Abstract
The vacuolar H+-ATPase (V-ATPase) is a major contributor to luminal acidification in epithelia of Wolffian duct origin. In both kidney-intercalated cells and epididymal clear cells, cAMP induces V-ATPase apical membrane accumulation, which is linked to proton secretion. We have shown previously that the A subunit in the cytoplasmic V1 sector of the V-ATPase is phosphorylated by protein kinase A (PKA). Here we have identified by mass spectrometry and mutagenesis that Ser-175 is the major PKA phosphorylation site in the A subunit. Overexpression in HEK-293T cells of either a wild-type (WT) or phosphomimic Ser-175 to Asp (S175D) A subunit mutant caused increased acidification of HCO3−-containing culture medium compared with cells expressing vector alone or a PKA phosphorylation-deficient Ser-175 to Ala (S175A) mutant. Moreover, localization of the S175A A subunit mutant expressed in HEK-293T cells was more diffusely cytosolic than that of WT or S175D A subunit. Acute V-ATPase-mediated, bafilomycin-sensitive H+ secretion was up-regulated by a specific PKA activator in HEK-293T cells expressing WT A subunit in HCO3−-free buffer. In cells expressing the S175D mutant, V-ATPase activity at the membrane was constitutively up-regulated and unresponsive to PKA activators, whereas cells expressing the S175A mutant had decreased V-ATPase activity that was unresponsive to PKA activation. Finally, Ser-175 was necessary for PKA-stimulated apical accumulation of the V-ATPase in a polarized rabbit cell line of collecting duct A-type intercalated cell characteristics (Clone C). In summary, these results indicate a novel mechanism for the regulation of V-ATPase localization and activity in kidney cells via direct PKA-dependent phosphorylation of the A subunit at Ser-175.
Keywords: ATPases, H+-ATPase, Kidney, Protein Kinase A (PKA), Vacuolar ATPase, ATP6V1A, Clone C, Bicarbonate, Proximal Tubule
Introduction
V-ATPases are ubiquitous and essential transport protein complexes that acidify many cellular organelles, such as endosomes, lysosomes, and the Golgi complex (1). The V-ATPase has 14 subunits distributed into two domains. The V1 peripheral or cytoplasmic domain, which catalyzes ATP hydrolysis, is composed of eight different subunits, including subunit A (reviewed in Ref. 2). The V0 integral membrane domain, which is responsible for H+ translocation, consists of six different subunits, including subunit a (2). Some epithelial cells, such as kidney proximal tubular cells, A-type intercalated cells, and epididymal clear cells express abundant V-ATPase at their apical membrane, where it participates in endocytosis and luminal acidification of the kidney tubule and the male reproductive tract, two epithelia of Wolffian duct origin (3–7). V-ATPase dysfunction in kidney cells has been implicated in the development of Fanconi syndrome and renal tubular acidosis, with severe kidney and generalized health sequelae (8–13). In mice, a lack of V-ATPase expressing clear cells leads to male infertility (14).
Acid secretion in epithelial cells is actively regulated by environmental signals, although the mechanisms by which these cues are translated into activation of H+ transport pathways remains the subject of intense research (11, 15–19). For example, the V-ATPase is regulated by several pathways, which involve CO2, phosphatidylinositol 3-kinase, aldolase, phosphofructokinase, actin, microtubules, and angiotensin in a variety of mammalian cellular systems (20–27). The number of V-ATPases at the apical membrane of intercalated cells in the kidney increases rapidly under conditions of systemic acidosis (28, 29). Acidosis also induces H+ secretion via the V-ATPase through changes in intracellular [Ca2+] concentration, calmodulin activation, the cytoskeleton, and by altering the rate of endocytosis and exocytosis in kidney cells (30).
We and others have shown that regulation of the V-ATPase at the apical membrane of intercalated and clear cells is tightly linked to alkaline luminal pH, HCO3−, carbonic anhydrase activity, activation of the soluble adenylyl cyclase (sAC),2 cAMP, and PKA (19, 31–33). In cells with abundant carbonic anhydrase, such as intercalated cells, increases in extracellular CO2 during acidosis may result in significant transient increases in intracellular [HCO3−] and [H+]. Increased [HCO3−] may in turn activate the sAC/cAMP/PKA pathway and the V-ATPase at the apical membrane of A-intercalated cells resulting in enhanced proton extrusion and subsequent basolateral recovery of HCO3− into the extracellular space (19).
The role of direct phosphorylation of V-ATPase subunits in H+ secretion in mammalian epithelial cells has not been well characterized (19, 34, 35). Although PKA agonists have been shown to regulate the V-ATPase in a variety of systems, it was not until recently that the direct phosphorylation of V-ATPase subunits by this kinase was linked to its regulation. In insect cells phosphorylation of the C subunit in the V1 sector by PKA contributes to the apical assembly and activity of the salivary gland V-ATPase (36, 37). We have recently shown that the V-ATPase A subunit is phosphorylated by PKA in vitro and in the intact cellular environment in HEK-293 cells, suggesting that direct A subunit phosphorylation by PKA could be involved in the trafficking of the V-ATPase complex to the apical membrane from cytoplasmic pools (19, 38, 39).
In this study we have used mass spectrometry and phosphorylation assays to identify and confirm the main PKA phosphorylation site in the V-ATPase A subunit, which is highly conserved across species. Moreover, we have established the relevance of this residue in the phosphorylation of the A subunit in HEK-293 cells that express native and active V-ATPase at their plasma membrane (40). We have also developed a useful technique to monitor V-ATPase activity in live cells and to test for the effects of mutations in the pump A subunit. Moreover, we have used established morphometric methods to confirm that this PKA phosphorylation site is required for apical accumulation of the pump in response to PKA activators in the Clone C cell line, a previously characterized rabbit cell line of kidney-intercalated cell characteristics that expresses V-ATPase.
EXPERIMENTAL PROCEDURES
Reagents and Chemicals
All chemicals used in the studies presented here were purchased from Sigma or Fisher Scientific unless otherwise stated. The cell-permeant PKA-specific activator N6-monobutyryl-cAMP (6-MB-cAMP) was obtained from Biomol.
Mass Spectrometry of Wild-type Mouse FLAG-A V-ATPase Subunit
The wild-type (WT) mouse FLAG-A subunit construct characterized in our previous study (19) was transfected into HEK-293T cells and immunoprecipitated using the M2 anti-FLAG monoclonal antibody (Sigma) coupled to protein A/G beads (Pierce), as described (19, 41, 42). This FLAG-WT-A V-ATPase subunit was phosphorylated by bacterially expressed active PKA (43) at 37 °C in kinase buffer (10 mm HEPES-Cl, pH 7.4, 200 μm ATP, 40 μm AMP, 5 mm MgCl2) supplemented with [γ-32P]ATP for 2 h. The reaction was stopped by adding SDS-containing sample buffer and heating to 95 °C for 5 min. The following steps were performed as described previously (43) with only one minor modification. Briefly, following SDS-PAGE and Coomassie Blue staining, the gel band corresponding to the FLAG-A V-ATPase subunit was excised from the wet gel and subjected to in-gel digestion with trypsin. Concentrated tryptic peptides were applied to a microbore reversed-phase column connected to a capillary liquid chromatography system and equipped with a microcollection/spotting system, thus allowing the microfractionation onto a prespotted AnchorChip (44). After autoradiography, selected fractions of the prespotted AnchorChip target indicating the presence of radiolabeled phosphopeptides were analyzed by matrix-assisted laser desorption ionization-mass spectrometry (MALDI-MS) (44).
To confirm the phosphorylation site identified by mass spectrometry analysis, the candidate phosphorylation site was mutated using the Stratagene QuikChange kit according to the manufacturer's instructions using as a template the pMO-FLAG-A plasmid, which we have used for mammalian cell expression (38). All mutations were confirmed by DNA sequencing.
A Subunit in Vitro Phosphorylation Assays
In vitro phosphorylation assays were performed essentially as described previously (38, 41). Briefly, HEK-293T cells were transiently transfected using Lipofectamine 2000 (Invitrogen) to express either FLAG-V-ATPase A subunit wild-type (FLAG-A-WT, mouse sequence) or this subunit with a specific point mutant (Ser-175 to Ala; FLAG-A-S175A) identified as the PKA target phosphorylation site. Cells were lysed 2 days after transfection, and the FLAG-V-ATPase A subunits (WT and S175A) were immunoprecipitated from cell lysates using the M2 anti-FLAG monoclonal antibody (Sigma) coupled to protein A/G beads (Pierce). In vitro phosphorylation was performed using purified active PKA catalytic subunit (Promega) with [γ-32P]ATP labeling, as described (38). After SDS-PAGE and transfer to nitrocellulose membranes, immunoblotting for expression of the FLAG-V-ATPase A subunit was first performed and quantified using a Versa-Doc Imager with Quantity One software (Bio-Rad). After the chemiluminescent signal decayed, phosphorylated bands on the membrane were identified by exposure of the same membrane to a phosphoscreen, and the detected bands were quantitated using a Bio-Rad PhosphorImager with Quantity One software. The intensity of each phosphoscreen band was corrected by subtracting out the local background in the same lane.
A Subunit in Vivo Phosphorylation Assays
HEK-293T cells were transiently transfected with 3 μg of either WT or S175A mutant FLAG-V-ATPase A subunit plasmid DNA 1 day before experimentation. Phosphorylation assays in HEK-293T cells were performed essentially as previously described (38). In addition, to assess potential differences in PKA-mediated [32P]orthophosphate labeling in the cells across conditions, 10 μg of lysate was pre-cleared by incubation with protein A/G beads (∼1.5 μl) and then spotted for each condition onto a nitrocellulose membrane. The [32P]orthophosphate-labeled proteins in each spot were detected by exposing the membranes to a phosphoscreen and quantified using a phosphorimager. Thereafter, the membranes were blocked in 5% bovine serum albumin in Tris-buffered saline, Tween and probed with an antibody recognizing a phosphorylated PKA consensus epitope (1:15,000; Cell Signaling Technologies) to account for potential differences in PKA activity modulation across conditions. Finally, membranes were stripped and re-probed using an anti-β-actin antibody (Sigma) to normalize for any differences in total protein content in the spotted samples. The total [32P]orthophosphate and PKA-phosphorylated substrate signals were corrected for the local background signal and normalized to the β-actin immunoblot signal in each spot. Each experimental condition was repeated three times for each V-ATPase A subunit construct. Measurements were analyzed and graphed as mean ± S.E.
Immunofluorescence Labeling of HEK-293T Cells Transfected with V-ATPase A Subunit Mutants
HEK-293T cells were seeded onto poly-l-lysine-coated coverslips at 2.5 × 105 cells/cm2, and transfected with either pMO vector alone or pMO-FLAG-A-WT, -FLAG-A-S175A (Ser-175 to Ala), or -FLAG-A-S175D (Ser-175 to Asp) A subunit using the techniques described above for phosphorylation experiments. At least three independent coverslips were used in immunolabeling experiments for each group of transfections (either vector alone, WT, S175A, or S175D A subunit). Cells on coverslips were incubated for 5 min in concanavalin A coupled to CY3 (Vector Laboratories) diluted at 1:200 for 5 min in PBS, pH 7.4, at 37 °C followed by a brief wash in PBS as described (45). All antibodies were diluted in DAKO diluent (DAKO Laboratories) at various concentrations. The coverslips were fixed in 2% paraformaldehyde in PBS for 30 min and immunolabeled using an anti-FLAG antibody raised in mouse (M2 anti-FLAG) for 60 min at room temperature (1:50 dilution), followed by incubation in secondary goat anti-mouse antibody coupled to Alexa 488 (1:800 dilution; Jackson Immunologicals) for 60 min, and then TO-PRO-3 (Invitrogen, 1:400 in PBS) to stain the nuclei for 5 min, using our previously published protocol in other cell lines (46). Images from these coverslips were acquired with identical laser confocal microscope settings across all transfection conditions. Images were imported into Adobe Photoshop for presentation as described previously (38).
Measurement of Changes in Extracellular Medium pH (pHo)
HEK-293T cells were passaged every 24–36 h for 1 week and then seeded onto poly-l-lysine-coated 24-well plates at an initial concentration of 2.5 × 105 cells/well. The cells were transiently transfected the next day with 0.3 μg/well of plasmid DNA (either vector alone, WT, S175A, or S175D; 6 wells per DNA sample). Transfected cells were grown in high-glucose, bicarbonate-containing Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum at 37 °C in the presence of 5% CO2, 95% air. At 28–31 h after transfection the medium from each well was collected, and the pH measured using a pH meter (Fisher) after careful equilibration of the sample with 5% CO2, 95% air. Cells were then incubated for 5 min at 37 °C in 1 ml of a Na+-free, low buffering capacity solution containing (in mm): 135 N-methyl-d-glucamine, 5 KCl, 2 CaCl2, 1.2 MgSO4, 5.5 d-glucose, 6 l-alanine, 4 lactic acid, 1 HEPES, titrated to pH 7.43 using 1 HCl (modified from Ref. 47). The solutions from each well were replaced with 1 ml of fresh solution and then incubated for 7 min at 37 °C before collecting them for pHo measurements, which were also performed at 37 °C. This procedure (7-min incubation followed by pHo measurement) was repeated two more times for each well either in the presence of vehicle or 100 μm 6-MB-cAMP, 500 μm IBMX. Reported acidification rates (in pH units/min) were calculated from the pH drop of the solutions (final minus initial pH) over the third 7-min incubation period. After these incubations the same procedure was repeated with three successive 7-min incubations in the presence of 1 μm bafilomycin A1. The V-ATPase-dependent rate of pHo acidification for each sample well was defined as the difference in the acidification rate measured in the absence versus the presence of bafilomycin A1 (i.e. bafilomycin A1-sensitive pH acidification rate). Comparing extracellular pH change rates originating from the same starting pH in the absence versus presence of bafilomycin allowed us to quantify the V-ATPase-dependent extracellular acidification rate within the same tissue culture well within a short period of time. The time frame for action of bafilomycin in these cells was ∼12–30 min, as evidenced by a significant inhibition of extracellular acidification starting at ∼12 min, and an increase in cell death after ∼30 min. Thus, by comparing the third of three 7-min incubations both in the absence and presence bafilomycin, we obtained the extracellular acidification rates in the window of time where bafilomycin is active against the V-ATPase but not yet significantly toxic to the cells.
At the end of each experiment cells were collected to determine total cell number, as counted with a hemocytometer, and cell viability, as assessed by trypan blue exclusion. We prepared at least 12 wells of cells for each plasmid type expressed (vector alone, WT, S175A and S175D), and half of those wells were treated with PKA agonist and the other half with buffer alone. To assess FLAG-A subunit expression in the transfected cells, we immunoblotted cell lysates at the end of the pHo experiments using both anti-FLAG and anti-β-actin antibodies, as described above. In addition, we performed dot blots of these cell lysates using the anti-PKA substrate antibody to confirm PKA activity changes in these cells with the different treatments, as described above.
Immunofluorescence Labeling of V-ATPase FLAG-A Subunit Mutants Transiently Transfected into a Cell Line of Intercalated Cell Characteristics
We used a previously characterized rabbit cell line (“Clone C,” a generous gift of Dr. Qais Al-Awqati), which displays kidney collecting duct intercalated cell characteristics (48, 49). This cell line expresses high levels of functional V-ATPase at the apical membrane when cells are seeded at high density. Briefly, Clone C cells were grown at 32 °C in medium containing Dulbecco's modified Eagle's medium/F-12 (Invitrogen), 1.8% heat-inactivated fetal calf serum, 27.6 μm hydrocortisone (Sigma), 0.45% insulin-transferrin-sodium selenite media supplement (Sigma), 15 mg/liter of epidermal growth factor (Sigma), 200 mm glutamine (Sigma), and 5% penicillin/streptomycin (Invitrogen) as previously described (50). Cells were placed into serum and antibiotic-free medium for 4 h prior to transfection. The cells were then transiently transfected according to the manufacturer's recommendations using Lipofectamine 2000 with either FLAG-A-WT or FLAG-A-S175A (3 μg of plasmid DNA). One day after transfection, cells were plated under conditions that confer apical V-ATPase proton secretion (51) onto 0.33-cm2 Transwell filters (Costar) coated with rat tail collagen (BD Biosciences) at a concentration of 5 × 105 cells/cm2 and grown at 40 °C in Dulbecco's modified Eagle's medium with 1.8% fetal bovine serum (52).
Five days after transfection, monolayers on filters were incubated in serum-free Dulbecco's modified Eagle's medium for 2 h and then treated with either vehicle or 1 mm 6-MB-cAMP and 0.5 mm IBMX for 30 min at 40 °C in PBS at pH 7.1. After treatment with agonists, cells on filters were incubated in CY3-concanavalin A (1:100) in PBS at 40 °C. After a brief PBS wash, cells were fixed and immunolabeled using the M2 anti-FLAG antibody, followed by a secondary antibody coupled to Alexa 488 (GAM-Alexa 488; 1:50, Invitrogen) and the nuclear label TO-PRO-3 using the same protocol as described above for HEK-293T cells. Filters were imaged from above the first appearance of apical fluorescence down to below the lowest basolateral fluorescence labeling using a Leica confocal microscope. For each set of filters the stacks were acquired using identical stack dimensions and Z-steps using a ×40 objective (zoom 3). Transfected cells on the filters were selected when they showed both concanavalin A and bright anti-FLAG immunolabeling by epifluorescence. Approximately 30% of the cells in the monolayers fulfilled these characteristics. At the time of selection of the transfected cells (as determined by their bright fluorescein isothiocyanate-associated fluorescence) for imaging and acquisition of the stacks, investigators were unable to judge the subcellular localization of the V-ATPase. Three-dimensional reconstructions of confocal stacks were used to obtain X-Z or X-Y projections of the transfected cells in the monolayers, which were then imported into Metamorph for quantification. V-ATPase apical accumulation was determined by measuring the mean pixel intensity of FLAG-associated fluorescence in an apical ROI (ROI-1, co-localizing with CY3-concanavalin A) and a cytoplasmic ROI of identical size immediately below concanavalin A labeling (ROI-2), using a very similar procedure to ones previously described by us (19, 38, 53). The brightest area of the cell at the apical membrane was chosen for measuring the ROI-1 (approximately, 200 pixels). After X-Z reconstruction, at least two additional investigators blinded as to the nature of the transfected subunits evaluated the distribution of anti-FLAG labeling in TIF images of these cells. The degree of apical accumulation of the V-ATPase FLAG A subunit mutants was determined by the ratio of apical-to-cytoplasmic ROI (ROI-1/ROI-2) of FLAG-associated fluorescence for each cell. At least five separate Clone C filters were evaluated for each of four conditions: FLAG-A-WT incubated in PBS, pH 7.1, ± 6-MB-cAMP/IBMX and FLAG-A-S175A incubated in PBS, pH 7.1, ± 6-MB-cAMP/IBMX. For each of these four studied conditions in Clone C monolayers, we quantified 20–45 cells.
Co-immunoprecipitation Studies
Clone C cells were transfected with FLAG-WT A subunit plasmid as described above. One day after transfection, cells were harvested in ice-cold lysis buffer using our established techniques (41). We used 1 mg of pre-cleared lysate for immunoprecipitations performed at 4 °C on each sample using the M2 anti-FLAG antibody (0.5 μg/immunoprecipitation) coupled to protein A/G beads. As a control, immunoprecipitation in the absence of the anti-FLAG antibody was also performed. After three washes in lysis buffer, the immunoprecipitation samples were eluted in sample buffer and, along with the cell lysate samples, subjected to SDS-PAGE. Immunoblotting was performed with: 1) V0 a subunit antibody (1:2,000 dilution, raised in rabbit, Santa Cruz), 2) V1 A subunit antibody (1:5,000 dilution, raised in chicken, Sigma), or 3) V1 E subunit antibody (1:10,000 dilution, raised in chicken, Sigma), followed by the appropriate secondary antibodies coupled to horseradish peroxidase (Jackson Immunologicals) (38).
Statistics
Data shown represent mean ± S.E. for each group. Significance was determined using two-tailed, unpaired Student's t tests assuming unequal variances between the treatment groups. p values <0.05 were considered significant.
RESULTS
PKA Phosphorylates the V-ATPase A Subunit at Ser-175
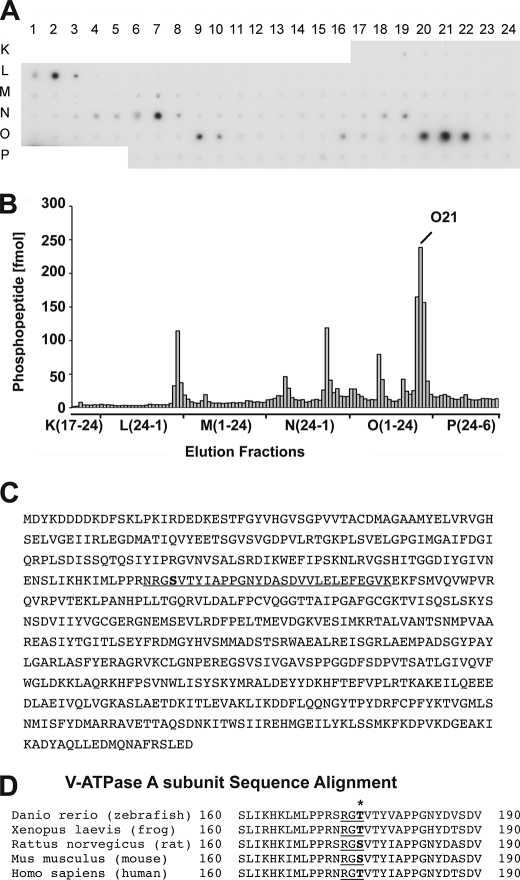
We have previously shown that the V-ATPase A subunit can be phosphorylated both in vitro and in vivo by AMPK and PKA when heterologously expressed in HEK-293 kidney cells (38). However, actual PKA phosphorylation sites within the A subunit have not yet been identified. Using a novel approach involving a liquid chromatography MALDI-MS workflow, we were able to localize and quantify phosphorylated peptides on a MALDI target plate prior to MS analysis (43). Using this technique with in vitro PKA-phosphorylated V-ATPase FLAG-tagged A-WT subunit, we identified a major phospholabeled tryptic peptide fragment that eluted in fraction O21 (Fig. 1, A and B). In this elution peak we detected a phosphorylated peptide corresponding to the expected molecular mass of a tryptic fragment with sequence NRGSVTYIAPPGNYDASDVVLELEFEGVK, for which sequence confirmation was obtained by fragmentation using MS/MS (see supplemental Fig. S1). This peptide fragment (underlined in Fig. 1C) contains two serines, however, only Ser-175 (bolded in Fig. 1C) fits within a consensus PKA phosphorylation site. The amino acid sequence around this residue is highly conserved in vertebrates (Fig. 1D), suggesting that PKA phosphorylation at this site could play a fundamental role in V-ATPase function.
FIGURE 1.
Identification of Ser-175 as a major site for PKA phosphorylation in the V-ATPase A subunit. FLAG-A V-ATPase subunit expressed in HEK-293T cells was incubated with a substoichiometric amount of PKA in the presence of [γ-32P]ATP, the proteins were subjected to SDS-PAGE, and the band corresponding to FLAG-A V-ATPase A-subunit was excised. A, autoradiographic film showing the fractionation profile of phosphopeptide mixtures after in-gel digestion and microfractionation onto prespotted AnchorChips. B, analysis by densitometry of individual radioactive spots identified after the microfractionation (shown in A) and quantification of the phosphorylated peptides using appropriate standards. The major radioactive peak eluted in fraction E10 and the corresponding phosphorylated peptide was identified by mass spectrometry. C, mouse FLAG-tagged V-ATPase A subunit amino acid sequence highlighting the major peptide phosphorylated by PKA (O21 fraction). D, Ser-175 (bold) is part of a highly conserved PKA consensus target phosphorylation site in this peptide.
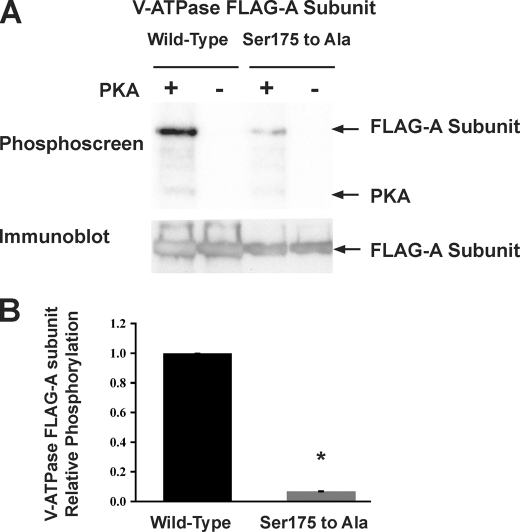
To further confirm that Ser-175 is a specific PKA phosphorylation site in vitro, we generated a putative phosphorylation-deficient FLAG-tagged A-subunit mutant with Ser-175 mutated to Ala (S175A) and then performed in vitro phosphorylation experiments, using methods previously described (38). We then compared in vitro phosphate labeling of WT versus the S175A V-ATPase A subunits exposed to purified active PKA catalytic subunit in the presence of [γ-32P]ATP (Fig. 2, A and B). The S175A V-ATPase A subunit mutant had a >90% reduction in 32P labeling relative to the WT A subunit after normalization to the amount of immunoprecipitated protein, confirming PKA-dependent phosphorylation at this residue in vitro. Together, the mass spectrometry analysis and in vitro phosphorylation results reveal that PKA phosphorylates the V-ATPase subunit at this highly conserved site in mammalian and other vertebrate animals.
FIGURE 2.
PKA phosphorylation of the A subunit in vitro occurs at Ser-175. A, typical phosphoscreen image (upper) revealing the signal of PKA in vitro phosphorylated A subunit compared with the Ser-175 to Ala mutant. The immunoblot blot (lower) confirms similar protein expression and loading of the gel for the different conditions. B, quantification of mean (±S.E.) V-ATPase A subunit phosphorylation signal normalized for protein loading as assessed by densitometry of Western blot. Compared with WT FLAG-A subunit, the phosphorylation-deficient (Ser to Ala) mutant showed a significant 90–95% decrease in phosphorylation by PKA in vitro (*, p < 0.05 relative to WT; n = 3).
PKA-dependent in Vivo Phosphorylation of the V-ATPase A Subunit in HEK-293T Cells Occurs at Ser-175
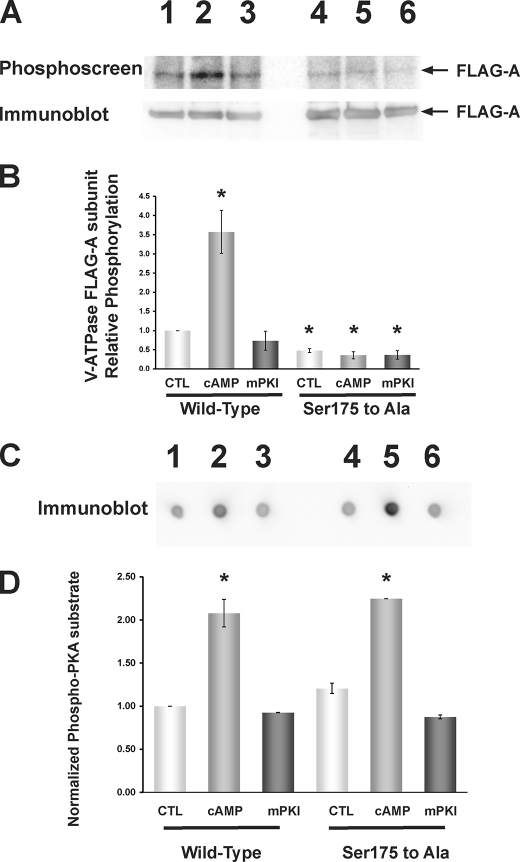
To determine whether Ser-175 is a target for PKA-dependent phosphorylation in intact HEK-293T cells, we compared [32P]orthophosphate labeling of the FLAG-A-WT and FLAG-A-S175A mutant subunits under control conditions or following treatment with PKA activators (100 μm 6-MB-cAMP + 500 μm IBMX) or a specific PKA inhibitor (10 μm mPKI) (Fig. 3A). The phosphate-labeling signal on the phosphoscreen (upper panel) was normalized to the respective FLAG-A subunit expression signal on the immunoblot (lower panel) from the same membrane.
FIGURE 3.
PKA-dependent in vivo phosphorylation of the V-ATPase A subunit in HEK-293T cells occurs at Ser-175. FLAG-tagged WT or S175A mutant A subunit was transfected into HEK-293T cells 1 day before experimentation, and cells were then incubated with [32P]orthophosphate for 2 h under control conditions or in the presence of PKA activator (1 mm 6-MB-cAMP; last 20 min of labeling period) or PKA inhibitor (10 μm mPKI; for entire labeling period). Cell lysis, immunoprecipitation using an anti-FLAG antibody, SDS-PAGE, immunoblotting using an anti-FLAG antibody, and exposure of the same membrane to a phosphoscreen were then performed as described (38). A, typical phosphoscreen image (upper panel) revealing the signal of phosphorylated A subunit in cells expressing FLAG-A-WT subunit (lanes 1–3) or FLAG-A-S175A subunit (lanes 4–6). Lanes 1 and 4 were derived from control-treated cells, lanes 2 and 5 were derived from PKA-stimulated cells, and lanes 3 and 6 were derived from PKA-inhibited cells. The Western blot (lower panel) confirms similar protein expression and loading of the gel for the different conditions. B, quantification of mean (±S.E.) V-ATPase A subunit phosphorylation signal relative to FLAG-A-WT control condition and normalized for protein expression. PKA activator increased FLAG-A-WT phosphorylation to ∼3.5 times that of the control condition, whereas FLAG-A-S175A mutant subunit phosphorylation was reduced across all conditions to ∼0.5 times that of the FLAG-A-WT subunit under the control condition (*, p < 0.05 relative to WT control by analysis of variance; n = 3 replicate experiments). C, dot blots using the PKA phosphorylation substrate-specific antibody of whole cell lysate samples taken from cells transfected and treated under the same conditions as shown in A and B and spotted onto a nitrocellulose filter. D, quantification of mean (±S.E.) PKA-phosphorylated substrate signal relative to that FLAG-A-WT-transfected cell lysates under the control condition and normalized to β-actin blot signal re-probed on the same dot blot (not shown). PKA activator 6-MB-cAMP increased the PKA-phosphorylated substrate signal to 2–2.5 times that of control (*, p < 0.05, relative to FLAG-A-WT control; unpaired t tests), whereas mPKI had no significant effect. As a further control, we measured the levels of [32P]orthophosphate protein labeling in cellular lysates. We did not observe any statistically significant difference across conditions, independently of the A-subunit mutant expressed and pharmacologic treatments (n = 3 per condition; data not shown).
Stimulation of cellular PKA activity by PKA activators significantly increased phosphorylation of the FLAG-A-WT subunit to ∼3.5 times that of control-treated cells (Fig. 3B, lanes 1 and 2). Treatment with the PKA inhibitor mPKI did not significantly decrease the level of WT A subunit phosphorylation compared with untreated cells, although there was an inhibitory trend with mPKI treatment (Fig. 3B, lanes 1 and 3). The changes in WT and mutant A subunit phosphorylation with PKA modulation were qualitatively similar to the changes in overall cellular PKA-phosphorylated substrates, as measured by a dot blot detecting PKA-phosphorylated proteins in the total cellular lysate (compare Fig. 3, B, lanes 1–3, with D, lanes 1–3). These results are consistent with our earlier published results on WT A subunit phosphorylation by PKA in cells (38).
Untreated cells expressing the S175A A subunit mutant had ∼50% lower phosphorylation levels compared with untreated cells expressing the WT A subunit (Fig. 3, A and B, lanes 1 and 4). This result indicates that Ser-175 in the A subunit is a likely target of baseline phosphorylation in unstimulated cells. Moreover, the robust enhancement in phosphorylation of the FLAG-A-WT subunit following PKA stimulation was completely blocked in the FLAG-A-S175A mutant subunit (compare Fig. 3, A and B, lanes 1 and 2, with lanes 4 and 5). This blockade could not be attributed to a lack of PKA activation in the S175A mutant subunit-expressing cells, as total cellular PKA-phosphorylated substrates approximately doubled with PKA stimulation in both WT- and mutant A subunit-expressing cells (Fig. 3D, lanes 2 and 5). Together, these results strongly indicate that Ser-175 in the V-ATPase A subunit is the main target of PKA phosphorylation in vivo (i.e. in an intact cellular environment).
Ser-175 Is Required for V-ATPase Subcellular Localization and Activity in HEK-293T Cells
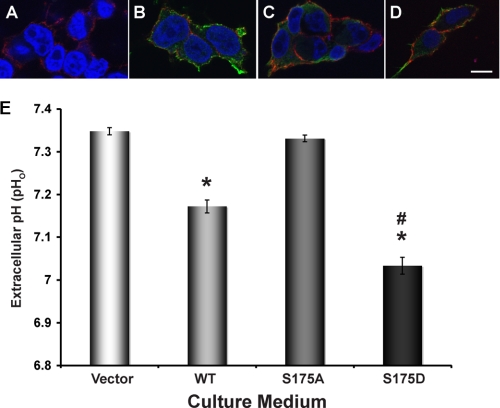
HEK-293 cells express the V-ATPase at their plasma membrane, where it has been shown to extrude protons (40). Lang et al. (40) demonstrated that other H+-secreting transporters, such as Na+/H+ exchangers (NHEs), are not active in HEK-293 cells with neutral to alkaline intracellular pH. To examine the subcellular localization of the V-ATPase A subunit expressed in HEK-293T cells, a commercially available subclone of HEK-293 cells (45), we transfected these cells with either FLAG-tagged WT or mutant A subunits. We then performed immunofluorescence labeling of the A subunit immediately after removing the cells from culture medium using an anti-FLAG antibody followed by confocal fluorescence microscopy (Fig. 4, A–D). Cells transfected with either FLAG-A-WT or with FLAG-A-S175D exhibited immunolabeling largely at or near the plasma membrane (co-labeled with concanavalin A-CY3, in red), with less prominent cytosolic staining (Fig. 4, B and D). However, HEK-293T cells transfected with FLAG-A-S175A displayed a more predominant cytosolic distribution than HEK-293T cells expressing WT or S175D A subunits (Fig. 4C). Immunolabeled cells that were transfected with vector alone showed very little nonspecific staining (Fig. 4A). All confocal images shown were acquired using identical laser settings in cells immunolabeled on the same day under the same conditions. Together, these results suggest that phosphorylation at Ser-175 in the A subunit may play a functional role in subcellular distribution of the V-ATPase in these cells.
FIGURE 4.
Expression of WT and mutant V-ATPase A subunit in HEK-293T cells modulates subcellular localization of the A subunit and extracellular pH. HEK-293 cells were transfected with either vector alone (A) or FLAG-tagged WT (B), S175A (C), or S175D (D) mutant A subunit 1 day prior to immunofluorescence staining for expression of FLAG (green), concanavalin A coupled to CY3 as a membrane marker (red), and TO-PRO-3 nuclear stain (blue). Scale bar = 15 μm. E, mean (±S.E.) extracellular pH (pHo) of the culture medium from HEK-293T cells transfected with different plasmids after 28–31 h incubation (*, p < 0.0001 relative to vector alone; #, p < 0.0001 relative to WT; n = 15–18).
To more directly assess the role of A subunit residue Ser-175 in the activity of the V-ATPase at the plasma membrane, we transfected HEK-293T cells with vector alone, WT, or mutant A subunits (S175A or S175D), incubated in the same volume of culture medium and then measured the extracellular pH (pHo) of the medium 28–31 h after transfection (Fig. 4E). Fresh HEK-293T cell culture medium had a measured pH of 7.48 ± 0.01 at 37 °C in a 5% CO2 environment. In cells transfected with either vector alone or the PKA phosphorylation-deficient S175A A subunit mutant, only modest acidification of the medium occurred over the subsequent 28–31 h (to a pHo of 7.33–7.35; Fig. 4E, first and third lanes). Expression of the WT A subunit significantly enhanced acidification of the medium relative to vector alone (to a pHo of 7.17 ± 0.02; Fig. 4E, lane 2). Finally, expression of the phosphomimic S175D A subunit mutant caused an even more profound and significant acidification of the culture medium (to a pHo of 7.03 ± 0.02; Fig. 4E, lane 4). Thus, the degrees of medium acidification observed after transfection of WT and Ser-175 mutant A subunits into HEK-293T cells (Fig. 4E) were consistent with qualitative changes in expression of the transfected subunits at or near the plasma membrane (Fig. 4, A–D). The total cell number, as counted on a hemocytometer at the end of each experiment, and viability, as measured by trypan blue exclusion, did not differ significantly across transfection conditions (between 0.99 × 106 and 1.10 × 106 cells per well and between 94.1 and 95.6% cell viability across all transfection conditions). Moreover, nuclear staining did not reveal blebbing of cell nuclei under any of the conditions tested (Fig. 4, A–D), which suggests that apoptosis was not occurring to a significant extent (54). Expression levels of FLAG-tagged A subunits in transfected cells were quite comparable, as demonstrated in the immunoblots of the phosphorylation experiments in intact HEK-293T cells (Fig. 3). These considerations suggest that changes in the pH of medium in cultures were not due to differences in cell death, growth rates, transfected subunit expression levels, or plating across conditions.
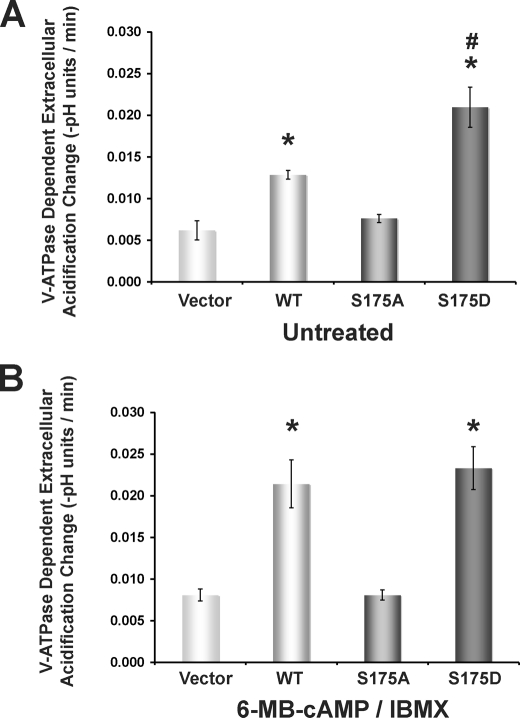
In additional experiments we monitored pHo changes under acute conditions (over 7-min intervals) in HEK-293T cells seeded at equal densities into 24-well plates and transfected with either vector alone or plasmid to express WT, S175A, or S175D mutant A subunit (Fig. 5). V-ATPase-dependent acidification was measured as described under “Experimental Procedures” after replacing the culture medium with a low buffering capacity solution. This buffer was prepared without Na+ to minimize any contribution of Na+/H+ exchange to pHo and in the nominal absence of HCO3− to minimize sAC activity, which could independently increase intracellular [cAMP] and PKA activity (31, 40, 55). We confirmed that HEK-293T cells incubated in this weak buffered solution express sufficient V-ATPase at their plasma membrane to generate significant changes in pHo. When comparing untreated (Fig. 5A) versus 6-MB-cAMP-treated cells (Fig. 5B), only overexpression of the WT A subunit caused a significant change in the V-ATPase-dependent acidification rate (expressed as: − (final buffer pH − initial buffer pH)/Δt). Specifically, expression of vector alone and S175A maintained relatively low acidification rates, and S175D maintained a high acidification rate under both untreated and PKA-stimulated conditions. On the other hand, the WT A subunit had a low acidification rate under untreated conditions that converted to a high acidification rate in the presence of the PKA activators 6-MB-cAMP and IBMX. Comparing the different transfection conditions in cells incubated with buffer alone (untreated), only vector alone and S175A had V-ATPase-dependent acidification rates that were not statistically different from one another. The relative measured acidification rates were: vector ∼ S175A < WT < S175D (Fig. 5A). Comparing the different transfection conditions in the presence of the PKA activators, both vector alone versus S175A and WT versus S175D had V-ATPase-dependent acidification rates not statistically different from one another. The relative measured acidification rates with PKA stimulation were: vector ∼ S175A < WT ∼ S175D (Fig. 5B). Of note, as observed in Fig. 3, during the time frame of these pHo measurements, there was comparable expression of each FLAG A subunit, and the level of total PKA-phosphorylated substrate in cells significantly increased with 6-MB-cAMP and IBMX treatment (data not shown). Taken together, the results presented so far suggest that PKA-mediated phosphorylation of the A subunit at Ser-175 in HEK-293T cells may be both necessary and sufficient to confer V-ATPase activity at the plasma membrane under conditions that minimize intracellular HCO3− and the contribution to H+ secretion by NHEs. In the presence of bafilomycin and in the absence of Na+, we still observed a slight but consistent drop in pHo (supplemental Fig. S2), which could be due to other Na+-independent, H+-extruding transporters, such as the H+-monocarboxylate co-transporters of the SLC16 family, which are known to be expressed in the HEK-293 cell line (56, 57).
FIGURE 5.
Bafilomycin-sensitive, V-ATPase-dependent extracellular acidification is modulated by Ser-175 A subunit mutants and PKA activators in HEK-293T cells. Cells were transfected with vector alone, WT, S175A, or S715D mutant FLAG-tagged A subunit for 28–31 h, and the rate of extracellular acidification in each set of transfected cells was measured in a low buffering capacity solution before and after the addition of bafilomycin A1, a specific V-ATPase inhibitor (see “Experimental Procedures”). The mean (±S.E.) rate of extracellular acidification (−[final buffer pH − initial buffer pH]/Δt) was obtained in the absence or presence of bafilomycin, for cells incubated either with (A) or without (B) PKA activators (n = 6 for each transfection condition; n = 6 for each treatment condition) (*, p < 0.005 relative to vector alone; #, p < 0.02 relative to WT).
Ser-175 Is Required for PKA-mediated V-ATPase A Subunit Apical Accumulation in Clone C Intercalated Cells
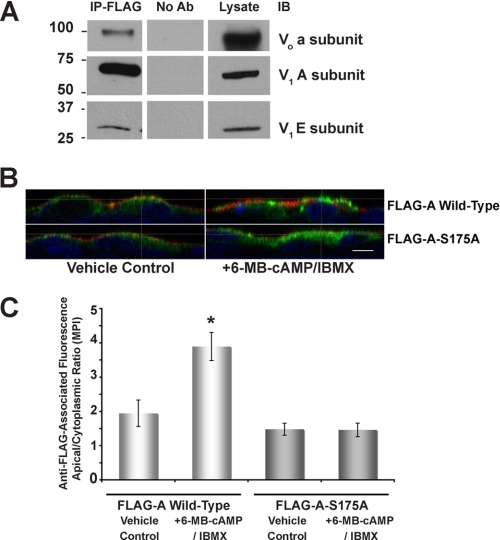
To evaluate the role of PKA-dependent phosphorylation at Ser-175 in the A subunit vis à vis subcellular localization changes of the V-ATPase in a more physiologically relevant cell system, we expressed the FLAG A subunit in a rabbit cell line of intercalated cell characteristics, Clone C. This cell line has been previously characterized and, when plated at high density, expresses active V-ATPase at the apical membrane (49). First, we performed co-immunoprecipitation experiments to determine whether the exogenously expressed FLAG-A-WT subunit incorporates into endogenous V-ATPase complexes. As demonstrated by immunoblotting with specific antibodies that recognize endogenous V-ATPase subunits, we confirmed that both the V-ATPase a subunit of the membrane-embedded Vo sector (Fig. 6A, upper panels) and the E subunit of the V1 sector (Fig. 6A, lower panels) co-immunoprecipitated with transfected FLAG-A-WT subunit expressed in Clone C cells (Fig. 6A, middle panel).
FIGURE 6.
The phosphorylation-deficient V-ATPase A subunit Ser-175 to Ala mutant does not accumulate at the apical membrane of intercalated cells in response to PKA activators. The Clone C cell line of intercalated cell characteristics was used for independent transient transfections using either WT or S175A A subunit. A, immunoprecipitation using an anti-FLAG antibody (IP-FLAG; left column) followed by immunoblotting using antibodies against the a (upper), A (middle), or E (lower) V-ATPase subunits revealed that the transfected FLAG-tagged A subunit forms a complex with the native V0 sector a subunit and with the V1 sector E subunit. No co-immunoprecipitation was observed when no antibody was added to the immunoprecipitation reaction (center column). Samples of the whole cell lysate (5%) were also directly immunoblotted for each of the three subunits (right column). B, 1 day after transfection with either FLAG-A wild-type subunit (top panels) or FLAG-A S175A mutant subunit (lower panels), Clone C cells were plated onto Transwell filters. After 4 days the filters were incubated in PBS, pH 7.1, with 100 μm 6-MB-cAMP and 0.5 mm IBMX (right panels) or with PBS, pH 7.1, alone (left panels) for 30 min. These incubations were followed by an incubation with concanavalin A coupled to CY3 (red) for 5 min in PBS, pH 7.1, fixation, and immunofluorescence labeling using anti-FLAG antibody (green) and TO-PRO-3 nuclear stain (blue). Scale bar = 10 μm. C, quantification of V-ATPase-associated mean pixel intensity (MPI) ratio of apical ROI-1 (where the A-subunit co-localizes with concanavalin A) and cytoplasmic ROI-2 (A-subunit alone). This ROI-1/ROI-2 ratio under the different conditions reveals a significant PKA-mediated apical V-ATPase accumulation in cells expressing the WT A subunit compared with cells expressing the S175A mutant (ROI-1/ROI-2 ratio presented as mean (±S.E.); *, p < 0.05 versus V-ATPase A WT; n = 20–45 cells analyzed for both conditions).
Next, we performed immunofluorescence labeling of V-ATPase FLAG-A-WT or S175A mutant subunit expressed in polarized Clone C cells followed by confocal microscopy. The FLAG-A-WT subunit was distributed in both apical and cytosolic domains when expressed in Clone C cells and incubated in PBS at pH 7.1 (Fig. 6B, left upper panel). Under this incubation condition we minimized any exposure of the cells to hormones and agonists present in serum (by serum starving the monolayers for 2 h), which could potentially influence intracellular cAMP/PKA. The use of PBS, pH 7.1, during the incubation also minimized HCO3−-induced trafficking of the V-ATPase in proton-secreting cells as previously described (31). When Clone C cells expressing the FLAG-A-WT subunit were treated with the PKA activators 6-MB-cAMP and IBMX, these cells accumulated FLAG-associated fluorescence at their apical poles (Fig. 6B, upper right panel). In contrast, the FLAG-A-S175A mutant subunit showed a more diffuse cytoplasmic distribution both in the presence and absence of PKA agonists (Fig. 6B, lower panels).
Quantification of the apical-to-cytoplasmic mean pixel intensity of FLAG-associated fluorescence in Clone C cells transfected with WT versus S175A A subunit confirmed a significantly lower apical accumulation of the S175A A-subunit mutant (Fig. 6C), and its unresponsiveness to trafficking to the apical membrane by PKA agonists. Our quantification also reveals that transfected FLAG-A-WT subunit in Clone C cells traffics to the apical membrane in the same 30-min time frame that the E subunit accumulates at the apical membrane of intercalated cells in kidney tissue slices in response to 6-MB-cAMP (19, 39). In summary, these results suggest that phosphorylation at Ser-175 is required for the PKA-mediated apical accumulation in polarized kidney-intercalated cells.
DISCUSSION
An area of particular research interest in kidney and epithelial cell physiology is in the understanding of how changes in intracellular and extracellular pH are sensed acutely by proton-secreting cells and translated into the activation of transporters such as the V-ATPase (17, 32). It has been shown that V-ATPase subcellular localization and/or V-ATPase-dependent proton secretion are regulated by a variety of stimuli, including changes in intracellular and extracellular pH, extracellular [CO2], intracellular [Ca2+], and [HCO3−] in proton-secreting cells derived embryologically from the Wolffian duct (20, 51, 58, 59). Carbonic anhydrase and sAC are very abundant in kidney-intercalated cells, where sAC activation generates cAMP upon increases of CO2 that may occur under conditions of acute respiratory acidosis (31, 60). We envision that in response to an acute intracellular increase of [CO2], HCO3− production catalyzed by carbonic anhydrase in the intercalated cell (11, 58) would generate cAMP via sAC and thereby induce acute PKA-dependent trafficking of the V-ATPase to the apical membrane for rapid proton secretion (19).
To date, the mechanisms of kinase-dependent regulation of V-ATPase trafficking in mammalian cells have been unknown (34, 38, 61). Our previous work demonstrated that direct phosphorylation of the V-ATPase A subunit occurred in HEK-293 cells, and we proposed that such phosphorylation could potentially play an important role in the regulation of subcellular localization and activity of the V-ATPase in kidney-intercalated cells (19, 38, 39). This mode of regulation of V-ATPase activity could also prove to be relevant in the proximal tubule of the kidney to the extent that PKA or other relevant kinases may become activated there. However, the levels of sAC in the epithelium of that nephron segment are lower than in the collecting duct (31). These findings may also be relevant to trafficking of the V-ATPase in other epithelia such as epididymal clear cells, where the V-ATPase accumulates at the apical membrane in response to PKA (61).
In this study we have identified Ser-175 as the dominant PKA site in the V-ATPase A subunit by two complementary approaches, mass spectrometry and candidate site mutagenesis with subsequent in vitro and in vivo phosphorylation studies performed in HEK-293T cells (Figs. 1–3). Immunolocalization studies performed in HEK-293T cells indicated that in the presence of HCO3−-containing medium, the WT and S175D mutants accumulated in submembrane regions, a finding that mirrored acidification of the culture medium under those transfection conditions (Fig. 4). Furthermore, in the nominal absence of extracellular bicarbonate and Na+, the proton-secreting activity of HEK-293T cells transfected with the A subunit was largely bafilomycin-sensitive and thus V-ATPase-dependent (Fig. 5). Moreover, under these conditions cells transfected with the WT A subunit responded to PKA activation, whereas PKA phosphorylation-deficient and phosphomimic Ser-175 mutants were unresponsive to PKA, having either constitutively reduced or elevated V-ATPase activity at the plasma membrane, respectively. PKA-mediated phosphorylation of the A subunit at Ser-175 thus appears to be both necessary and sufficient to confer V-ATPase activity at the plasma membrane in HEK-293 cells. Finally, the immunolocalization studies performed in Clone C cells expressing either WT or S175A mutant A subunit suggest that phosphorylation at Ser-175 is also required for the apical membrane accumulation of the V-ATPase following treatment with PKA activators in a relevant polarized epithelial cell line of intercalated cell characteristics (Fig. 6).
The finding that overexpression of the WT A subunit significantly enhanced V-ATPase-dependent extracellular acidification (Fig. 5) suggests that abundance of the A subunit of the V-ATPase may be rate-limiting for the formation of active V-ATPase holoenzyme complex at the plasma membrane in HEK-293T cells. Of note, the kinetics of assembly of the V-ATPase have largely been investigated in the yeast system (reviewed in Ref. 62). It has been described that the A subunit of the V1 sector associates at a faster rate with the V0 sector a subunit than with other subunits of the V1 sector (reviewed in Ref. 1).
Additional studies are needed to better define the molecular mechanism(s) by which PKA-dependent phosphorylation at Ser-175 in the A subunit is associated with increased plasma membrane accumulation and activity of the V-ATPase. Specifically, it is conceivable that PKA phosphorylation of the A subunit modulates protein-protein interactions within the multisubunit V-ATPase complex and/or with other interacting proteins involved with cellular trafficking of the pump. For example, the sequence around our identified PKA phosphorylation site is highly conserved in eukaryotes and lies within a so-called “non-homologous” region (63). This region received its name because it is not present in the F-ATPase β subunit, which otherwise has high homology to the V-ATPase A subunit. It has been described that the highly conserved non-homologous region in the A subunit of the V1 sector binds to the V0 sector in a glucose-dependent manner in yeast, where it has been proposed to be a glucose sensor, thereby linking pump function to metabolic status (2, 64).
To our knowledge, this study is the first to identify and characterize the functional role of a specific phosphorylation site of any V-ATPase subunit in mammalian cells. Our previous work demonstrated that in addition to PKA, the metabolic-sensing kinase AMPK can directly phosphorylate the A subunit of the V-ATPase (38). An antagonistic regulatory relationship between PKA and AMPK with respect to subcellular localization of the pump in both epididymal clear cells and kidney-intercalated cells was also suggested (19). Specifically, AMPK appeared to inhibit PKA-dependent phosphorylation of the A subunit in cells and blocked the PKA-mediated accumulation of the pump at the apical membrane in proton-secreting cells in kidney and epididymis. However, the mechanistic details of how V-ATPase phosphorylation by these two kinases may be translated into an integrated response of the pump to disparate cellular signals is unclear. Specifically, it will be important in future studies to define how PKA-dependent phosphorylation of the pump may couple the sensing of the acid base status to pump activity, whereas AMPK-dependent phosphorylation of the V-ATPase may couple its activity to metabolic and other cellular stresses.
Supplementary Material
Acknowledgments
We thank Dr. Qais Al-Awqati for the generous gift of Clone C rabbit-intercalated cells and Drs. Ossama Kashlan, Ora Weisz, and Thomas Kleyman for helpful discussions. We also thank Alexander and Andrew Zerby for careful assistance with data management.
This work was supported, in whole or in part, by National Institutes of Health Grants P30 DK079307 (to the Pittsburgh Kidney Research Center), R01 DK-075048 (to K. R. H.), and R01 DK-084184 (to N. M. P. -S.), the American Society of Nephrology Carl W. Gottschalk Research Scholar Award (to N. M. P. -S.), American Heart Association Grants AHA 09GRNT2060539 (to N. M. P. -S.) and 0825540D (to R. A.), Cystic Fibrosis Foundation Grant CFF R883-CR02 (to C. A. B.), Swiss National Science Foundation Grant 3100A0-11437/1, European Union FP6 contract LSHM-CT-2004-005272 (EXGENESIS), and a graduate training fellowship ETHIIRA from ETH Zurich (to R. F. T.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- sAC
- soluble adenylyl cyclase
- 6-MB-cAMP
- N6-monobutyryl-cAMP
- AMPK
- AMP-activated protein kinase
- HEK
- human embryonic kidney
- MALDI-MS
- matrix-assisted laser desorption ionization-mass spectrometry
- IBMX
- 3-isobutyl-1-methylxanthine
- mPKI
- myristoylated protein kinase A inhibitor
- NHE
- Na+/H+ exchanger
- pHo
- extracellular pH
- PKA
- protein kinase A
- ROI
- region of interest
- V-ATPase
- vacuolar H+-ATPase
- WT
- wild-type
- PBS
- phosphate-buffered saline.
REFERENCES
- 1.Nelson N., Harvey W. R. (1999) Physiol. Rev. 79, 361–385 [DOI] [PubMed] [Google Scholar]
- 2.Forgac M. (2007) Nat. Rev. Mol. Cell Biol. 8, 917–929 [DOI] [PubMed] [Google Scholar]
- 3.Zeidel M. L., Silva P., Seifter J. L. (1986) J. Clin. Invest. 77, 113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gluck S., Al-Awqati Q. (1984) J. Clin. Invest. 73, 1704–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gluck S., Caldwell J. (1988) Am. J. Physiol. 254, F71–79 [DOI] [PubMed] [Google Scholar]
- 6.Brown D., Hirsch S., Gluck S. (1988) J. Clin. Invest. 82, 2114–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breton S., Smith P. J., Lui B., Brown D. (1996) Nat. Med. 2, 470–472 [DOI] [PubMed] [Google Scholar]
- 8.Marshansky V., Ausiello D. A., Brown D. (2002) Curr. Opin. Nephrol. Hypertens. 11, 527–537 [DOI] [PubMed] [Google Scholar]
- 9.Hamm L. L., Nakhoul N. L. (2008) in Brenner and Rector's The Kidney (Brenner B. M. ed) 8th Ed., pp. 248–279, Saunders Elsevier, Philadelphia, PA [Google Scholar]
- 10.Hamm L. L., Hering-Smith K. S. (1993) Semin. Nephrol. 13, 246–255 [PubMed] [Google Scholar]
- 11.Steinmetz P. R. (1986) Am. J. Physiol. 251, F173–F187 [DOI] [PubMed] [Google Scholar]
- 12.Karet F. E., Finberg K. E., Nelson R. D., Nayir A., Mocan H., Sanjad S. A., Rodriguez-Soriano J., Santos F., Cremers C. W., Di Pietro A., Hoffbrand B. I., Winiarski J., Bakkaloglu A., Ozen S., Dusunsel R., Goodyer P., Hulton S. A., Wu D. K., Skvorak A. B., Morton C. C., Cunningham M. J., Jha V., Lifton R. P. (1999) Nat. Genet. 21, 84–90 [DOI] [PubMed] [Google Scholar]
- 13.Smith A. N., Skaug J., Choate K. A., Nayir A., Bakkaloglu A., Ozen S., Hulton S. A., Sanjad S. A., Al-Sabban E. A., Lifton R. P., Scherer S. W., Karet F. E. (2000) Nat. Genet. 26, 71–75 [DOI] [PubMed] [Google Scholar]
- 14.Blomqvist S. R., Vidarsson H., Söder O., Enerbäck S. (2006) EMBO J. 25, 4131–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hays S., Kokko J. P., Jacobson H. R. (1986) J. Clin. Invest. 78, 1279–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gluck S., Nelson R. (1992) J. Exp. Biol. 172, 205–218 [DOI] [PubMed] [Google Scholar]
- 17.Pastor-Soler N., Piétrement C., Breton S. (2005) Physiology 20, 417–428 [DOI] [PubMed] [Google Scholar]
- 18.Wagner C. A., Finberg K. E., Breton S., Marshansky V., Brown D., Geibel J. P. (2004) Physiol. Rev. 84, 1263–1314 [DOI] [PubMed] [Google Scholar]
- 19.Gong F., Alzamora R., Smolak C., Li H., Naveed S., Neumann D., Hallows K. R., Pastor-Soler N. M. (2010) Am. J. Physiol. Renal Physiol. 298, F1162–F1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz G. J., Al-Awqati Q. (1985) J. Clin. Invest. 75, 1638–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sautin Y. Y., Lu M., Gaugler A., Zhang L., Gluck S. L. (2005) Mol. Cell. Biol. 25, 575–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su Y., Zhou A., Al-Lamki R. S., Karet F. E. (2003) J. Biol. Chem. 278, 20013–20018 [DOI] [PubMed] [Google Scholar]
- 23.Shum W. W., Da Silva N., Brown D., Breton S. (2009) J. Exp. Biol. 212, 1753–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pech V., Kim Y. H., Weinstein A. M., Everett L. A., Pham T. D., Wall S. M. (2007) Am. J. Physiol. Renal Physiol. 292, F914–F920 [DOI] [PubMed] [Google Scholar]
- 25.Rothenberger F., Velic A., Stehberger P. A., Kovacikova J., Wagner C. A. (2007) J. Am. Soc. Nephrol. 18, 2085–2093 [DOI] [PubMed] [Google Scholar]
- 26.Beaulieu V., Da Silva N., Pastor-Soler N., Brown C. R., Smith P. J., Brown D., Breton S. (2005) J. Biol. Chem. 280, 8452–8463 [DOI] [PubMed] [Google Scholar]
- 27.Breton S., Brown D. (1998) J. Am. Soc. Nephrol. 9, 155–166 [DOI] [PubMed] [Google Scholar]
- 28.Bastani B., Purcell H., Hemken P., Trigg D., Gluck S. (1991) J. Clin. Invest. 88, 126–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabolić I., Brown D., Gluck S. L., Alper S. L. (1997) Kidney Int. 51, 125–137 [DOI] [PubMed] [Google Scholar]
- 30.Schwartz J. H., Masino S. A., Nichols R. D., Alexander E. A. (1994) Am. J. Physiol. 266, F94–F101 [DOI] [PubMed] [Google Scholar]
- 31.Pastor-Soler N., Beaulieu V., Litvin T. N., Da Silva N., Chen Y., Brown D., Buck J., Levin L. R., Breton S. (2003) J. Biol. Chem. 278, 49523–49529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paunescu T. G., Da Silva N., Russo L. M., McKee M., Lu H. A., Breton S., Brown D. (2008) Am. J. Physiol. Renal Physiol. 294, F130–F138 [DOI] [PubMed] [Google Scholar]
- 33.Păunescu T. G., Ljubojevic M., Russo L. M., Winter C., McLaughlin M. M., Wagner C. A., Breton S., Brown D. (2010) Am. J. Physiol. Renal Physiol. 298, F643–F654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers M., Forgac M. (1993) J. Biol. Chem. 268, 9184–9186 [PubMed] [Google Scholar]
- 35.Tuerk R. D., Thali R. F., Auchli Y., Rechsteiner H., Brunisholz R. A., Schlattner U., Wallimann T., Neumann D. (2007) J. Proteome Res. 6, 3266–3277 [DOI] [PubMed] [Google Scholar]
- 36.Dames P., Zimmermann B., Schmidt R., Rein J., Voss M., Schewe B., Walz B., Baumann O. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 3926–3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voss M., Vitavska O., Walz B., Wieczorek H., Baumann O. (2007) J. Biol. Chem. 282, 33735–33742 [DOI] [PubMed] [Google Scholar]
- 38.Hallows K. R., Alzamora R., Li H., Gong F., Smolak C., Neumann D., Pastor-Soler N. M. (2009) Am. J. Physiol. Cell Physiol. 296, C672–C681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pastor-Soler N. M., Alzamora R., Naveed S., Smolak C., Gong F., Hallows K. R. (2009) FASEB J. 23, 602.13 [Google Scholar]
- 40.Lang K., Wagner C., Haddad G., Burnekova O., Geibel J. (2003) Cell. Physiol. Biochem. 13, 257–262 [DOI] [PubMed] [Google Scholar]
- 41.Bhalla V., Oyster N. M., Fitch A. C., Wijngaarden M. A., Neumann D., Schlattner U., Pearce D., Hallows K. R. (2006) J. Biol. Chem. 281, 26159–26169 [DOI] [PubMed] [Google Scholar]
- 42.Carattino M. D., Edinger R. S., Grieser H. J., Wise R., Neumann D., Schlattner U., Johnson J. P., Kleyman T. R., Hallows K. R. (2005) J. Biol. Chem. 280, 17608–17616 [DOI] [PubMed] [Google Scholar]
- 43.Djouder N., Tuerk R. D., Suter M., Salvioni P., Thali R. F., Scholz R., Vaahtomeri K., Auchli Y., Rechsteiner H., Brunisholz R. A., Viollet B., Mäkelä T. P., Wallimann T., Neumann D., Krek W. (2010) EMBO J. 29, 469–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuerk R. D., Auchli Y., Thali R. F., Scholz R., Wallimann T., Brunisholz R. A., Neumann D. (2009) Anal. Biochem. 390, 141–148 [DOI] [PubMed] [Google Scholar]
- 45.Gao Z., Lei D., Welch J., Le K., Lin J., Leng S., Duhl D. (2003) J. Pharmacol. Exp. Ther. 307, 870–877 [DOI] [PubMed] [Google Scholar]
- 46.Hallows K. R., Fitch A. C., Richardson C. A., Reynolds P. R., Clancy J. P., Dagher P. C., Witters L. A., Kolls J. K., Pilewski J. M. (2006) J. Biol. Chem. 281, 4231–4241 [DOI] [PubMed] [Google Scholar]
- 47.Constantinescu A., Silver R. B., Satlin L. M. (1997) Am. J. Physiol. 272, F167–F177 [DOI] [PubMed] [Google Scholar]
- 48.Al-Awqati Q. (1996) Am. J. Physiol. Cell Physiol. 270, C1571–C1580 [DOI] [PubMed] [Google Scholar]
- 49.van Adelsberg J., Edwards J. C., Takito J., Kiss B., al-Awqati Q. (1994) Cell 76, 1053–1061 [DOI] [PubMed] [Google Scholar]
- 50.Edwards J. C., van Adelsberg J., Rater M., Herzlinger D., Lebowitz J., al-Awqati Q. (1992) Am. J. Physiol. Cell. Physiol. 263, C521–C529 [DOI] [PubMed] [Google Scholar]
- 51.van Adelsberg J., Al-Awqati Q. (1986) J. Cell Biol. 102, 1638–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bens M., Vallet V., Cluzeaud F., Pascual-Letallec L., Kahn A., Rafestin-Oblin M. E., Rossier B. C., Vandewalle A. (1999) J. Am. Soc. Nephrol. 10, 923–934 [DOI] [PubMed] [Google Scholar]
- 53.Bouley R., Pastor-Soler N., Cohen O., McLaughlin M., Breton S., Brown D. (2005) Am. J. Physiol. Renal Physiol. 288, F1103–1112 [DOI] [PubMed] [Google Scholar]
- 54.O'Brien M. C., Bolton W. E. (1995) Cytometry 19, 243–255 [DOI] [PubMed] [Google Scholar]
- 55.Zippin J. H., Chen Y., Nahirney P., Kamenetsky M., Wuttke M. S., Fischman D. A., Levin L. R., Buck J. (2003) FASEB J. 17, 82–84 [DOI] [PubMed] [Google Scholar]
- 56.Ahlin G., Hilgendorf C., Karlsson J., Szigyarto C. A., Uhlén M., Artursson P. (2009) Drug Metab. Dispos. 37, 2275–2283 [DOI] [PubMed] [Google Scholar]
- 57.Hallows K. R., Restrepo D., Knauf P. A. (1994) Am. J. Physiol. 267, C1057–C1066 [DOI] [PubMed] [Google Scholar]
- 58.Boron W. F. (1986) Annu. Rev. Physiol. 48, 377–388 [DOI] [PubMed] [Google Scholar]
- 59.Gluck S., Cannon C., Al-Awqati Q. (1982) Proc. Natl. Acad. Sci. U.S.A. 79, 4327–4331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dobyan D. C., Magill L. S., Friedman P. A., Hebert S. C., Bulger R. E. (1982) Anat. Rec. 204, 185–197 [DOI] [PubMed] [Google Scholar]
- 61.Pastor-Soler N. M., Hallows K. R., Smolak C., Gong F., Brown D., Breton S. (2008) Am. J. Physiol. Cell Physiol. 294, C488–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kane P. M. (2006) Microbiol. Mol. Biol. Rev. 70, 177–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shao E., Nishi T., Kawasaki-Nishi S., Forgac M. (2003) J. Biol. Chem. 278, 12985–12991 [DOI] [PubMed] [Google Scholar]
- 64.Shao E., Forgac M. (2004) J. Biol. Chem. 279, 48663–48670 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.