Abstract
In order to overcome host mechanisms that prevent blood loss, the bloodsucking bug Rhodnius prolixus has evolved a complex salivary secretion containing dozens of different proteins. A number of these have been characterized and found to have roles in inhibiting various hemostatic or inflammatory systems. Interestingly, many of these biologically active salivary proteins belong to the lipocalin protein family. A proliferation of lipocalin genes has occurred via gene duplication and subsequent divergence. Functional genomic, proteomic and functional studies have been performed to probe the role of salivary lipocalins in blood feeding. In the course of these investigations, anticoagulant, antiplatelet, antiinflammatory and vasodilatory molecules have been described.
In the process of blood feeding arthropods must overcome the physiological defense responses of the host. Ingestion of blood would be impossible without some mechanism to inhibit normal clotting processes. Additionally, penetration of the skin and vasculature during feeding evokes an inflammatory response that may lead to defensive behavior by the host, and longer term attachment can cause immune responses that negatively affect feeding. The major role of salivary proteins in blood-feeding arthropods is to overcome these obstacles and maintain a free flow of blood through the mouthparts and into the gut. This is accomplished through the combined action of numerous salivary proteins and in some cases small molecules acting together to inhibit the coagulation cascade, limit platelet activation and prevent vasoconstriction responses. Depending on necessity, additional substances may be present that modulate inflammatory and immune responses.
Since blood feeding has evolved independently many times in arthropods, different types of molecules are used to overcome host hemostatic defenses in different species (Ribeiro 1995). In the bug Rhodnius prolixus, a vector of Chagas’ disease, an expansion of salivary gland proteins belonging to the lipocalin family has produced a large number of different molecules having unique antihemostatic activities while the fundamental structure of the protein fold is maintained (Montfort and others 2000). Similar proteins have also been described from other triatomine bugs. From detailed analyses of salivary gland cDNAs it appears that gene duplications have produced several major lipocalin groups in the saliva, while further duplication within each group has led to a remarkable level of functional diversity (Ribeiro and others 2004). The salivary components can be functionally redundant, with numerous components attacking the same physiological target. A relatively small number of these proteins have been subjected to functional studies, but the groups that have been intensively studied demonstrate that sequence variation within each major group of lipocalins has important functional consequences.
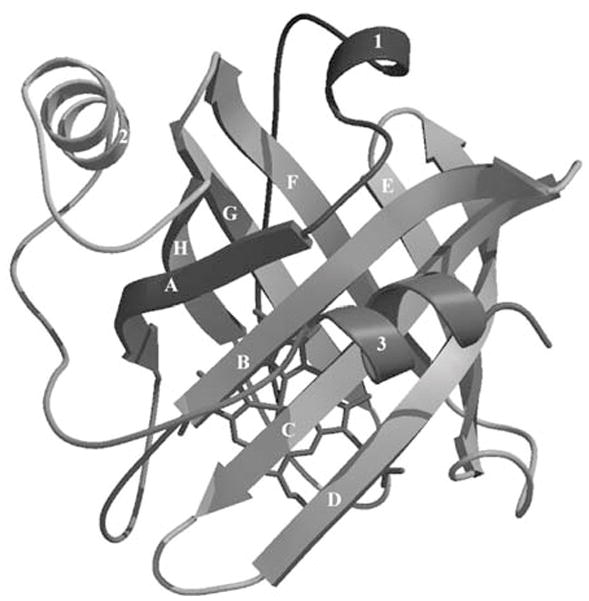
The lipocalin structure consists of an eight-stranded antiparallel β-barrel forming a central hydrophobic cavity (Fig. 1) (Flower 1996). The loops connecting the strands of the β-barrel and forming the entrance to the ligand binding cavity are flexible and can tolerate relatively free substitution of amino acid side chains without alteration of the basic protein fold. Lipocalins are found in all types of living organisms and serve a variety of functions from hormone transport to coloration (Flower 1996). Normally, lipocalins act by binding a small-molecule ligand, but they can also act by binding proteins in solution or receptors (Logdberg and Wester 2000).
Figure 1.
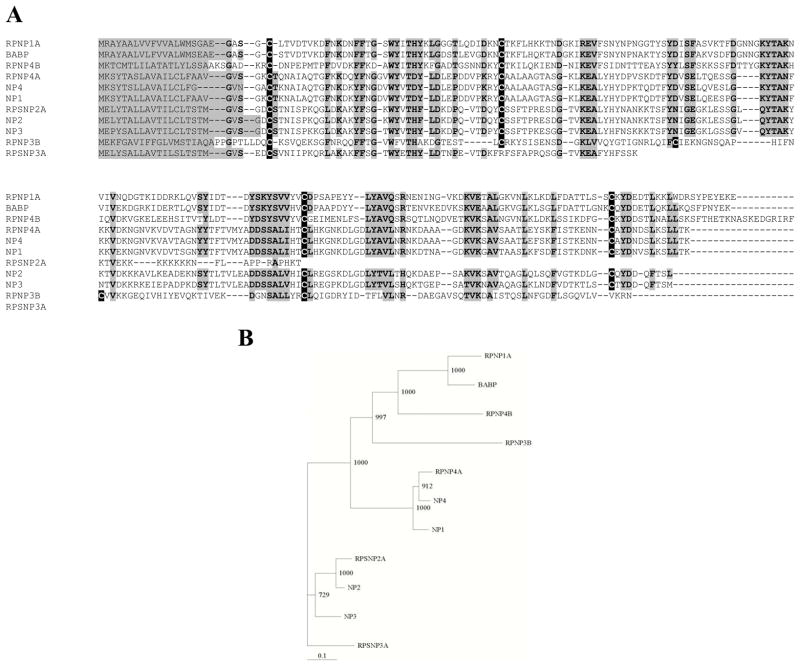
A. Clustal amino acid alignment and phylogenetic tree of lipocalins belonging to the nitrophorin (NP) group in R. prolixus saliva. Cysteine residues are highlighted in black, while other conserved regions are highlighted in gray. The original four NPs are labeled NP1-4. New NP sequences identified as ESTs are given the prefix RP. The biogenic amine binding protein is identified as BABP. B. A phylogenetic tree showing relationships within the nitrophorin group. The numbers refer to the bootstrap value for 1000 iterations (Taken from Ribeiro and others 2004).
In this review we describe the various roles played by lipocalin proteins in blood feeding by R. prolixus. This system provides an excellent example of adaptive diversification of a single protein family in a specialized tissue.
Methodological considerations
Until recently, salivary proteins were isolated using chromatographic procedures and various functional assays. This was followed by cloning of cDNAs from libraries based on amino acid sequence information from Edman degradation experiments (Champagne and others 1995; Valenzuela and others 1995). It is now feasible using high speed capillary sequencers to sequence hundreds of clones from salivary gland libraries (Ribeiro and others 2004). These data can be used to identify proteins with various biochemical activities based on sequence homology, or to hypothesize functions and test these hypotheses using recombinant proteins. We have developed methods that allow the expression in Escherichia coli and refolding of R. prolixus lipocalins for functional analyses (Andersen and others 1997). With these procedures, recombinant proteins can be produced as preparations of 100 mg or greater.
A common mechanism of salivary lipocalin action involves the binding of small-molecule effectors such as histamine. For this reason, methods for measuring binding affinity have been very important in elucidating the functions of various lipocalin forms. The nitrophorins (NPs) are nitric oxide (NO)-binding heme proteins. Ligand binding to NPs can be measured by changes in the spectral features of the protein in the bound and unbound state (Ribeiro and others 1993). This feature has been extremely useful for detailing the kinetics of NO binding using stopped flow or flash photolysis methods. For proteins that do not bind heme, ligand binding has been demonstrated using isothermal titration calorimetry, and the Hummel-Dreyer chromatographic method of equilibrium titration (Andersen and others 2003; Francischetti and others 2002). Furthermore, protein-protein and protein-lipid interactions have been characterized using surface plasmon resonance, an enormously sensitive and flexible physical method of detecting interactions between macromolecules (Andersen and others 2004; Isawa and others 2000).
Bio- and enzymatic assay systems have been essential in characterizing salivary lipocalins. The vasodilatory activity of NPs and other salivary lipocalins have been demonstrated using aortic preparations and measuring relaxation (Ribeiro 1982) (Ribeiro and others 1990). The antiserotonin activity of saliva and a salivary biogenic amine-binding protein (ABP) was first demonstrated by measuring inhibition of the serotonin-induced contraction of the rat uterus (Ribeiro 1982). Platelet aggregation assays performed in an aggregometer have been used to elucidate the activities of RPAI1 (a platelet aggregation inhibitor), ABP and nitric oxide released from the NPs (Francischetti and others 2000). Inhibition of aggregation responses to ADP, serotonin and epinephrine have been essential to determining the ligand-binding specificities and likely biological functions of these molecules.
Relationships diversity and structure of triatomine salivary lipocalins
A recent study of the transcriptome of R. prolixus salivary gland has provided the most complete picture of sequence diversity of triatomine salivary proteins (Ribeiro and others 2004). The saliva is extremely complex with 74 distinct transcripts being associated with predicted secretory products. Many of the Rhodnius salivary transcripts were shown directly to encode secreted products by analysis of salivary gland homogenates via Edman degradation following preparative HPLC. Among the putative secreted proteins, at least 62 distinct lipocalins are indicated by sequences in the salivary gland library. The vast majority of these have not been characterized functionally, but many are related by sequence similarity to previously characterized lipocalin forms.
Three types of R. prolixus salivary lipocalins have been functionally characterized. The most abundant lipocalins of the saliva are the NPs, a group of nitric oxide carrier proteins (Fig. 2). This lipocalin group also contains ABP which is related to the NPs by sequence similarity. Instead of nitric oxide, however, it binds a category of biologically active small molecules, the biogenic amines. The second lipocalin group contains the ADP-binding protein RPAI1 which bind adenosine and its phosphates with high affinity and acts to inhibit platelet activation and aggregation. The third group is comprised of uncharacterized lipocalins related to the Triatoma pallidipennis thrombin inhibitor triabin (Fuentes-Prior and others 1997). Inhibition of thrombin by R. prolixus saliva has not been seen suggesting that proteins in this group are functionally different than triabin.
Figure 2.
Ribbon diagram of NP4 with the eight β-strands labeled A-H. Note the heme prosthetic group located in the central cavity (From Andersen and others 1998).
The nitrophorins
In the mammalian host, NO is a hormone-like molecule produced by the vascular endothelium and regulates vascular tone. By activating soluble guanylate cyclase, NO stimulates signaling pathways that ultimately result in relaxation of the vascular wall. NO also functions in many other tissues and physiological systems. It is produced by immune effector cells as a toxicant aimed at invading microorganisms, and is important as a platelet aggregation inhibitor.
R. prolixus produces NO in the salivary gland and delivers it to the circulation where it induces a vasodilatory response (Ribeiro and others 1990). The stability of NO in solution is low, however, and it must be protected from oxidation by protein binding (Ding and others 1999; Weichsel and others 2000). Protection is provided by the NPs which transport a single molecule of NO per molecule of protein from the gland to the host while protecting it from oxidation. Once in the host tissue or circulation the NO is released where it can act as a vasodilator and platelet aggregation inhibitor.
The crystal structures of several NPs have been determined and each is found to bind a single molecule of heme in the central cavity of the β-barrel (Andersen and Montfort 2000; Andersen and others 1998; Roberts and others 2001; Weichsel and others 1998). The heme is ligated to the protein via a coordination of the iron atom with a histidine residue on the second β-strand of the barrel. In the salivary gland, nitric oxide is synthesized in the epithelium, secreted into the salivary gland lumen and coordinated with iron on the distal side of the heme. The binding and release of NO can be readily observed by the position of the Soret absorbance in the optical spectrum at approximately 420 nm in the NO complex, and approximately 404 nm in the NO free molecule (Ribeiro and others 1993). The heme iron of NPs is maintained in the FeIII oxidation state. This results in NO binding of moderate affinity, rather than the extremely tight binding that is seen with an FeII complexes (Ding and others 1999).
The NO complex of the NPs is more stable at pH 5-6, than at pH 7.4. This difference is thought to be physiologically significant in enhancing the delivery of NO to the host. The pH of the saliva is reportedly near 6.0, providing for a relatively stable complex in the salivary gland (Yuda and others 1997). Injection into the host raises the pH surrounding the NP to 7.4, resulting in an increase in the release rate of NO of approximately 5-10-fold (Andersen and others 2000) (Table 1).
Table 1.
Nitric oxide release rate constants for nitrophorins 1-4a. Two rate constants are observable, koff1 = fast and koff2 = slow (Andersen and others 2000).
| pH 5.0 | pH 8.0 | |||
|---|---|---|---|---|
| koff1a | koff2 | koff1 | koff2 | |
| NP1 | 0.2 | 0.02 | 2.2 | 0.6 |
| NP2 | 0.05 | 0.006 | 0.12 | 0.01 |
| NP3 | 0.05 | 0.005 | 0.08 | 0.01 |
| NP4 | 0.14 | 0.01 | 2.6 | 0.6 |
sec−1
NPs can also bind one molecule of histamine in the binding pocket (Andersen and others 2000; Ribeiro and Walker 1994; Weichsel and others 1998). The binding is not coincident with that of NO since both coordinate with iron on the distal side of the heme. Histamine release by mast cells in response to a feeding wound causes swelling and pain that elicits host defensive responses, and the histamine-binding activity of the NPs is apparently aimed at preventing this. In this case, NPs act as a sort of “molecular sponge” by absorbing the biologically active small molecule histamine and preventing it from serving as a physiological effector. As will be seen below in discussions of platelet aggregation inhibitors, the “sponge” mechanism is a common and extremely adaptable method of antihemostasis involving R. prolixus salivary lipocalins.
Anticoagulant effects of NPs
The saliva of R. prolixus has long been known to possess potent anticoagulant activity (Hellman and Hawkins 1965). After direct isolation from salivary gland homogenates the primary anticlotting agent was shown to be NP2 (Ribeiro and others 1995). The anticoagulant activity of this molecule is independent of NO or histamine binding, and is not related to the presence of the heme prosthetic group. NP2 inhibits clotting by preventing assembly of the intrinsic factor Xase complex on the anionic phospholipid membranes of activated platelets (Zhang and others 1998). The protein binds directly with factor IXa, the protease component of the complex, but does not inhibit the hydrolysis of chromogenic small-peptide substrates, indicating that it does not block the catalytic site (Isawa and others 2000). Rather, NP2 interferes either with interaction of factor IXa with factor VIIIa or with the phospholipid membrane. Interestingly, despite having 80 % sequence identity with NP2, NP3 possesses only weak anticoagulant activity.
Membrane binding and NP function
In resting platelets, anionic phospholipids (particularly phosphatidylserine (PS)) are sequestered in the inner leaflet of the plasma membrane. On activation, this asymmetry is lost as PS is rapidly flips to the outer leaflet of the membrane in a calcium-dependent process (Sims and Wiedmer 2001). The result is a randomization of phospholipids in the membrane, with formation of a negatively-charged exterior surface that provides a substrate for assembly of coagulation complexes. Assembly on the membrane results in large rate accelerations in the intrinsic factor Xase and prothrombinase reactions. Recognition and binding to activated platelet membranes could serve to concentrate the salivary proteins in areas where the binding and release of biologically active ligands would have the most effect.
Many of the predicted translation products of R. prolixus salivary proteins show extended tracts of basic amino acids or have predicted positively charged surfaces that could potentially interact with negatively-charged phospholipids in membranes (Ribeiro and others 2004). Molecular modeling of NP7, a new member of the NP family showed it to have a positively charged area on its molecular surface relative to other members of the NP group (Andersen and others 2004). Recombinant NP7 was found to bind tightly to vesicles containing PS or mixtures of PS and phosphatidylcholine (PC), but not to homogeneous PC vesicles (Andersen and others 2004) (Table 2). The binding was sufficient to inhibit the prothrombinase reaction by blocking enzyme complex assembly sites on both phospholipid vesicles and activated platelets.
Table 2.
Binding and kinetic parameters (two-site model) for association of NP7 to 3:1 PC:PS monolayer as determined by surface plasmon resonance (Andersen and others 2004).
M−1s−1
s−1
nM
It is not clear whether the physiological significance of NP7 membrane binding lies in the inhibition of coagulation or in the increased efficiency of binding and release of ligands. It does appear, however, that recognition of anionic lipids is a feature of a number of R. prolixus salivary proteins, and is probably of importance in other arthropod blood feeders.
Reasons for NP multiplicity
Initially, four distinct forms of NP were isolated from saliva and identified as NP1-4. Detailed studies on the nitric oxide binding capabilitites of these proteins showed that they differed in their rate constants for nitric oxide release (Table 1). NP1 and NP4 release nitric oxide rapidly (koff ~ 2.5 sec-1) while NP2 and NP3 release it more slowly (koff ~ 0.1 sec−1) at physiological pH (Andersen and others 2000). These differences in release rate are probably functionally significant, serving to spread the vasodilatory NO in both space and time relative to a single NO-binding protein. NP1 and 4 would release NO quickly and near the feeding site while NP2 and 3 would release it more slowly and over a larger area.
The of the anticoagulant and histamine-binding activities of NPs demonstrates that secondary and tertiary functions can be acquired by salivary lipocalins. These observations strongly suggest that the sequence diversification seen with other R. prolixus salivary lipocalin groups is also related to functional diversification (Ribeiro and others 2004).
RPAI1, an ADP-binding protein
The most obvious antiplatelet activity in R. prolixus salivary gland homogenates could be assigned to the enzyme apyrase, which converts the potent platelet agonist ADP to AMP and inorganic phosphate (Ribeiro and Garcia 1981). Apyrase has not yet been isolated from saliva, but fractionation of gland extracts revealed a second, less prominent antiplatelet substance that did not act enzymatically. The activity was assigned to a lipocalin protein designated as RPAI1 (Rhodnius Platelet Aggregation Inhibitor 1) that was distinct from NP or ABP but showed sequence homology with pallidipin, a platelet aggregation inhibitor from the related bug Triatoma pallidipennis (Francischetti and others 1999; Noeske-Jungblut and others 1994).
RPAI1 proved to be an inhibitor of ADP induced platelet aggregation, but was only effective at low concentrations of ADP. The reason for this became clear when RPAI1 was found to bind a single molecule of ADP at high affinity (K0.5 = 49 nM) (Francischetti and others 2002) (Table 3). The protein is an effective inhibitor of platelet aggregation up to equimolar concentrations of RPAI1 and ADP. However, in the presence of excess ADP the inhibitory effect is not seen since the protein binding sites are saturated. The mechanism of inhibition of RPAI1 lies in its ability to remove secreted ADP from the vicinity of the platelet and prevent it from performing its role in propagation of aggregation (Francischetti and others 2000). The protein shows little binding specificity among adenosine nucleotides, with relatively small differences in affinity being seen between ATP, ADP and AMP (Francischetti and others 2002) (Table 3). Clearly, the phosphate moieties do not pose any large steric constraints to binding, and may protrude from the binding pocket into solution.
Table 3.
Affinities of adenosine and its phosphates for the lipocalin RPAI1 as determined by the Hummel-Dreyer method of equilibrium gel filtration (Francischetti and others 2002).
| Nucleotide | K0.5a |
|---|---|
| ATP | 14.8 |
| ADP | 48.9 |
| AMP | 31.8 |
| ADO | 60.0 |
nM
Biogenic amine binding protein
Random generation of ESTs from a R. prolixus salivary gland library revealed a group of genes encoding a relatively abundant but uncharacterized lipocalin (Andersen and others 2003). An antiserotonin activity had previously been detected in R. prolixus saliva by noting its ability to prevent the serotonin induced contraction of the rat uterus (Ribeiro 1982). We hypothesized that a mechanism analogous to the antihistamine function of the NPs might be operating and this novel lipocalin was a good candidate as the active agent.
After expression of the cDNA in E. coli and refolding of the protein, bioassay showed it to be a potent inhibitor of serotonin induced uterine contraction (Andersen and others 2003). The stoichiometry of serotonin binding to protein concentration was 1:1, and the inhibition could be completely overcome by adding an excess of serotonin. This suggests that the protein was indeed acting by binding the agonist directly and preventing it from binding with the 5-HT receptor. Subsequent experiments using isothermal titration calorimetry demonstrated that serotonin as well as epinephrine and norepinephrine bound directly to ABP with nanomolar affinity (Table 4). Because of its binding specificity the protein was given the name amine-binding protein (ABP).
Table 4.
Dissociation constants (Kd) for biogenic amines with the R. prolixus biogenic amine-binding protein (ABP) as determined by isothermal titration calorimetry (Andersen and others 2003)
| Ligand | Kda |
|---|---|
| Serotonin | 102 |
| Epinephrine | 345 |
| Norepinephrine | 24 |
nM
The functional significance of this protein in blood feeding is manifold. Serotonin and epinephrine are contained in the dense granules of platelets and contribute significantly to activation and aggregation. Neither serotonin nor epinephrine given alone are able to induce platelet aggregation, but do induce a full aggregation response when given together. Importantly, both serotonin and epinephrine potentiate the response of platelets to the important agonists collagen and ADP (Andersen and others 2003). Salivary ABP would neutralize the effect of these agonists on platelet aggregation by binding them as they are secreted from the platelet. Binding of norepinephrine could also have a significant antihemostatic effect. On wounding, norepinephrine is released by sympathetic nerves in the vessel wall and interacts with adrenergic receptors causing vasoconstriction, and important mechanism for preventing blood loss. Binding of norepinephrine by ABP would reduce the circulating concentration of norepinephrine and prevent vasoconstriction. In this regard, ABP can be thought of as acting in concert with the NPs by preventing a physiological response that is antagonistic to the action of NO.
Conclusions
The lipocalin protein family has undergone tremendous diversification in the salivary gland of R. prolixus. This enormously adaptable protein fold has taken on numerous roles in blood feeding. The NP group alone has vasodilatory, anticoagulant, antiinflammatory, and anti-platelet activities. The suitability of the lipocalin fold as a general antihemostatic template lies in its remarkable ability to tolerate extensive amino acid substitution, particularly in the loops surrounding the ligand-binding pocket. This binding pocket plasticity has allowed the evolution of many specific interaction sites for small molecules, lipids and proteins. From this single type of scaffold R. prolixus has fashioned an entire arsenal of antihemostatic and antiinflammatory molecules that allow it to efficiently consume a blood meal.
References
- Andersen JF, Champagne DE, Weichsel A, Ribeiro JMC, Balfour CA, Dress V, Montfort WR. Nitric Oxide Binding and Crystallization of Recombinant Nitrophorin I, a Nitric Oxide Transport Protein from the Blood-Sucking Bug Rhodnius prolixus. Biochemistry. 1997;36(15):4423–4428. doi: 10.1021/bi9628883. [DOI] [PubMed] [Google Scholar]
- Andersen JF, Ding XD, Balfour C, Shokhireva TK, Champagne DE, Walker FA, Montfort WR. Kinetics and equilibria in ligand binding by nitrophorins 1–4: Evidence for stabilization of a nitric oxide-ferriheme complex through a lignad-induced conformational trap. Biochemistry. 2000;39(33):10118–10131. doi: 10.1021/bi000766b. [DOI] [PubMed] [Google Scholar]
- Andersen JF, Francischetti IMB, Valenzuela JG, Schuck P, Ribeiro JMC. Inhibition of hemostasis by a high affinity biogenic amine-binding protein from the saliva of a blood-feeding insect. J Biol Chem. 2003;278:4611–4617. doi: 10.1074/jbc.M211438200. [DOI] [PubMed] [Google Scholar]
- Andersen JF, Gudderra NP, Francischetti IMB, Valenzuela JG, Ribeiro JMC. Recognition of anionic phospholipid membranes by an antihemostatic protein from a blood-feeding insect. Biochemistry. 2004 doi: 10.1021/bi049655t. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JF, Montfort WR. The crystal structure of nitrophorin 2: A trifunctional antihemostatic protein form the saliva of Rhodnius prolixus. J Biol Chem. 2000;275(39):30496–30503. doi: 10.1074/jbc.M002857200. [DOI] [PubMed] [Google Scholar]
- Andersen JF, Weichsel A, Balfour CA, Champagne DE, Montfort WR. The crystal structure of nitrophorin 4 at 1.5 A resolution: transport of nitric oxide by a lipocalin-based heme protein. Structure. 1998;6(10):1315–27. doi: 10.1016/s0969-2126(98)00131-2. [DOI] [PubMed] [Google Scholar]
- Champagne DE, Nussenzvieg RH, Ribeiro JMC. Purification, partial characterization and cloning of nitric oxide-carrying heme proteins (nitrophorins) from salivary glands of the blood-sucking insect Rhodnius prolixus. The Journal of Biological Chemistry. 1995;270(15):8691–8695. doi: 10.1074/jbc.270.15.8691. [DOI] [PubMed] [Google Scholar]
- Ding XD, Weichsel A, Andersen JF, Shokhireva TK, Balfour C, Pierik AJ, Averill BA, Montfort WR, Walker FA. Nitric oxide binding to the ferri- and ferroheme states of nitrophorin 1, a reversible NO-binding heme protein from the saliva of the blood-sucking insectRhodnius prolixus. J Am Chem Soc. 1999;121:128–138. [Google Scholar]
- Flower DR. The lipocalin protein family: structure and function. Biochemical Journal. 1996;318:1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM, Andersen JF, Ribeiro JM. Biochemical and functional characterization of recombinant Rhodnius prolixus platelet aggregation inhibitor 1 as a novel lipocalin with high affinity for adenosine diphosphate and other adenine nucleotides. Biochemistry. 2002;41(11):3810–3818. doi: 10.1021/bi011015s. [DOI] [PubMed] [Google Scholar]
- Francischetti IM, Ribeiro JM, Champagne D, Andersen J. Purification, cloning, expression, and mechanism of action of a novel platelet aggregation inhibitor from the salivary gland of the blood-sucking bug, Rhodnius prolixus. Journal of Biological Chemistry. 2000;275(17):12639–12650. doi: 10.1074/jbc.275.17.12639. [DOI] [PubMed] [Google Scholar]
- Francischetti IMB, Ribeiro JMC, Champagne D, Andersen JF. Purification, cloning, expression, and mechanism of action of a novel salivary platelet aggregation inhibitor frm the blood sucking bugRhodnius prolixus. The Journal of Biological Chemistry. 1999 doi: 10.1074/jbc.275.17.12639. in press. [DOI] [PubMed] [Google Scholar]
- Fuentes-Prior P, Noeske-Jungblut C, Donner P, Schleuning W-D, Huber R, Bode W. Structure of the thombin complex with triabin, a lipocalin-like exosite-binding inhibitor derived from a triatomine bug. Proceedings of the National Acadamy of Sciences USA. 1997;94:11845–11850. doi: 10.1073/pnas.94.22.11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman K, Hawkins RI. Prolixin-S and prolixin-G: Two anticoagulants from Rhodnius prolixus STAHL. Nature. 1965;207:265–268. doi: 10.1038/207265a0. [DOI] [PubMed] [Google Scholar]
- Isawa H, Yuda M, Yoneda K, Chinzei Y. The insect salivary protein prolixin-S inhibits factor IXa generation and Xase complex formation in the blood coagulation pathway. The Journal of Biological Chemistry. 2000;275:6636–6641. doi: 10.1074/jbc.275.9.6636. [DOI] [PubMed] [Google Scholar]
- Logdberg L, Wester L. Immunocalins: a lipocalin subfamily that modulates immune and inflammatory responses. Biochim Biophys Acta. 2000;1482:284–297. doi: 10.1016/s0167-4838(00)00164-3. [DOI] [PubMed] [Google Scholar]
- Montfort WR, Weichsel A, Andersen JF. Nitrophorins and related antihemostatic lipocalins fronm Rhodnius prolixus and other blood-sucking arthropods. Biochimica et Biophysica Acta. 2000 doi: 10.1016/s0167-4838(00)00165-5. in press. [DOI] [PubMed] [Google Scholar]
- Noeske-Jungblut C, Kratzschmar J, Haendler B, Alagon A, Possani L, Verhallen P, Donner P, Shleuning W-D. An inhibitor of collagen-induced platelet aggregation from the saliva of Triatoma pallidipennis. The Journal of Biological Chemistry. 1994;269:5050–5053. [PubMed] [Google Scholar]
- Ribeiro JMC. The antiserotonin and antihistamine activities of salivary secretion of Rhodnius prolixus. J Insect Physiol. 1982;28(1):69–75. [Google Scholar]
- Ribeiro JMC. Blood-feeding arthropods - live syringes or invertebrate pharmacologists. Infectious Agents and Disease. 1995;4(3):143–152. [PubMed] [Google Scholar]
- Ribeiro JMC, Andersen J, Silva-Neto MAC, Pham VM, Garfield MK, Valenzuela JG. Exploring the sialome of the blood-sucking bug Rhodnius prolixus. Ins Biochem Mol Biol. 2004;34:61–79. doi: 10.1016/j.ibmb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Ribeiro JMC, Garcia ES. The role of the salivary glands in feeding in Rhodnius prolixus. J Exp Biol. 1981;94:219–230. [Google Scholar]
- Ribeiro JMC, Hazzard JMH, Nussenzveig RH, Champagne DE, Walker FA. Reversible binding of nitric oxide by a salivary heme protein from a bloodsucking insect. Science. 1993;260:539–541. doi: 10.1126/science.8386393. [DOI] [PubMed] [Google Scholar]
- Ribeiro JMC, Marinotti O, Gonzales R. A salivary vasodilator in the blood-sucking bug, Rhodnius prolixus. British Journal of Pharmacology. 1990;101:932–936. doi: 10.1111/j.1476-5381.1990.tb14183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JMC, Schneider M, Guimaraes JA. Purification and characterization of prolixin S (nitrophorin 2), the salivary anticoagulant of the blood-sucking bug Rhodnius prolixus. Biochemical Journal. 1995;308:243–249. doi: 10.1042/bj3080243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JMC, Walker FA. High affinity histamine-binding and antihistaminic activity of the salivary nitric oxide-carrying heme protein (nitrophorin) of Rhodnius prolixus. Journal of Experimental Medicine. 1994;180:2251–2257. doi: 10.1084/jem.180.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SA, Weichsel A, Qiu Y, Shelnutt JA, Walker FA, Montfort WR. Ligand-induced heme ruffling and bent NO geometry in ultra-high-resolution structures of nitrophorin 4. J Biol Chem. 2001;40:11327–11337. doi: 10.1021/bi0109257. [DOI] [PubMed] [Google Scholar]
- Sims PJ, Wiedmer T. Unraveling the mysteries of phospholipid scrambling. Thromb Haemost. 2001;86:266–275. [PubMed] [Google Scholar]
- Valenzuela JG, Walker FA, Ribeiro JMC. A salivary nitrophorin (nitric-oxide-carrying hemoprotein) in the bedbug Cimex lectularius. Journal of Experimental Biology. 1995;198:1519–1526. doi: 10.1242/jeb.198.7.1519. [DOI] [PubMed] [Google Scholar]
- Weichsel A, Andersen JF, Champagne DE, Walker FA, Montfort WR. Crystal structures of a nitric oxide transport protein from a blood-sucking insect. Nature Structural Biology. 1998;5:304–309. doi: 10.1038/nsb0498-304. [DOI] [PubMed] [Google Scholar]
- Weichsel A, Andersen JF, Roberts SA, Montfort WR. Nitric oxide binding to nitrophorin 4 induces complete distal pocket burial. Nature Structural Biology. 2000;7(7):551–554. doi: 10.1038/76769. [DOI] [PubMed] [Google Scholar]
- Yuda M, Higuchi K, Sun J, Kureishi Y, Ito M, Chinzei Y. Expression, reconstitution and characterization of prolixin-S as a vasodilator--a salivary gland nitric-oxide-binding hemoprotein of Rhodnius prolixus. Eur J Biochem. 1997;249(1):337–42. doi: 10.1111/j.1432-1033.1997.00337.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ribeiro JM, Guimaraes JA, Walsh PN. Nitrophorin-2: a novel mixed-type reversible specific inhibitor of the intrinsic factor-X activating complex. Biochemistry. 1998;37(30):10681–10690. doi: 10.1021/bi973050y. [DOI] [PubMed] [Google Scholar]