Abstract
Adult neural stem cells (NSCs) are located in the subventricular zone (SVZ), a specialized brain niche located on the walls of the lateral ventricle. Under physiological conditions, NSCs generate a large number of young neurons and some oligodendrocytes, however the mechanisms controlling cell proliferation and migration are unclear. In vitro, epidermal growth factor (EGF) signaling has been shown to be an important mediator of cell proliferation and migration in the adult brain; however, the primary SVZ progenitors that respond to EGF are not well known. In this study, we isolated SVZ type-B astrocytes and cultured them under different EGF concentrations. We found a dose-dependent effect of EGF on proliferation rates and migration of SVZ type-B astrocytes. We found that GFAP+ type-B astrocytes gave rise to highly migratory and proliferating cells that expressed Olig2 and NG2. After EGF withdrawal, a significant number of EGF-stimulated cells differentiated into S100β+ / O4+ oligodendrocytes. This study provides new insights about the production of oligodendrocytes derived from the astrocyte NSCs residing in the adult SVZ. To be able to manipulate the endogenous adult progenitors, it is crucial to identify and isolate the responding primary precursors and determine the extracellular signals that regulate their cell division, migration and fate.
INTRODUCTION
The subventricular zone (SVZ) is located on the walls of the lateral ventricle (Lois and Alvarez-Buylla 1993; Morshead et al. 1994) and contains adult neural stem cells (NSCs), which not only generate a large number of young neurons that migrate in chains to the olfactory bulb (Lois and Alvarez-Buylla 1993; Lois and Alvarez-Buylla 1994), but also some oligodendrocytes that migrate mostly into the neighboring corpus callosum (Gonzalez-Perez et al. 2009; Menn et al. 2006). In the SVZ, primary precursors of new neurons in vivo correspond to type B astrocytes, which divide and give rise to actively proliferating transit amplifying type-C cells. Type C cells differentiate into neuroblasts (Type A cells) (Alvarez-Buylla and Garcia-Verdugo 2002).
SVZ neural stem cells proliferate and self-renew in response to EGF in vitro (Craig et al. 1996; Doetsch et al. 1999; Gritti et al. 1999; Kuhn et al. 1997; Morshead et al. 1994; Reynolds and Weiss 1992). Infusion of EGF into the brain results in a dramatic amplification of endogenous SVZ precursor cells (Craig et al. 1996; Doetsch et al. 2002; Kuhn et al. 1997), promoting–oligodendrogenesis (Gonzalez-Perez et al. 2009). EGFR signaling regulates the migratory pattern of neural precursors in the fetal telencephalon (Burrows et al. 1997; Caric et al. 2001; Ciccolini et al. 2005), confers motile phenotype to neural stem cells in vitro (Boockvar et al. 2003) and induces migration of SVZ-derived cells (Aguirre et al. 2005). This suggests that EGFR signaling is an important mediator of cell proliferation and migration. In the adult SVZ, EGFR has been found in the Type-B and Type-C cells (Doetsch et al. 2002), however the primary SVZ precursor that first responds to EGF signaling is not well-known. A previous study in vivo indicates that EGF induces adult SVZ astrocytes to differentiate into the oligodendrocyte lineage(Gonzalez-Perez et al. 2009). However, EGF can also induce the production of local factors that could affect the responsiveness of the SVZ primary progenitors. Therefore, isolating specific populations of cells allows for monitoring the effects of EGF under controlled conditions. In this study, we isolated and cultured SVZ type-B astrocytes and show that EGF induces a dose-dependent effect on proliferation and migration of type-B astrocytes which, in turn, give rise to highly migratory Olig2+ cells. Upon EGF removal, Olig2+ cells differentiate into s100β+ / O4+ cells. This study provides new insights about the production of oligodendrocytes derived from adult neural stem cells. Since SVZ stem cells may function as source of neural precursors for brain repair, elucidating the primary precursors that respond to growth factor stimulation is a crucial step in manipulating SVZ progenitors.
MATERIALS AND METHODS
Animal Care and Tissue Processing
P45 CD-1 mice were housed in the animal care facility and were maintained in accordance with the Committee on Animal Research Guidelines of the University of Guadalajara, Mexico. For matrigel assays and primary cell cultures, animals were anesthetized with an intraperitoneal injection of 25-30μl/g body weight of 2.5% Avertin®. Then, mice were decapitated and their brains immersed in ice-cold PIPES buffer.
Matrigel assay
Under a dissecting microscope, the SVZ from P45- mice was dissected from 200-μm coronal slices (from +0.5 to +0.2, antero-posterior coordinates relative to Bregma) by cutting a 1 mm long (dorsal to ventral) x 0.3 mm wide (from lateral ventricle to brain parenchyma) tissue piece with a 22.5° stab knife (Reading, PA; http//:www.surgicalsspecialities.com). Special care was taken to avoid tissue derived from corpus callosum and striatum. Tissue was then cut into pieces 50–300 μm in diameter. The explants were mixed with growth-factor-reduced basement membrane matrix (BD MatrigelTM; BD Biosciences, San Jose, CA) at 4°C in a 1:3 ratio and allowed to solidify into a glass-bottom 35-mm petri dish (Corning). The gel containing the explants was overlaid with 1.5 ml of serum-free Neurobasal-A medium (Gibco/BRL, Bethesda, MD) containing B-27 supplement (Gibco/BRL), 0.5 mM L-glutamine (Gibco/BRL), and penicillin–streptomycin antibiotics (Gibco/BRL). Cultures were maintained in a humidified, 5% CO2 incubator at 37°C. EGF was added to the media at concentrations of 0, 2.5, 5, 10, 20, 50 or 100 ng/ml of EGF (Upstate biotechnology). Tissue cultures were fixed at different days in vitro (DIV) by replacing media with 2% paraformaldehyde (PFA) in 0.1M phosphate buffer (PB) for 1 h and post-fixed for 6 h at 4°C in the same fixative.
Primary cell culture preparation
Cortices, striatum or SVZ from P45- mice were dissected from 200-μm coronal slices, the meninges were removed, and samples were minced and dissociated enzymatically by incubation with 1 ml of 0.25% Trypsin + 0.5 mM EDTA (Gibco/BRL, Bethesda, MD) at 37° C for 10 min. The trypsin was removed, fresh DMEM F-12 medium was added and tissue was triturated with a sterile Pasteur pipette with flame-rounded tip (the tip was rounded in a Bunsen flame and cooled in PBS). Trypsin was inhibited with DMEM/F12 + 10% fetal bovine serum medium. Remaining non-homogenized pieces of tissue were removed using a 40-μm cell strainer. Cells were counted with a hemocytometer and cell viability was determined with the use of trypan blue. 1×106 cells were plated in laminin-pre-coated 25-cm2 flasks with vented caps. Flasks were incubated at 37°C and 5% CO2. After a week, the culture medium was replaced with fresh medium; from this point, culture medium was changed three times a week. Cell cultures were checked daily for viability and cell density with an inverted microscope (Leica). Astrocyte cell cultures were purified as described below.
Astrocyte cell culture purification
This procedure was performed as previously described (Chaichana et al. 2009; Lim and Alvarez-Buylla 1999). At confluence, caps of 25-cm2 flasks were tightened. All flasks were firmly taped on a rotating shaker. Then, cell cultures were shook for 8 hours at 1,000 RPM at room temperature, so that only astrocytes remained adhered to the flask. The media in the flask was removed along with detached non-astrocytic cells and cell debris was eliminated by gently washing the flask twice for 1 min with 5 ml of sterile PBS pre-warmed at 37° C. After that, 5 ml of DMEM + 10% fetal bovine serum medium, pre-warmed at 37°C, was placed into flasks. To reduce the risk of tissue culture contamination, all flask caps were replaced with new sterile vented caps. After that, cultures were immediately returned to the incubator. Two days later, the same procedure was repeated. Cultures were then let to settle down for at least 48h before use for spot assays.
Spot assay
This assay was performed as described previously (Wichterle et al. 2003) and modified as follows: Purified astrocyte cultures were detached from flasks using 1 ml Trypsin/EDTA (Gibco/BRL, Bethesda, MD) at 37°C and flasks were tapped to mechanically assist the enzymatic detachment. Trypsin was inhibited with 10 mL of Neurobasal-A (NB-A) medium + 10% FBS + 10 μg/ml DNase I (Sigma). Cell suspension was placed into a sterile 15-ml tube and centrifuged at 1000 RPM, 4°C for 3-5 min to pellet. Medium was removed and cells re-suspended in a 5-ml Eppendorf tube using NB-A solution supplemented with B-27(Gibco/BRL, Bethesda, MD). Cells were re-concentrated by centrifuging at 1000 RPM at 4°C for 3 min. Finally, astrocytes were re-suspended with 3-5 μl of NB-A medium at a final concentration of 1-5 ×105 cells/μl. Cell suspension was spotted (~1μl per spot) onto polycarbonate filters floating on NB-A medium. Filters were fixed at different DIV by replacing media with fixative at 37°C as described above.
Immunocytochemistry (ICC)
For freshly-dissociated cell staining, animals were decapitated and their brains immersed in ice-cold PIPES buffer. Dorsal SVZ was dissected (1 mm long × 0.3 mm wide tissue piece from lateral wall of ventricle). SVZ was minced and incubated in 0.25% trypsin-EDTA solution at 37° C for 10 min. Then, trypsin was removed, fresh F-12 medium was added and tissue was triturated with a fire-polished pipette. The resulting cell suspension was placed, dried, and fixed with 3% PFA onto glass slides. Staining was performed as follows. For fluorescent ICC, samples were rinsed (10 min × 3) in 0.1 M PBS. After blocking in 0.1M PBS containing 10% normal goat serum for 1 h at room temperature, samples were incubated with primary antibodies overnight at 4°C in blocking solution plus 0.1% Triton-X, when appropriate. The following mouse monoclonal primary antibodies were used: anti-GFAP (1:500; Millipore), anti-TuJ1 (1:1000; Covance), anti-PSA-NCAM (1:1000; AbCys, France) and anti-EGFR (1:200; Millipore). The following polyclonal rabbit antibodies were used: anti-Olig2 (1:5000; kindly provided by Dr. H. Takebayashi), and anti-NG2 (1:250; Millipore). After that, tissue sections were rinsed three times with 0.1 M PBS, incubated with the appropriate Alexa Fluor conjugated secondary antibodies (all 1:1000; Molecular Probes) in blocking solution for 1h at room temperature, and washed three times in PBS. O4 staining was performed with staining medium (NB-A medium + 1:200 mouse anti-O4 antibody from Millipore). Culture medium was replaced by 0.5 ml of staining medium per well to the live unfixed cells for 30 minutes at room temperature with slow rocking. Staining medium was replaced by 3% PFA fixative and rinsed 2 times with PBS 0.1M. ICC was then performed as described above. Controls in which primary antibodies were omitted resulted in no detectable staining. For imaging, a Leica SP-2 laser scanning confocal microscope was used and optical 0.5 μm serial sections were obtained to co-localize fluorescent signals.
Quantification
Cells found in the cell culture were quantified by counting the total number of ICC-labeled cells found in at least five cell cultures per group, under the 40X magnification objective. Double-labeling was confirmed and quantified by matching cellular morphologies with clearly discernible nuclei and by analyzing at least 30, randomly selected, non-overlapping high-power fields of view. For all assays, at least five experiments per group were performed. All quantifications were performed with a fluorescence microscope (Leica QWin5001, Germany). All data are expressed as mean ± standard deviation. For mean comparisons among multiple groups, we used the one-way ANOVA followed by Tukey post-hoc test. For mean comparisons between two groups we used the Student's “t” test. In all cases, the P < 0.05 value was chosen to establish significant differences.
RESULTS
EGF has a dose-dependent effect on proliferation and phenotype of SVZ progenitors
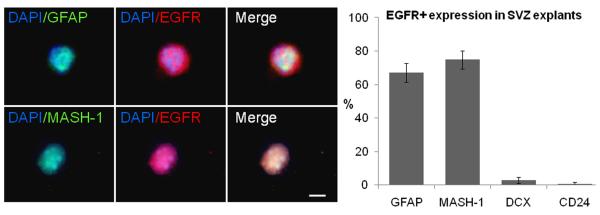
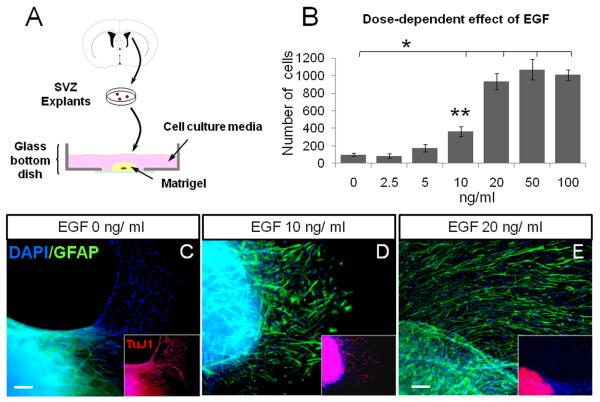
First, we characterized the EGFR expression in freshly dissociated cells obtained from SVZ explants. We used GFAP to label Type-B cells (Doetsch et al. 1999), MASH-1 for transit amplifying progenitors (Pastrana et al. 2009), doublecortin for neuroblasts (Gleeson et al. 1999) and CD24 for ependymal cells (Spassky et al. 2005). In the adult SVZ explants, 67 ± 5% of EGFR+ cells co-expressed GFAP+, 74 ± 5% co-expressed MASH-1, 2.9 ± 1% co-expressed doublecortin and 0.8 ± 0.8% co-expressed CD24 (figure 1). This suggested that most of the EGFR+ cells found in the explants corresponded to Type-B and Type-C cells. To characterize the effect of different concentrations of EGF on SVZ tissue explants, we incubated the tissue pieces in matrigel using different amounts of EGF (0, 2.5, 5, 10, 20, 50 and 100 ng/ml). Forty-eight hours later, EGF-treated explants showed cells leaving the core and incorporating into the matrigel. To quantify the number of cells that emerged from the explants, we fixed explants at 7 DIV. We found that the number of cells remained practically constant at doses ≥ 20 ng/ml (figure 2A-B). The proliferation rate, evaluated by the number of Ki67+ cells, showed a ~3.5-fold increase in the number of proliferating cells in the groups that received 20, 50 and 100 ng/ml of EGF as compared to lower doses (0, 2.5 and 5 ng/ml; P < 0.05, ANOVA-Tukey). These findings suggest a dose dependent effect of EGF on SVZ explants. No statistical differences were found in the number of cells among the groups that received 20 ng/ml (933 ± 94 cells), 50 ng/ml (1070 ± 115 cells) and 100 ng/ml (1007 ± 62 cells) of EGF.
Figure 1. EGFR expression in adult SVZ explants.
SVZ cell freshly dissociated and immunostained with antibodies anti-GFAP and MASH-1. Quantitative analysis indicates that the majority of EGFR+ cells in the SVZ co-express GFAP and MASH1 as compared to doublecortin (DCX) and CD-24. Scale bar = 5 μm.
Figure 2. Effect of EGF on SVZ tissue explants.
A. Schematic drawing illustrating the matrigel assay. 200-μm SVZ tissue pieces were immersed into solidified matrigel and covered by neurobasal medium.
B. Histogram of the number of DAPI+ cells found at 7 DIV after exposure to different doses of EGF. (*) indicates differences between groups 20, 50 and 100 ng/ml EGF vs. the low-dose groups; (**) indicates significant differences between the 10 ng/ml group vs. the groups treated with lower doses of EGF. P < 0.05; ANOVA-Tukey. No differences were found among the groups of 20, 50 and 100 ng/ml.
C - E. SVZ tissue explants at 7 DIV stained for astrocytes (GFAP in green) and neuroblasts (Tuj1 in red). EGF induced an increase in the number of GFAP+ cells associated with a reduction in the number of Tuj1+ cells (insets). Scale bar (C and D) = 20 μm and 10 μm (in E). DAPI-counterstained nuclei are in blue.
Remarkably the migration pattern of cells leaving the explants was affected by EGF dose. At 7 DIV, we performed double ICC to typify the phenotype of migratory cells. At dose of 0 ng/ml EGF, most cells, if not all, leaving the explants expressed the neuronal marker β-III-tubulin (as shown by TuJ1 expression), whereas GFAP-expressing cells were restricted to the tissue explant (figure 2C). At an EGF dose of 10 ng/ml, the number of TuJ1+ neuroblasts was reduced as the number of GFAP+ cells increased (figure 2D). At 20 ng/ml EGF no TuJ-1 expression was observed, instead a number of GFAP+ cells were found migrating out of the explant (figure 2E).
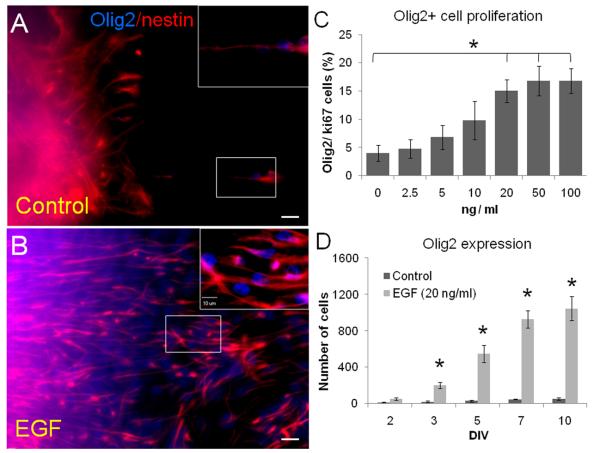
The above results suggest that EGF drove SVZ neuronal progenitors into a glial lineage. To further characterize the SVZ-derived cells stimulated by EGF, we counterstained sections for Olig2, nestin and S100β. We found that EGF induced a significant expression of Olig2 and nestin in migratory cells (figure 3A-B). Olig2+ cells proliferate in dose-dependent pattern as shown by Olig2/Ki67 expression: 0 ng / ml (4 ± 1.4 %), 2.5 ng/ml (4.8 ± 1.6 %), 5 ng/ml (6.8 ± 2.1 %), 10 ng/ml (9.8 ± 3.4 %), 20 ng/ml (15 ± 2 %; P ≤ 0.05 as compared to 0, 2.5, 5 and 10 ng/ml), 50 ng/ml (16.8 ± 2.5 %) and 100 ng/ml (16.8 ± 2.1 %)(figure 3C). Since no statistical differences were found among the groups 20, 50 and 100 ng/ml of EGF, we decided to use the dose 20 ng/ml of EGF to perform the remainder of the experiments. We then quantified the number of Olig2+ cells at 2, 3, 5, 7 and 10 DIV. The number of Olig2+ cells increases by the effect of EGF until 7 DIV when cell counts remained relatively constant (figure 3D). At 7 DIV, we found a ~20-fold increase in the number of Olig2+ cells in the EGF-cultivated explants (929 ± 95 cells per culture) as compared to controls (47 ± 4 cells per culture; P < 0.001, Student's “t” test). Taken together, these data suggest that EGF acts in a dose-dependent manner to induce the proliferation of SVZ progenitors and their differentiation towards a glial lineage.
Figure 3. Olig2 expression on SVZ tissue explants at 7 DIV.
A - B. Nestin (red) and Olig2 (blue) expression in SVZ explants treated with or without EGF for 7 days.
C. Cell proliferation of Olig2+ cells shows a dose-dependent effect of EGF as determined by Ki67 co-expression. (*) indicates differences between groups 20, 50 and 100 ng/ml EGF vs. the low-dose groups. P < 0.05, ANOVA-Tukey. No differences were found among the groups of 20, 50 and 100 ng/ml.
D. Absolute number of Olig2+ cells found at different time points at 20 ng/ml dose EGF. Statistically significant differences were found from the 3 DIV (asterisks indicate P < 0.01; Student's “t” test). Scale bars = 25 μm.
EGF-responding SVZ astrocytes give rise to Olig2/NG2+ migratory cells
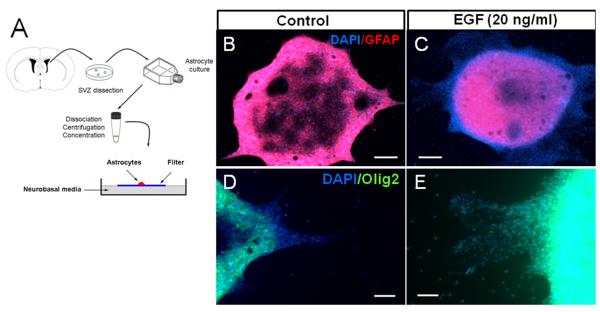
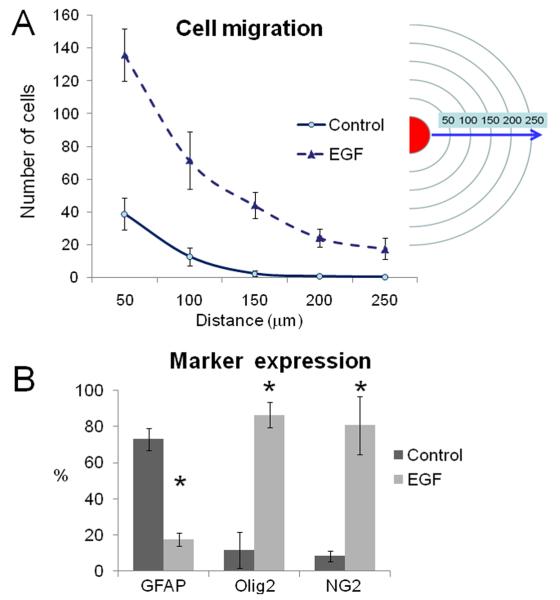
The above results indicate that local progenitors within the SVZ respond to EGF. To elucidate whether SVZ astrocytes (Type-B cells) are target cells that give rise to the EGF-expanded cell population, we purified SVZ astrocytes as described in the methods section. Using this technique, we observed that 99.2% (1364 / 1375 cells) of DAPI+ cells expressed GFAP. To characterize the population of purified GFAP+ astrocytes, we co-stained the cultures with antibodies against nestin, vimentin, Olig2 or EGFR. We found that of GFAP+ cells, 98.3% (857 / 872 cells) co-expressed nestin, 97.2% (830 / 854 cells) of GFAP+ cells co-expressed vimentin, 4 % (28 / 702 cells) GFAP+ cells co-expressed Olig2 and 11% (83 / 759 cells) of GFAP+ cells co-expressed EGFR. These findings confirm that an significant percentage of purified astrocytes express the EGFR. We then spotted purified astrocytes onto floating filters with EGF-supplemented media (20 ng/ml) or without EGF (figure 4A). At 3 DIV, cells were observed migrating out of the spot. At 7 DIV, this effect was more evident and under the effect of EGF most, if not all, migratory cells were GFAP-/Olig2+ (figure 4B-E). To determine the magnitude of the EGF effect on migration at 7 DIV, we quantified the number of cells found at different distances from 50 to 250 micrometers. We found a sustained migratory effect on astrocyte-derived Olig2+ cells stimulated by EGF (figure 5A). We then quantified the number of cells found out of the spot at 7 DIV. In the control group, 73 ± 6 % of the cells were GFAP+ vs. 17 ± 4 % of the EGF group (P < 0.05). In contrast, Olig2 and NG2 expression were more pronounced in the EGF group (86 ± 7% and 80 ± 16%, respectively) than in the controls (11 ± 9 and 8 ± 3 %, respectively; P < 0.05). These cells did not express TuJ1 or PSA-NCAM neuronal markers. These findings indicate that EGF-stimulated SVZ astrocytes give rise to highly migratory Olig2/NG2 progenitors.
Figure 4. Effect of EGF on SVZ purified astrocytes.
A. Schematic drawing illustrating the spot assay. GFAP+ astrocytes were spotted onto polycarbonate filters to evaluate the effect of EGF on marker expression and cell migration.
B - E. At 7 DIV, many GFAP-negative (red) cells migrating out of the core of the spot were observed in the EGF-treated group. Most, if not all, of these migratory cells expressed Olig2 (green) transcription factor. Magnifications: x4 (in B and C) and x20 (in D and E). Nuclei were counterstained with DAPI (blue). Bars in B - C = 25 μm; D - E = 10 μm.
Figure 5. Cell migration and marker expression induced by EGF.
A. Quantification of the number of DAPI+ cells found at 7 DIV. In the EGF-treated group many cells were found far away from the core of the spot.
B. In the EGF group (20 ng/ml), the cells observed out of the spots predominantly expressed Olig2 and NG2 markers, whereas in the control group most of the cells were GFAP+ astrocytes. Asterisks indicate P < 0.01; Student's “t” test.
To analyze whether astrocytes from other brain regions give rise to Olig2/NG2+ migratory cells, we cultured and purified astrocytes from cortex and striatum, and spotted them onto floating filters with EGF-supplemented media (20 ng/ml) or without EGF. At 3 and 7 DIV, very few cells were observed out of the spot of cortical and striatal astrocytes. In both cases, no statistical differences were found between the EGF group and controls (supplementary figure 1).
Olig2/NG2+ cells derived from SVZ astrocytes differentiate into an oligodendrocyte lineage
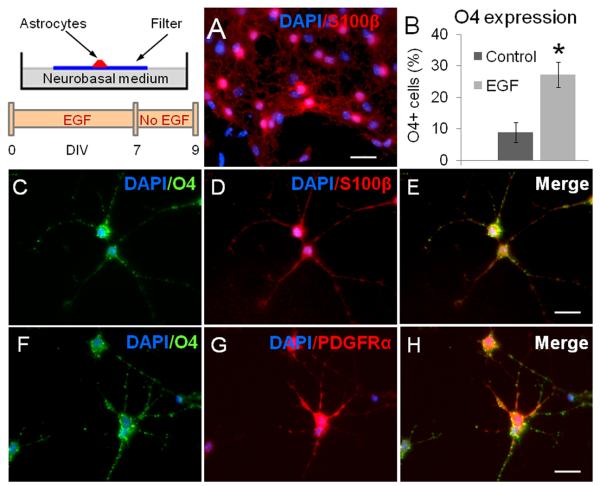
To elucidate the ultimate fate of the Olig2/NG2 progenitors derived from SVZ type B cells, EGF was removed from cell cultures and the spots were analyzed 48 hours later. We found that while the expression of Olig2 and NG2 was clearly reduced after EGF removal, a high number of cells found outside of the spots expressed S100β (figure 6A) in both the control (96 ± 3 % of S100β+ cells) and the group pre-exposed to EGF (92 ± 3 % of S100β+ cells). However, in the group pre-exposed to EGF, the proportion of cells that expressed O4 (an oligodendrocyte precursors marker) was higher (27 ± 4 %) when compared to controls (9 ± 3 %; P < 0.01) (figure 6B). Most, if not all, O4+ cells found in our cultures co-expressed S100β+ and PDFGRα (figure 6 C-H). These data indicate that the progeny of NG2/Olig2+ cells, derived from EGF-stimulated SVZ astrocytes, differentiate into the oligodendrocyte lineage upon EGF removal in vitro.
Figure 6. Phenotype of cells derived from SVZ astrocytes upon EGF removal.
A. 48 h after EGF withdrawal, most of the cells showed expanded processes and S100β expression (red).
B. Interestingly, ~30% of cells found after EGF stimulation expressed the oligodendrocyte marker O4. (*) indicates significant differences between the EGF-pretreated group vs. the control group (P < 0.05, Student's “t” test).
C - E. S100β+ (red) / O4+ (green) cells found in the EGF-pretreated spots. O4+ cells also expressed PDGFRα (F –H). DAPI-counterstained nuclei are in blue. Scale bar in A = 20 μm; in E and H = 10. μm.
DISCUSSION
In this study, we show that: 1) EGF induces proliferation, promotes migration and drives SVZ progenitors into a glial lineage in a dose-dependent manner; 2) SVZ astrocyte-derived migratory cells express Olig2/NG2, but not neuronal markers; and 3) when the EGF supplementation is withdrawn, Olig2/NG2 migratory cells stop migrating and give rise to cells expressing an oligodendrocyte marker. This in vitro approach offers the possibility of isolating specific populations of SVZ cells and monitoring the effects of EGF under controlled conditions
EGF is one of the main regulators of SVZ cell proliferation and self-renewal (Craig et al. 1996; Doetsch et al. 1999; Kuhn et al. 1997; Morshead et al. 1994; Reynolds and Weiss 1992). EGFR signaling plays an important role in the migration of embryonic neural precursors (Burrows et al. 1997; Caric et al. 2001; Ciccolini et al. 2005), neural stem cell lines (Boockvar et al. 2003) and early postnatal NG2-expressing cells (Aguirre et al. 2005). Interestingly, EGFR seems to have a negative effect on neuroblast migration (Kim et al. 2009). In the early postnatal SVZ, EGFR+/NG2- cells are transit amplifying precursors (Type C cells) (Cesetti et al. 2009). In the adult SVZ, recent findings indicate that EGFR+ Type B astrocytes functions as neural stem cells. According to our initial characterization of SVZ explants, the majority of these EGF-responding cells correspond to GFAP+ Type-B cells (Doetsch et al. 1999) and MASH1+ Type-C cells (Aguirre et al. 2004), but novel findings indicate that many of MASH1+ cells may actually be Type B cells (Pastrana et al. 2009). Our results in the matrigel assay indicate that EGF induces a dose-dependent expansion of highly-migratory adult SVZ progenitors. These EGF-expanded progenitors are in the glial lineage and express Olig2. Under physiological conditions, Olig2 expression in the adult SVZ is modest (Menn et al. 2006), and only a subpopulation of Type B and Type C cells expresses this transcription factor (Hack et al. 2005; Hack et al. 2004; Menn et al. 1998). Olig2 is essential for the generation of oligodendrocytes and motor neurons (Lu et al. 2002; Lu et al. 2000; Takebayashi et al. 2002; Zhou et al. 2001). Despite the crucial role of Olig2 in cell-fate decisions during glial development, little is known about how Olig2 is regulated in the adult brain. A recent study indicates that EGF is an important up-regulator of Olig2 expression in the adult SVZ (Gonzalez-Perez et al. 2009). PDGFRα and NG2 expression has been identified in Olig2-positive oligodendrocyte progenitors (Hack et al. 2005; Liu et al. 2003). Olig2−/− knockout mice lack NG2- and PDGFRα- expressing cells (Takebayashi et al. 2002). This suggested that the Olig2-positive cells expressing NG2 found after EGF supplementation could be committed to an oligodendrocyte lineage. For all cultures, we used B27-supplemented medium to provide an enhanced environment for cell division and to preserve multi-potential precursor cells in vitro (Svendsen et al. 1995). Although we also used non-neurogenic astrocytes as controls, it is possible that the combination of EGF plus B27 supplement had a synergistic effect on cell survival/proliferation, but removal of B27 supplement from media might promote differentiation of SVZ precursors.
Growth-Factor-Reduced Matrigel™ is a solubilized basement membrane rich in extracellular matrix proteins, such as laminin, collagen IV and heparan sulfate proteoglycans (Kleinman et al. 1982). Spot assays preclude this bias and allow examining the effect of EGF specifically on Type-B cells and in absence of extracellular matrix proteins. Using purified astrocyte cultures from the adult SVZ and the spot assay, we determined that EGF expanded-Olig2/NG2-expressing cells derive from the Type-B cells. Remarkably, we did not find a significant effect of EGF on non-SVZ astrocytes. These data support the notion that SVZ Type-B astrocytes actively respond to EGF (Gritti et al. 1999; Pastrana et al. 2009). However, we did not directly examine the effects of EGF on EGFR+/GFAP- progenitors in the SVZ and thus, the relative importance of the different populations of EGF-responsive cells in the adult SVZ could not be addressed.
We did not find cells that expressed GFAP or neuronal markers leaving the spot, which suggests that EGF drives a glial-precursor cell lineage in SVZ Type-B cells. Developmental studies indicate that EGF signaling has also been involved in cell fate and its overexpression pushes cells into glial lineage at the expense of neuron formation (Burrows et al. 1997; Caric et al. 2001; Viti et al. 2003). Olig2 expression has been associated with oligodendrocyte and astrocyte formation in the neonatal brain (Marshall et al. 2005). Additionally, recent findings have shown that Olig2 expressing cells can also give rise to astrocytes and ependymal cells (Masahira et al. 2006). Therefore, we decided to analyze the cell type to which these Olig2-positive cells give rise after EGF withdrawal. Interestingly, most of the cells found expressed markers of pre-myelinating oligodendrocytes (Aguirre et al. 2007). The role of EGF in adult oligodendrogenesis has been suggested in the ventral spinal cord (Chandran et al. 1998) and the adult SVZ (Aguirre et al. 2007; Gonzalez-Perez et al. 2009). Reduced EGFR signaling in progenitor cells of the adult SVZ attenuates the production of oligodendrocytes (Aguirre and Gallo 2007), whereas EGFR overexpression in SVZ and corpus callosum during early postnatal development expands oligodendrocyte population (Aguirre et al. 2007).
Our results indicate that when EGF is removed from cell culture media, the EGF-expanded cells give rise to S100β+ cells. These results support the possible role of Olig2 in astrocytic and oligodendroglial lineages as suggested in neonatal brain (Marshall et al. 2005). Although S100β has usually been associated with the astrocytic lineage, recent evidence suggests that S100β plays a role in oligodendroglial lineage and modulates oligodendrocyte maturation (Deloulme et al. 2004; Hachem et al. 2005). Therefore, a significant proportion of the S100β+ cells, found after EGF withdrawn, may correspond to oligodendrocyte precursors. Our data indicate that approximately one third of the S100β+ cells co-expressed O4 and PDGFRα, and thus were considered to be in the oligodendrocyte lineage (Danesin et al. 2006; Sugimori et al. 2007; Sugimori et al. 2008). O-antigens are expressed by oligodendrocytes during differentiation; in particular, O4 is expressed in pro-oligodendrocytes and is a marker for mature oligodendrocytes types I and II (Gard and Pfeiffer 1990; Reynolds and Hardy 1997). Our findings suggest that a subset of the Olig2/NG2-expressing cells derived from SVZ astrocytes give rise to cells in the oligodendrocyte lineage. Previous studies suggested that oligodendrocyte production strongly depends on EGFR signaling level (Aguirre and Gallo 2007). Thus, EGF-responsive progenitor cells in the SVZ may contribute to the repair of demyelinating lesions (Gonzalez-Perez et al. 2009; Menn et al. 2006; Nait-Oumesmar et al. 1999; Petratos et al. 2004; Picard-Riera et al. 2002). The present work indicates that EGF can promote the production of oligodendrocyte precursors derived from SVZ astrocytes.
In summary, our results indicate that EGF has a dose-dependent effect on adult SVZ astrocytes. The progeny of these multipotent astrocytes give rise to a high proportion of Olig2 progenitors that are driven into the oligodendrocyte lineage. The possibility of amplifying the population of mature oligodendrocytes derived from the adult SVZ may have important implications for cell replacement therapies.
Supplementary Material
The schematic drawing depicts the area of the cortex and striatum from which astrocytes were isolated. Histograms show the quantification of cells migrating out of the core of spots. In both cases, no statistically significant differences were found between groups (Student's “t” test).
ACKNOWLEDGEMENTS
This work was funded by NIH Grant K08NS055851 and the Howard Hughes Medical Institute to A.Q-H. O.G-P was financed by CONACyT (CB-2008-101476)
References
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10(8):990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Gallo V. Reduced EGFR signaling in progenitor cells of the adult subventricular zone attenuates oligodendrogenesis after demyelination. Neuron Glia Biol. 2007;3(3):209–20. doi: 10.1017/S1740925X08000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A, Rizvi TA, Ratner N, Gallo V. Overexpression of the epidermal growth factor receptor confers migratory properties to nonmigratory postnatal neural progenitors. J Neurosci. 2005;25(48):11092–106. doi: 10.1523/JNEUROSCI.2981-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165(4):575–89. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22(3):629–34. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boockvar JA, Kapitonov D, Kapoor G, Schouten J, Counelis GJ, Bogler O, Snyder EY, McIntosh TK, O'Rourke DM. Constitutive EGFR signaling confers a motile phenotype to neural stem cells. Mol Cell Neurosci. 2003;24(4):1116–30. doi: 10.1016/j.mcn.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Burrows RC, Wancio D, Levitt P, Lillien L. Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron. 1997;19:251–267. doi: 10.1016/s0896-6273(00)80937-x. [DOI] [PubMed] [Google Scholar]
- Caric D, Raphael H, Viti J, Feathers A, Wancio D, Lillien L. EGFRs mediate chemotactic migration in the developing telencephalon. Development. 2001;128(21):4203–16. doi: 10.1242/dev.128.21.4203. [DOI] [PubMed] [Google Scholar]
- Cesetti T, Obernier K, Bengtson CP, Fila T, Mandl C, Holzl-Wenig G, Worner K, Eckstein V, Ciccolini F. Analysis of stem cell lineage progression in the neonatal subventricular zone identifies EGFR+/NG2- cells as transit-amplifying precursors. Stem Cells. 2009;27(6):1443–54. doi: 10.1002/stem.74. [DOI] [PubMed] [Google Scholar]
- Ciccolini F, Mandl C, Holzl-Wenig G, Kehlenbach A, Hellwig A. Prospective isolation of late development multipotent precursors whose migration is promoted by EGFR. Dev Biol. 2005;284(1):112–25. doi: 10.1016/j.ydbio.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, Van der Kooy D. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. JNeurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaichana KL, Guerrero-Cazares H, Capilla-Gonzalez V, Zamora-Berridi G, Achanta P, Gonzalez-Perez O, Jallo GI, Garcia-Verdugo JM, Quinones-Hinojosa A. Intra-operatively obtained human tissue: protocols and techniques for the study of neural stem cells. J Neurosci Methods. 2009;180(1):116–25. doi: 10.1016/j.jneumeth.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran S, Svendsen C, Compston A, Scolding N. Regional potential for oligodendrocyte generation in the rodent embryonic spinal cord following exposure to EGF and FGF-2. Glia. 1998;24(4):382–9. doi: 10.1002/(sici)1098-1136(199812)24:4<382::aid-glia3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Danesin C, Agius E, Escalas N, Ai X, Emerson C, Cochard P, Soula C. Ventral neural progenitors switch toward an oligodendroglial fate in response to increased Sonic hedgehog (Shh) activity: involvement of Sulfatase 1 in modulating Shh signaling in the ventral spinal cord. J Neurosci. 2006;26(19):5037–48. doi: 10.1523/JNEUROSCI.0715-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloulme JC, Raponi E, Gentil BJ, Bertacchi N, Marks A, Labourdette G, Baudier J. Nuclear expression of S100B in oligodendrocyte progenitor cells correlates with differentiation toward the oligodendroglial lineage and modulates oligodendrocytes maturation. Mol Cell Neurosci. 2004;27(4):453–65. doi: 10.1016/j.mcn.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular Zone Astrocytes Are Neural Stem Cells in the Adult mammalian Brain. Cell. 1999;97:1–20. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36(6):1021–34. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Gard AL, Pfeiffer SE. Two proliferative stages of the oligodendrocyte lineage (A2B5+O4− and O4+GalC−) under different mitogenic control. Neuron. 1990;5:615–625. doi: 10.1016/0896-6273(90)90216-3. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan L, Walsh C. Doublecortin Is a Microtubule-Associated Protein and Is Expressed Widely by Migrating Neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez O, Romero-Rodriguez R, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Epidermal Growth Factor Induces the Progeny of Subventricular Zone Type B Cells to Migrate and Differentiate into Oligodendrocytes. Stem Cells. 2009;27(8):2032–2043. doi: 10.1002/stem.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti A, Frolichsthal-Schoeller P, Galli R, Parati EA, Cova L, Pagano SF, Bjornson CRR, Vescovi AL. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. JNeurosci. 1999;19:3287–3297. doi: 10.1523/JNEUROSCI.19-09-03287.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM, Gotz M. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005 doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- Hack MA, Sugimori M, Lundberg C, Nakafuku M, Gotz M. Regionalization and fate specification in neurospheres: the role of Olig2 and Pax6. Mol Cell Neurosci. 2004;25(4):664–78. doi: 10.1016/j.mcn.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Hachem S, Aguirre A, Vives V, Marks A, Gallo V, Legraverend C. Spatial and temporal expression of S100B in cells of oligodendrocyte lineage. Glia. 2005;51(2):81–97. doi: 10.1002/glia.20184. [DOI] [PubMed] [Google Scholar]
- Kim Y, Comte I, Szabo G, Hockberger P, Szele FG. Adult mouse subventricular zone stem and progenitor cells are sessile and epidermal growth factor receptor negatively regulates neuroblast migration. PLoS One. 2009;4(12):e8122. doi: 10.1371/journal.pone.0008122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. JNeurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci U S A. 1999;96(13. 13):7526–31. doi: 10.1073/pnas.96.13.7526. 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Cai J, Hu X, Tan M, Qi Y, German M, Rubenstein J, Sander M, Qiu M. Region-specific and stage-dependent regulation of Olig gene expression and oligodendrogenesis by Nkx6.1 homeodomain transcription factor. Development. 2003;130(25):6221–31. doi: 10.1242/dev.00868. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. ProcNatlAcadSciUSA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Marshall CA, Novitch BG, Goldman JE. Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J Neurosci. 2005;25(32):7289–98. doi: 10.1523/JNEUROSCI.1924-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26(30):7907–18. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Timsit S, Calothy G, Lamballe F. Differential Expression of TrkC Catalytic and Noncatalytic Isoforms Suggessts That They Act Independently or in Association. JcompPhysiol. 1998;401:47–64. doi: 10.1002/(sici)1096-9861(19981109)401:1<47::aid-cne4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, Van der Kooy D. Neural stem cells in the adult mammalian forebrain: A relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11(12):4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- Pastrana E, Cheng LC, Doetsch F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci U S A. 2009;106(15):6387–92. doi: 10.1073/pnas.0810407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petratos S, Gonzales MF, Azari MF, Marriott M, Minichiello RA, Shipham KA, Profyris C, Nicolaou A, Boyle K, Cheema SS. Expression of the low-affinity neurotrophin receptor, p75(NTR), is upregulated by oligodendroglial progenitors adjacent to the subventricular zone in response to demyelination. Glia. 2004;48(1):64–75. doi: 10.1002/glia.20056. and others. [DOI] [PubMed] [Google Scholar]
- Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Evercooren AB. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A. 2002;99(20):13211–6. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res. 1997;47(5):455–70. doi: 10.1002/(sici)1097-4547(19970301)47:5<455::aid-jnr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25(1):10–8. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimori M, Nagao M, Bertrand N, Parras CM, Guillemot F, Nakafuku M. Combinatorial actions of patterning and HLH transcription factors in the spatiotemporal control of neurogenesis and gliogenesis in the developing spinal cord. Development. 2007;134(8):1617–29. doi: 10.1242/dev.001255. [DOI] [PubMed] [Google Scholar]
- Sugimori M, Nagao M, Parras CM, Nakatani H, Lebel M, Guillemot F, Nakafuku M. Ascl1 is required for oligodendrocyte development in the spinal cord. Development. 2008;135(7):1271–81. doi: 10.1242/dev.015370. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, Fawcett JW, Bentlage C, Dunnett SB. Increased survival of rat egf-generated cns precursor cells using B27 supplemented medium. ExpBrain Res. 1995;102:407–414. doi: 10.1007/BF00230645. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12(13):1157–63. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Viti J, Feathers A, Phillips J, Lillien L. Epidermal growth factor receptors control competence to interpret leukemia inhibitory factor as an astrocyte inducer in developing cortex. J Neurosci. 2003;23(8):3385–93. doi: 10.1523/JNEUROSCI.23-08-03385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Alvarez-Dolado M, Erskine L, Alvarez-Buylla A. Permissive corridor and diffusible gradients direct medial ganglionic eminence cell migration to the neocortex. Proc Natl Acad Sci U S A. 2003;100(2):727–32. doi: 10.1073/pnas.242721899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The schematic drawing depicts the area of the cortex and striatum from which astrocytes were isolated. Histograms show the quantification of cells migrating out of the core of spots. In both cases, no statistically significant differences were found between groups (Student's “t” test).