Summary
CD40 is expressed on a variety of tumors; anti-CD40 agonists promote tumor cell apoptosis and subsequent tumor regression. Because the effectiveness of anti-CD40-agonists is dependent upon CD40 surface expression, the current study examined ligation-mediated changes in CD40 protein half-life (t1/2)at the cell surface. This study utilized a CD40+ epithelial cell line (9HTEo-), a CD40 null epithelial cell line (HT-29) engineered to express either wild-type (WT) or mutant (T254A, Q263A, E235A, Δ201) CD40, and the anti-CD40 antibody G28.5. Ligation of endogenous CD40 expressed on 9HTEo-cells decreased CD40 surface protein t1/2 from 13h to 4h (p<0.05). Ligation of WT-, Q263A-, or T254A-CD40 expressed on engineered HT-29 cells decreased CD40 surface protein t1/2 from an average of 8h to 4h (p<0.05); T254A and Q263A contain mutated TRAF2/3 binding sites. In contrast, ligation of E235A andΔ201-CD40 had no affect on its surface protein t1/2 (p<0.05); E235A contains a mutated TRAF6 binding site while Δ201 lacks an intact cytoplasmic tail. These results suggest that anti-CD40 agonists decrease CD40 surface protein t1/2 via a mechanism that involves TRAF6 but not TRAF2/3; the therapeutic implications for CD40-mediated tumor regression are discussed.
Keywords: CD40, epithelial cell, TRAF molecules
Introduction
The CD40 receptor, a member of the TNF receptor family, plays a central role in the regulation of humoral and cell-mediated immunity. It is expressed on the surface of a wide variety of cells including B lymphocytes, dendritic cells, macrophages, fibroblasts, thymic epithelial cells, and kidney epithelial cells. In addition, we and others have demonstrated that airway epithelial cells express the CD40 receptor [1;2]. CD40 receptor expression has also been detected in approximately 100% of B cell malignancies and 70% of solid tumors, including epithelial-derived tumors [3]. Recent studies suggest that engagement of the CD40 receptor with anti-CD40 antibody agonists inhibits the growth of solid tumors in vitro and in vivo [3;4].
Because the short cytoplasmic domain of the CD40 receptor lacks intrinsic kinase activity, it requires adaptor TRAF (TNF Receptor Associated Factor) molecules to initiate signals down-stream. To date, six TRAF molecules have been characterized; all except TRAF4 are known to bind CD40 [5–8]. Previous studies, performed largely in B lymphocytes, suggest that the CD40 receptor exists as a monomer and, upon CD40L or anti-CD40 antibody binding, clusters in lipid raft domains at sites of cell-cell contact [9–14]. Hostager and co-workers have demonstrated that CD40 receptor engagement leads to the recruitment of CD40, TRAF2, and TRAF3 into membrane microdomains [12]. Additional reports indicate that ligand-binding causes the CD40 receptor to multimerize as either a homodimer or a homotrimer to initiate down-stream signaling events [8;9]. Despite these previous reports, no studies to date have measured the protein half-life (t1/2) of the CD40 receptor at the plasma membrane of any cell type. The hypothesis of the current study was that ligation of the surface CD40 receptor would decrease its protein t1/2 at the cell surface via a mechanism that required an intact cytoplasmic domain. To test this hypothesis, we utilized the epithelial cell lines 9HTEo-, a surface CD40 positive model system, and HT-29, a CD40 null cell line engineered to express either wild-type (WT) or TRAF-binding mutants (Q263A, T254A, E235A,Δ201) CD40 protein stably. Because recent studies indicate that anti-CD40 antibody agonists inhibit the growth of solid tumors [3;4], the anti-CD40 antibody G28.5 was utilized; G28.5 has been shown to be agonistic [12]. Results presented herein demonstrate that ligation of CD40 decreased its protein t1/2 at the plasma membrane. Further, these results suggest that anti-CD40 agonists decrease CD40 surface protein t1/2 via a mechanism that involves TRAF6 but not TRAF2/3. The therapeutic implications of these data for CD40-mediated tumor regression are discussed.
Results
Ligation of cell surface wild-type CD40 decreased its protein half-life
To examine the surface half-life of the CD40 receptor, cells were surface biotinylated in the presence of G28.5 or an isotype-matched antibody control. The results presented in Figure 1 show that, in the presence of the isotype-matched antibody control, CD40 protein t1/2 was approximately 13h. Upon G28.5-mediated ligation, however, CD40 protein t1/2 was reduced significantly to 4h. Controls performed in the absence of the G28.5 secondary antibody cross-linker yielded CD40 protein t1/2 results (data not shown) that were equivalent to those of the isotype matched antibody control (Fig. 1).
Figure 1.
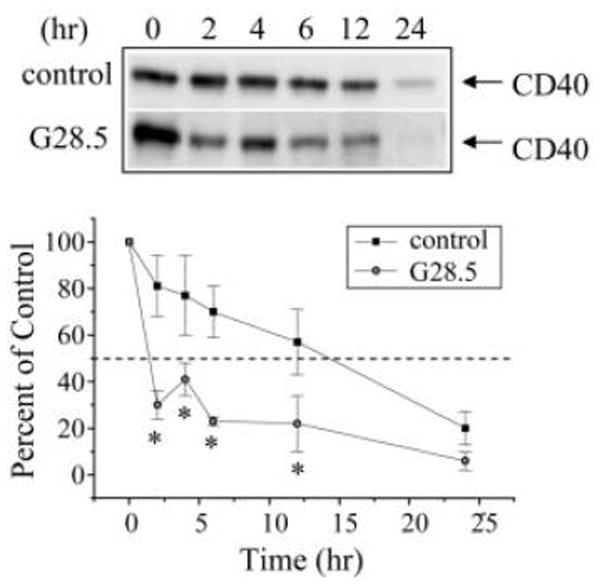
G28.5-mediated ligation of the CD40 receptor decreases its protein t1/2 at the cell surface 9HTEo- epithelial cells. 9HTEo- cells were surface biotinylated and then ligated with G28.5 followed by goat-anti-mouse Fab2 for the time-points indicated. A, Representative blots from three independent experiments are shown. B, Densitometric analyses were performed and are reported as percent remaining (n = 3; * p ≤ 0.05 relative to matched control).
Mutation of TRAF binding sites had differential effects on CD40 protein half-life
TRAF2, TRAF3 and TRAF6 bind the CD40 receptor with high affinity; TRAF2 and TRAF3, but not TRAF6, have been implicated in regulating CD40 surface expression [12;15]. To examine the role of TRAF2, TRAF3 and TRAF6 binding on CD40 surface protein t1/2, HT-29 cells engineered to express wild-type (WT) or mutant forms of human CD40 were utilized. HT-29 cells do not express CD40 endogenously [2;16], but do express TRAF 2, 3, and 6 proteins (data not shown). Specifically, HT-29 cells were engineered to express either WT or CD40 mutants that contain alanine substitutions at the TRAF3 binding domain (Q263), TRAF2/3 binding domains (T254), or TRAF6 binding domain (E235). We and other have shown that these mutant clones exhibit reduced CD40-mediated signaling upon ligation [16;17].
To examine the protein surface t1/2 of WT and mutant CD40 receptors expressed in engineered HT-29 cells, cells were surface biotinylated in the presence of G28.5 or an isotype-matched antibody control. Results shown in Figures 2 and 3 demonstrate that, in the presence of the isotype-matched antibody control, WT-CD40 protein t1/2 was approximately 8h; upon G28.5-mediated ligation, WT-CD40 protein t1/2 was decreased significantly to 4h (Fig. 2A, Fig. 3). Similarly, G28.5-mediated ligation of Q263A-CD40 and T254A-CD40 decreased CD40 protein t1/2 significantly from approximately 8h to 4h (Fig. 2B, C, Fig. 3). In contrast, G28.5-mediated ligation of E235A-CD40 had no significant affect on surface protein t1/2 as compared with isotype-matched controls (Fig. 2D, Fig. 3). Controls performed in the absence of the G28.5 secondary antibody cross-linker generated WT-, Q263A-T254A-CD40 and E235A-CD40 protein t1/2 results (data not shown) that were equivalent to those of the isotype matched antibody control (Figs. 2, 3).
Figure 2.
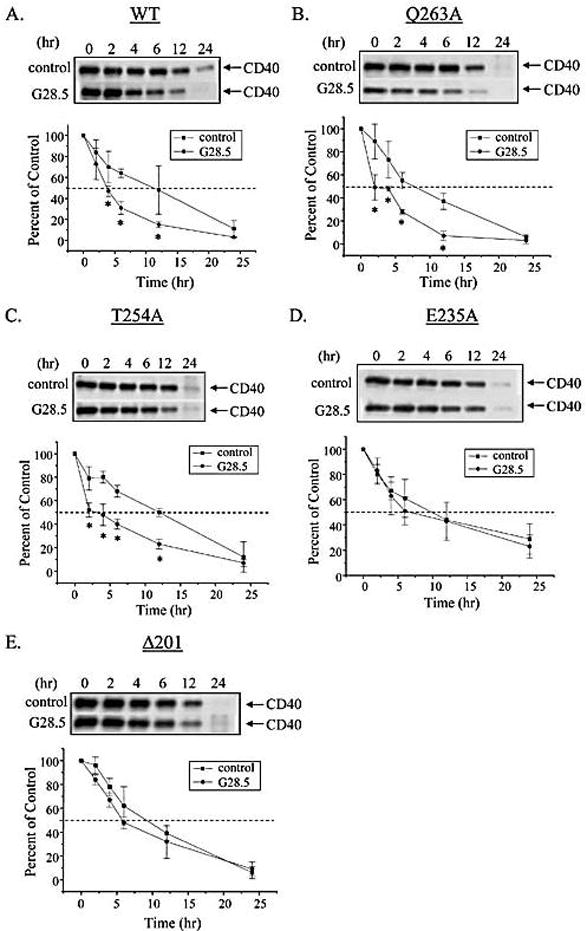
G28.5-mediated ligation of WT-CD40, Q263A-CD40 and T254A-CD40, but not of E235A-CD40 or Δ201-CD40, decreases its protein t1/2 at the cell surface of stably transfected HT-29 cells. HT-29 cells stably expressing A, WT-CD40, B, Q263A-CD40, C, T254A-CD40, D, E235A-CD40, or E,Δ201-CD40 were surface biotinylated and then ligated with G28.5 followed by goat-anti-mouse Fab2 for the time-points indicated. Representative blots from three independent experiments are shown. Densitometric analyses were performed and are reported as percent remaining (n = 3; * p ≤ 0.05 relative to matched control).
Figure 3.
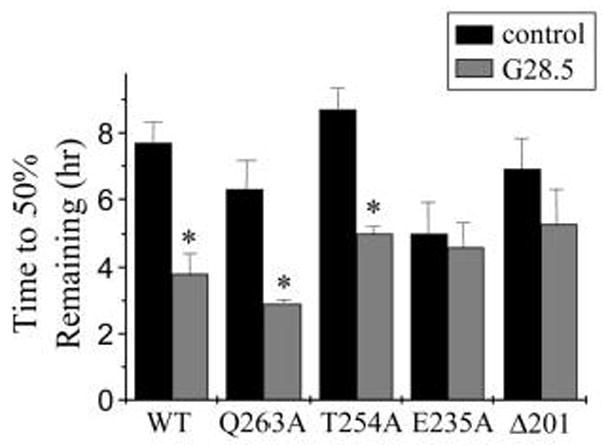
WT-, Q263A-, and T254A-CD40, but not E235A-CD40 orΔ201-CD40, exhibit differences in CD40 protein half-lives upon G28.5-mediated ligation as compared with controls. Protein half-lives (time to 50% remaining) were calculated as described in Materials and Methods (n = 3; * p ≤ 0.05 relative to matched control).
Truncation of CD40 blocked the effects of ligation on CD40 protein half-life
We have reported previously that HT29 cells expressing Δ201-CD40 lack CD40 mediated signaling [16]. Results presented herein show that G28.5-mediated ligation of Δ201-CD40 did not change its surface protein t1/2 as compared with isotype-matched antibody controls (Fig. 2E, Fig. 3). Controls performed in the absence of the G28.5 secondary antibody cross-linker exhibited Δ201-CD40 protein t1/2 results (data not shown) that were equivalent to those of the isotype matched antibody control (Figs. 2, 3).
Discussion
Results presented herein demonstrate that ligation of the endogenous or wild-type CD40 receptor decreases its protein t1/2 at the epithelial cell surface significantly. To date, this is the first report that examines the protein t1/2 of the CD40 receptor at the cell surface in any cell model system. Previous studies have examined the surface protein t1/2 of other members of the TNF receptor (TNFR) superfamily, including TNFR1 [18;19] and the Fas (CD95) receptor [20]. These studies show that ligation of the TNFR1 receptor reduced its surface protein t1/2 from 2 hours to 30 min [18;19]. Similarly, ligation of the Fas receptor decreased its surface expression by 50% within 15 minutes of ligation [20]. Together, these findings suggest that the surface expression of the CD40 receptor is more stable than other members of the TNFR superfamily.
The role of TRAF2, TRAF3 and TRAF6 molecules in CD40-mediated signaling has been well established in B lymphocytes and other cell model systems, such as epithelial cells [2;16;17]. Findings from this current study indicate that TRAF6, but neither TRAF2 nor TRAF3, play a role in the regulation of CD40 protein t1/2 at the cell surface. These results contrast previous work performed in B cells by Manning and colleagues [15]. Specifically, these authors reported that disruption of the TRAF2/TRAF3 binding site within the CD40 receptor cytoplasmic tail prohibited the loss of CD40 surface expression upon its ligation. These authors also reported that disruption of the TRAF6 binding site had no affect upon ligation-mediated changes in CD40 surface expression. Such contrasting results may be due to cell-specific mechanisms that underlie CD40 expression and turnover. We have reported previously that TRAF3, but not TRAF2, positively regulates CD40-mediated activation of the RANTES promoter in epithelial cells [16]; these findings are in sharp contrast with previously published reports examining the role of TRAF molecules in CD40-mediated signaling [17]. In addition, these contrasting results may be due to the utilization of different experimental approaches. Manning and co-workers monitored the disappearance of surface bound gp39/CD8α chimeric CD40 ligand (CD154) while the current study monitored CD40 receptor disappearance directly. Moreover, it should be noted that CD154 will activate CD40 and may have different effects on CD40 expression.
Upon CD40 receptor ligation, TRAF6 binds the CD40 cytoplasmic domain and initiates signaling cascades that are unique in comparison with other TRAF molecules, including TRAF1, TRAF2 and TRAF3. Studies utilizing kidney epithelial cells or T lymphocytes have shown that TRAF6, unlike other TRAF molecules, plays a role in CD40-mediated activation of the ERK-, p38- and PI-3-kinase/Akt- signaling pathways [17]. These signaling pathways play important roles in the coordination of cellular events, such as receptor expression and internalization. For example, recent studies demonstrate that the ERK1/2, p38 and/or the PI3-kinase/Akt signaling pathways regulate ligand-mediated endocytosis and internalization of a variety surface receptors, including β1-adrenergic receptors [21], the insulin receptor [22], β-arrestin [23], the EGF receptor [24] and the EphA2 receptor [25]. In light of these studies, it is possible that TRAF6 may regulate CD40 surface expression under ligated conditions via a mechanism that involves the ERK1/2, p38 and/or the PI3-kinase/Akt signaling pathways.
Alternatively, the ability of TRAF6 to function as an E3-ubiquitin ligase may underlie its role in regulating CD40 surface expression under ligated conditions. TRAF6 has been shown to function as an E3-ubiquitin ligase in a variety of cell model systems, including kidney epithelial cells and macrophages [17]. Ubiquitination of proteins is a post-translational modification in which ubiquitin is attached to a target protein and promotes the degradation of that protein; E3- ubiquitin ligases catalyze the last reaction of the ubiquitination process. Ubiquitination plays an important role in the downregulation of ligated membrane receptors, such as the EGF receptor and T cell receptor [26–28]. It should be noted that TRAF2 can also function as an E3-ubiquitin ligase; however, CD40 ligation triggers the ubiquitination and subsequent degradation of TRAF2, but not TRAF6 [17].
Several studies demonstrate that anti-CD40 agonists inhibit the growth of solid tumors both in vitro and in vivo [3;4]. Specifically, in vitro studies have demonstrated growth inhibition of B-cell lymphoma and carcinoma cell lines through the use of either antibody- or soluble ligand-mediated CD40 ligation; ligation was enhanced with an additional cross-linker [29;30]. Similarly, in vivo studies have shown anti-tumor activity in melanoma and lymphoma patients with the use of either anti-CD40 antibodies or soluble CD40 ligand in the absence of a cross-linker; however, it is possible that these reagents may be cross-linked by accessory cells in vivo [31]. The mechanisms that underlie CD40-mediated growth inhibition may involve initiation of tumor cell apoptosis, inhibition of tumor cell proliferation and/or stimulation of an anti-tumor immune response [3;4]. The effectiveness of anti-CD40 agonist therapy is governed partly by the surface protein half-life of CD40. The findings presented herein demonstrate that, in the absence of CD40 engagement, CD40 surface protein expression is stable; such stability may explain, in part, its utility in anti-tumor responses. Upon engagement, however, the stability of surface CD40 protein expression decreases. At present, it is unclear how such a decrease in CD40 expression affects its anti-tumor responses. Possible fates of CD40 following engagement include release from the cell surface, association with the cytoskeleton, translocation to lipid rafts and internalization. Future studies should include an examination of these possibilities in the analysis of CD40-mediated anti-tumor activity.
Materials and methods
Cell culture
Experiments employed the human airway epithelial cell line 9HTEo-(tracheal; a gift from Dr. Dieter Gruenert, UCSF, CA [32]) and the human colon carcinoma epithelial cell line HT-29 (American Type Culture Collection (ATCC) Manassas, VA) [33]. Cells were cultured in DMEM media (Biofluids, Inc., Rockville, MD) containing 10% (HT-29) FBS, 1% penicillin/streptomycin, and 0.2% fungizone (Gibco/BRL, Grand Island, NY). Cells were grown at 37°C in a 5% CO2 environment and on Vitrogen 100 (Cohesion, Inc., Palo Alto, CA) – coated flasks [2;16].
Stable CD40 transfectants
HT-29 epithelial cells were transfected stably with constructs encoding either wild-type or mutant forms of human CD40 (Q263A, T254A, E235A,Δ201) as described previously [16]. E235A was generous gift from Dr. Jun Ichiro Inoue [34]. Stable expression of CD40 constructs was maintained by supplementing the culture media with hygromycin (600μg/ml, Sigma Chemical).
CD40 receptor ligation
Cells were incubated in PBS pH 7.4 containing 5μg/ml of the anti-CD40 antibody, G28.5 (ATCC), or an isotype matched IgG control (Jackson Laboratories, West Grove, PA) for 20 min on ice as described previously [9]. Cells were then washed with PBS and incubated in serum-free feeding media containing 1μg/ml of goat anti-mouse Fab2 antibody fragment (Jackson Laboratories, West Grove, PA) for 10 min at 37oC in order to enhance cross-linking [9].
Cell surface biotinylation
Surface biotinylation analysis was performed as described previously [35]. Briefly, cells were grown to approximately 80% confluence, surface biotinylated with NHS S-S Biotin (Pierce Chemical, Rockford, IL), and lysed in RIPA lysis buffer (50 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl, pH 8.0). Lysates were probed with Neutravidin-coated sepharose beads (Pierce Chemical) to remove all biotinylated proteins, including biotinylated CD40 receptor protein [35–37]. All biotinylated proteins were resolved via SDS-PAGE, blotted, and probed with the rabbit anti-human CD40 polyclonal antibody sc-974 (Santa Cruz, Inc.). The immunoblots were developed via chemiluminescence (Millipore) and then imaged for 20 seconds using the Chemidoc XRS Imaging Station (BioRAD); results were quantitated via densitometry.
Calculation of CD40 protein half-life
Densitometry results were converted to percent of control (time 0hr) and graphed as first-order exponential decay (R>0.99). Protein half-lives (i.e. time to 50% remaining) were determined from the respective exponential decay equations.
Statistical analysis
Data are expressed as the mean ± SD of replicate determinations as indicated. Statistical significance was determined by two-tailed student t-test. A p ≤0.05 was considered significant.
Acknowledgments
The authors would like to thank Dr. James F. Collawn, Dr. Karoly Varga, and Ms. Kim Estell for technical assistance. This project was supported by NIH R01 HL075465 (LMS) and T32HL07553 (TAT; trainee).
Abbreviations
- TRAF
TNF Receptor Associated Factor
- TNF
Tumor Necrosis Factor
References
- 1.Cagnoni F, Oddera S, Giron-Michel J, Riccio A, Olsson S, Dellacasa P, Melioli G, Canonica G, Azzarone B. CD40 on adult human airway epithelial cells: expression and proinflammatory effects. J Immunol. 2004;172:3205–3214. doi: 10.4049/jimmunol.172.5.3205. [DOI] [PubMed] [Google Scholar]
- 2.Propst SM, Estell K, Schwiebert LM. Proinflammatory and Th2-derived cytokines modulate CD40-mediated expression of inflammatory mediators in airway epithelia: implications for the role of epithelial CD40 in airway inflammation. J Immunol. 2000;165:2214–2221. doi: 10.4049/jimmunol.165.4.2214. [DOI] [PubMed] [Google Scholar]
- 3.Vonderheide R. Prospect of targeting the CD40 pathway for cancer therapy. Clin Can Res. 2006;13:1083–1088. doi: 10.1158/1078-0432.CCR-06-1893. [DOI] [PubMed] [Google Scholar]
- 4.Eliopoulos A, Young L. The role of the CD40 pathway in the pathogenesis and treatment of cancer. Curr Opin Pharmacol. 2004;4:360–367. doi: 10.1016/j.coph.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Jabara HH, Buckley RH, Roberts JL, Lefrance G, Loiselet J, Khalil G, Geha RS. Role of JAK3 in CD40-mediated signaling. Blood. 1998;92:2435–2440. [PubMed] [Google Scholar]
- 6.Pullen SS, Dang T, Crute JJ, Kehry MR. CD40 signaling through tumor necrosis factor-associated factors (TRAFs). Binding site specificity and activation of downstream pathways by distinct TRAFs. J Biol Chem. 1999;274:14246–14254. doi: 10.1074/jbc.274.20.14246. [DOI] [PubMed] [Google Scholar]
- 7.Pullen SS, Miller HG, Everdeen DS, Dang TTA, Crute JJ, Kehry MR. CD40-tumor necrosis factor receptor-associated factor (TRAF) interactions: regulation of CD40 signaling through multiple TRAF binding sites and TRAF hetero-oligomerization. Biochem. 1998;37:11836–11845. doi: 10.1021/bi981067q. [DOI] [PubMed] [Google Scholar]
- 8.Pullen S, Labadia M, Ingraham R, Medzhitov R, Everdeen D, Alber T, Crute J, Kehry M. High affinity interactions of tumor necrosis factor receptor-associated factors (TRAFS) and CD40 require TRAF trimerization and CD40 multimerization. Biochem. 1999;38:10168–10177. doi: 10.1021/bi9909905. [DOI] [PubMed] [Google Scholar]
- 9.Reyes-Moreno C, Girouard J, Lapointe R, Darveau A, Mourad W. CD40/CD40 homodimers are required for CD40-induced phosphatidylinositol 3-kinase-dependent expression of B7.2 by human B lymphocytes. J Biol Chem. 2004;279:7799–7806. doi: 10.1074/jbc.M313168200. [DOI] [PubMed] [Google Scholar]
- 10.Grassme H, Bock J, Kun J, Gulbins E. Clustering of CD40 ligand is required to forma functional contact with CD40. J Biol Chem. 2002;277:30289–30299. doi: 10.1074/jbc.M200494200. [DOI] [PubMed] [Google Scholar]
- 11.Brown K, Hostager BS, Bishop GA. Regulation of TRAF2 signaling by self-induced degradation. J Biol Chem. 2002;277:19433–19438. doi: 10.1074/jbc.M111522200. [DOI] [PubMed] [Google Scholar]
- 12.Hostager B, Catlett I, Bishop G. Recruitment of CD40 and tumor necrosis factor receptor associated factors 2 and 3 to membrane microdomains during CD40 signaling. J Biol Chem. 2000;275:15392–15398. doi: 10.1074/jbc.M909520199. [DOI] [PubMed] [Google Scholar]
- 13.Jalukar SV, Hostager BS, Bishop GA. Characterization of the roles of TRAF6 in CD40-mediated B lymphocyte effector functions. J Immunol. 2000;164:623–630. doi: 10.4049/jimmunol.164.2.623. [DOI] [PubMed] [Google Scholar]
- 14.Moore C, Bishop G. Differential regulation of CD40-mediated TNF receptor-associated factor degradation in B lymphocytes. J Immunol. 2005;175:3780–3789. doi: 10.4049/jimmunol.175.6.3780. [DOI] [PubMed] [Google Scholar]
- 15.Manning E, Pullen S, Souza D, Kehry M, Noelle R. Cellular responses to murine CD40 in a mouse B cell line may be TRAF dependent or independent. Eur J Immunol. 2002;32:39–49. doi: 10.1002/1521-4141(200201)32:1<39::AID-IMMU39>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 16.Propst SM, Estell K, Schwiebert LM. CD40-mediated activation of NF-kB in airway epithelial cells. J Biol Chem. 2002;277:37054–37063. doi: 10.1074/jbc.M205778200. [DOI] [PubMed] [Google Scholar]
- 17.Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ. TRAF proteins in CD40 signaling. Adv Exp Med Biol. 2007;597:131–151. doi: 10.1007/978-0-387-70630-6_11. [DOI] [PubMed] [Google Scholar]
- 18.Higuchi M, Aggarwal B. TNF induces internalization of the p60 receptor and shedding of the p80 receptor. J Immunol. 1994;152:3550–3558. [PubMed] [Google Scholar]
- 19.Watanabe N, Kuriyama H, Sone H, Neda H, Yamauchi N, Maeda M, Nitsu Y. Continuous internalization of tumor necrosis factor receptors in a human myosarcoma cell line. J Biol Chem. 1988;263:10262–10266. [PubMed] [Google Scholar]
- 20.Lee K, Feig C, Tchikov V, Schnickel R, Hallas C, Schutze S, Peter M, Chan A. The role of receptor internalization in CD95 signaling. EMBO J. 2006;25:1009–1023. doi: 10.1038/sj.emboj.7601016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gavi S, Yin D, Shumay E, Wang H, Malbon C. Insulin-like growth factor-I provokes functional antagonism and internalization of β1-adrenergic receptors. Endocrin. 2007;148:2653–2662. doi: 10.1210/en.2006-1569. [DOI] [PubMed] [Google Scholar]
- 22.Hunker CM, Giambini H, Galvis A, Hall J, Kruk I, Veisaga ML, Barbieri MA. Rin1 regulates insulin receptor signal transduction pathways. Exp Cell Res. 2006;312:1106–1118. doi: 10.1016/j.yexcr.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Shenoy S, Barak L, Xiao K, Ahn S, Berthouze M, Shukla A, Luttrell L, Lefkowitz R. Ubiquitination of β-arrestin links seven-transmembrane receptor endocytosis and ERK activation. J Biol Chem. 2007;282:29549–29562. doi: 10.1074/jbc.M700852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winograd-Katz SE, Levitzki A. Cisplatin induces PKB/Akt activation and p38MAPK phosphorylation of the EGF receptor. Oncogene. 2006;25:7381–7390. doi: 10.1038/sj.onc.1209737. [DOI] [PubMed] [Google Scholar]
- 25.Zhaung G, Hunter S, Hwang Y, Chen J. Regulation of EphA2 receptor endocytosis by SHIP2 lipid phosphatase via phosphatidylinositol 3-kinase-dependent Rac1 activation. J Biol Chem. 2007;282:2683–2694. doi: 10.1074/jbc.M608509200. [DOI] [PubMed] [Google Scholar]
- 26.Frey MR, Dise RS, Edelblum KL, Polk DB. p38 kinase regulates epidermal growth factor receptor downregulation and cellular migration. EMBO J. 2006;25:5683–5692. doi: 10.1038/sj.emboj.7601457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geisler C. TCR trafficking in resting and stimulated T cells. Crit Rev Immunol. 2004;24:67–86. doi: 10.1615/critrevimmunol.v24.i1.30. [DOI] [PubMed] [Google Scholar]
- 28.Rotin D, Staub O, Haguenauer-Tsapis R. Ubitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J Membrane Biol. 2000;176:1–17. doi: 10.1007/s00232001079. [DOI] [PubMed] [Google Scholar]
- 29.Funakoshi S, Long D, Beckwith M, Conley D, Tsarfaty G, Tsarfaty I, Armitage R, Fanslow W, Spriggs MK, Murphy WJ. Inhibition of human B-cell lymphoma growth by CD40 stimulation. Blood. 1994;83:2787–2794. [PubMed] [Google Scholar]
- 30.Eliopoulos AG, Davies C, Knox P, Gallagher NJ, Afford S, Adams D, Young LS. CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily. Mol Cell Biol. 2000;20:5503–5515. doi: 10.1128/mcb.20.15.5503-5515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vonderheide RH, Flaherty K, Khalil M, Stumacher M, Bajor D, Hutnick N, Sullivan P, Mahany J, Gallagher M, Kramer A, Green S, O’Dwyer P, Running K, Huhn D, Antonia S. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 32.Gruenert DC, Basbaum CB, Welsh MJ, Li M, Finkbeiner WE, Nadel JA. Characterization of human tracheal epithelial cells transformed by an origin-defective simian virus 40. Proc Natl Acad Sci USA. 1988;85:5951–5955. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noguchi P, Wallace R, Johnson J, Earley EM, O’Brien S, Pellegrino MA, Milstein J, Needy C, Browne W, Petricciani J. Characterization of the WIDR: a human colon carcinoma cell line. In vitro. 1979;15:401–408. doi: 10.1007/BF02618407. [DOI] [PubMed] [Google Scholar]
- 34.Tsukamoto N, Kobayashi N, Azuma S, Yamamoto T, Inoue J. Two differently regulated nuclear factor kB activation pathways triggered by the cytoplasmic tail of CD40. Proc Natl Acad Sci USA. 1999;96:1234–1239. doi: 10.1073/pnas.96.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volz B, Orberger G, Porwoll S, Hauri HP, Tauber R. Selective reentry of recycling cell surface glycoproteins to the biosynthetic pathway in human hepatocarcinoma HepG2 cells. J Cell Biochem. 1995;277:537–551. doi: 10.1083/jcb.130.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varga K, Jurkuvenaite A, Wakefield J, Jong JS, Guimbellot J, Venglarik C, Niraj A, Mazur M, Sorscher EJ, Collawn J, Bebok Z. Efficient intracellular processing of the endogenous cystic fibrosis transmembrane conductance regulator in epithelial cell lines. J Biol Chem. 2004;279:22578–22584. doi: 10.1074/jbc.M401522200. [DOI] [PubMed] [Google Scholar]
- 37.Peter K, Varga K, Bebok Z, McNicholas-Bevensee C, Schwiebert L, Sorscher E, Schwiebert E, Collawn J. Ablation of internalization signals in the carboxy-terminal tail of the cystic fibrosis transmembrane conductance regulator enhances cell surface expression. J Biol Chem. 2002;277:49952–49957. doi: 10.1074/jbc.M209275200. [DOI] [PubMed] [Google Scholar]


