304-3835(97)04740-x. [DOI] [PubMed] [Google Scholar]
- 23.Smith M-L, Hawcroft G, Hull MA. The effect of non-steroidal anti-inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur J Cancer. 2000;36:664–674. doi: 10.1016/s0959-8049(99)00333-0. [DOI] [PubMed] [Google Scholar]
- 24.Ko SCW, Chapple KS, Hawcroft G, Coletta PL, Markham AF, Hull MA. Paracrine cyclooxygenase-2-mediated signalling by macrophages promotes tumorigenic progression of intestinal epithelial cells. Oncogene. 2002;21:7175–7186. doi: 10.1038/sj.onc.1205869. [DOI] [PubMed] [Google Scholar]
- 25.Gardner SH, Hawcroft G, Hull MA. Effect of nonsteroidal anti-inflammatory drugs of β-catenin protein levels and catenin-related transcription in human colorectal cancer cells. B J Cancer. 2004;91:153–163. doi: 10.1038/sj.bjc.6601901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, Johnson DH, Morrow JD. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Anal Biochem. 2004;334:266–275. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Calviello G, Di Nicuolo F, Gragnoli S, Piccioni E, Serini S, Maggiano N, Tringali G, Navarra P, Ranelletti FO, Palozza P. n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and -2 and HIF-1α induction pathway. Carcinogenesis. 2004;25:2303–2310. doi: 10.1093/carcin/bgh265. [DOI] [PubMed] [Google Scholar]
- 28.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a novel Gs-axin-β-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 29.Shoji Y, Takahashi M, Kitamura T, Watanabe K, Kawamori T, Maruyama T, Sugimoto Y, Negishi M, Narumiya S, Sugimura T, et al. Downregulation of prostaglandin E receptor subtype EP3 during colon cancer development. Gut. 2004;53:1151–1158. doi: 10.1136/gut.2003.028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Courtney ED, Matthews S, Finlayson C, Di Pierro D, Belluzzi A, Roda E, Kang JY, Leicester RJ. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. Int J Colorectal Dis. 2007;22:765–776. doi: 10.1007/s00384-006-0240-4. [DOI] [PubMed] [Google Scholar]
- 31.Wada M, DeLong CJ, Hong YH, Rieke CJ, Song I, Sidhu RS, Chong Y, Warnock M, Schmaier AH, Yokoyama C, et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem. 2007;282:22254–22266. doi: 10.1074/jbc.M703169200. [DOI] [PubMed] [Google Scholar]
- 32.Belury MA, Patrick KE, Locniskar M, Fischer SM. Eicosapentaenoic acid and arachidonic acid: comparison of metabolism and activity in murine epidermal cells. Lipids. 1989;24:423–429. doi: 10.1007/BF02535150. [DOI] [PubMed] [Google Scholar]
- 33.Hatala MA, Rayburn J, Rose DP. Comparison of linoleic acid and eicosapentaenoic acid incorporation into human breast cancer cells. Lipids. 1994;29:831–837. doi: 10.1007/BF02536250. [DOI] [PubMed] [Google Scholar]
- 34.Tull SP, Yates CM, Maskrey BH, O'Donnell VB, Madden J, Grimble RF, Calder PC, Nash GB, Rainger GE. Omega-3 fatty acids and inflammation. Novel interactions reveal a new step in neutrophil recruitment. PLoS Biol. 2009;7:e1000177. doi: 10.1371/journal.pbio.1000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arterburn LM, Bailey Hall E, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83(suppl):1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 36.Löffler I, Grün M, Böhmer FD, Rubio I. Role of cAMP in the promotion of colorectal cancer cell growth by prostaglandin E2. BMC Cancer. 2008;8:380. doi: 10.1186/1471-2407-8-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denkins Y, Kempf D, Ferniz M, Nileshwar S, Marchetti D. Role of ω-3 polyunsaturated fatty acids on cyclooxygenase-2 metabolismin brain-metastatic melanoma. J Lipid Res. 2005;46:1278–1284. doi: 10.1194/jlr.M400474-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from ω-6 and ω-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenzie KE, Bandyopadhyay GK, Imagawa W, Sun K, Nandi S. ω-3 and ω-6 fatty acids and PGE2 stimulate the growth of normal but not tumour mouse mammary epithelial cells. Prostaglandins Leukot Essent Fatty Acids. 1994;51:437–443. doi: 10.1016/0952-3278(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 40.Dommels YEM, Haring MMG, Keestra NGM, Alink GM, van Bladeren PJ, van Ommen B. The role of cyclooxygenase in n-6 and n-3 polyunsaturated fatty acid mediated effects on cell proliferation, PGE2 synthesis and cytotoxicity in human colorectal carcinoma cell lines. Carcinogenesis. 2003;24:385–392. doi: 10.1093/carcin/24.3.385. [DOI] [PubMed] [Google Scholar]
- 41.Aronson WJ, Glaspy JA, Reddy ST, Reese D, Heber D, Bagga D. Modulation of omega-3/omega-6 polyunsaturated ratios with dietary fish oils in men with prostate cancer. Urology. 2001;58:283–288. doi: 10.1016/s0090-4295(01)01116-5. [DOI] [PubMed] [Google Scholar]
- 42.Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. 2006;52:2265–2272. doi: 10.1373/clinchem.2006.072322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.