Abstract
Glucose metabolism in nervous tissue has been proposed to occur in a compartmentalized manner with astrocytes contributing largely to glycolysis and neurons being the primary site of glucose oxidation. However, mammalian astrocytes and neurons both contain mitochondria and it remains unclear why in culture neurons oxidize glucose, lactate, and pyruvate to a much larger extent than astrocytes. The objective of this study was to determine whether pyruvate metabolism is differentially regulated in cultured neurons vs. astrocytes. Expression of all components of the pyruvate dehydrogenase complex (PDC), the rate-limiting step for pyruvate entry into the Krebs cycle, was determined in cultured astrocytes and neurons. In addition, regulation of PDC enzymatic activity in the two cell types via protein phosphorylation was examined. We show that all components of the PDC are expressed in both cell types in culture but that PDC activity is kept strongly inhibited in astrocytes through phosphorylation of the pyruvate dehydrogenase alpha subunit (PDHα). In contrast, neuronal PDC operates close to maximal levels with much lower levels of phosphorlyated PDHα. Dephosphorylation of astrocytic PDHα restores PDC activity and lowers lactate production. Our findings suggest that the glucose metabolism of astrocytes and neurons may be far more flexible than previously believed.
Keywords: Glycolysis, oxidation, lactate, dichloroacetate
INTRODUCTION
The cellular metabolism of glucose by brain tissue has been proposed to occur in a compartmentalized manner with distinct brain cell types performing the bulk of anaerobic vs. aerobic processing (Sibson et al. 1998). Glycolysis, the cytosolic and anaerobic arm of glucose metabolism, has been proposed to take place largely in astrocytes, while mitochondrial oxidation of the glycolytic endproducts pyruvate and lactate is believed to occur predominantly in neurons (Hyder et al. 2006; Tsacopoulos and Magistretti 1996; Vega et al. 1998). The astrocyte-neuron lactate shuttle hypothesis proposes that excitatory glutamatergic neurotransmission in the cerebral cortex enhances astrocytic glycolysis, which in turn generates lactate for transfer to and utilization by active neurons (Bittar et al. 1996; Kasischke et al. 2004; Magistretti 2006).
The evidence for astrocytes being primarily glycolytic and neurons using more oxidative metabolism includes several studies performed with cultured astrocytes and neurons (e.g. (Walz and Mukerji 1988). For example, glutamate stimulates glycolysis in astrocyte cultures but promotes glucose oxidation in neuronal cultures (Pellerin and Magistretti 1994; Pellerin and Magistretti 2004; Takahashi et al. 1995). By contrast, many other studies indicate that astrocytes are fully capable of oxidizing glucose and other substrates and thus may have significant flexibility in utilizing fuel sources (reviewed in Hertz et al. 2007). NMR approaches have clearly demonstrated, both in vitro and in vivo, that Krebs cycle fluxes are higher in neurons than in astrocytes, but they also demonstrate that significant oxidative metabolism does occur in astrocytes in vivo (Bluml et al. 2002; Bouzier-Sore et al. 2006; Cruz and Cerdan 1999).
Although the in vitro cell culture approach offers several methodological advantages, neurons and astrocytes in culture are likely to have different properties than those in vivo. Despite this concern, several in vivo observations do support the notion of differential metabolic properties between mammalian astrocytes and neurons. For example, brain glycogen is found largely in astrocytes and can generate lactate for possible use by axons (Tekkok et al. 2005). Some metabolic fuels, such as acetate, are oxidized exclusively in astrocytes (Lebon et al. 2002; Muir et al. 1986). Neurons on the other hand can not only survive in glucose-free culture media containing lactate or pyruvate as the only energy source (Itoh et al. 2003), they can also be rescued from in vivo hypoglycemic injury with pyruvate infusion (Suh et al. 2005).
The molecular underpinnings driving the differential metabolic phenotype of astrocytes and neurons remain incompletely delineated. Significant focus has been placed on differential expression of lactate dehydrogenase (LDH) enzymes and monocarboxylate transporters (MCTs). High rates of pyruvate conversion into lactate via LDH are necessary in cells that derive ATP largely from glycolysis, since this action maintains a high cytosolic NAD+/NADH ratio and avoids an upstream block in glycolysis at glyceraldehyde 3-phosphate dehydrogenase (Cerdan et al. 2006). Astrocytes express LDH isoforms that favor lactate formation and MCT isoforms that favor lactate efflux (Bittar et al. 1996; Broer et al. 1997; Pierre and Pellerin 2005). However, both neurons and astrocytes in the mammalian brain contain mitochondria (Pysh and Khan 1972), and the molecular mechanisms that may limit pyruvate oxidation by astrocyte mitochondria have not been thoroughly evaluated. Surprisingly, a comprehensive transcriptional profiling of protoplasmic astrocytes indicates that most Krebs cycle enzymes are more enriched in astrocytes than in neurons (Lovatt et al. 2007). Low astrocytic level of aralar1, a component of the mitochondrial malate-aspartate shuttle (Ramos et al. 2003), could prevent shuttling of cytosolic NADH within mitochondria and favor conversion of pyruvate to lactate in order to regenerate cytosolic NAD, at the expense of the Krebs cycle and the respiratory chain activity. However, the potential contribution of other mechanisms used by cells in peripheral organs to reversibly route glycolytic endproducts for oxidation has not been explored in astrocytes (Bowker-Kinley et al. 1998).
A major molecular control point that allows liver and striated muscle cells to switch from mitochondrial oxidation of glycolytic endproducts to other fuels is the mitochondrial multienzyme pyruvate dehydrogenase complex (PDC) (Jeoung et al. 2006b; Lydell et al. 2002; Patel and Korotchkina 2006; Sugden and Holness 2003). This large complex performs three reactions via three distinct components referred to as E1 (pyruvate dehydrogenase or PDH; composed of two subunits, PDHα and PDHβ), E2 (dihydrolipoyl acetyltransferase, DLAT) and E3 (dihydrolipoyl dehydrogenase, DLD), which together catalyze the irreversible oxidative decarboxylation of pyruvate to acetyl-CoA, CO2, and NADH. PDC activity can be dynamically regulated by the differential expression of its constituent proteins or by phosphorylation of the PDHα subunit (Harris et al. 2001; Patel and Korotchkina 2006; Sugden and Holness 2003; Tovar-Mendez et al. 2003). The control of PDHα phosphorylation is accomplished by a set of 4 different pyruvate dehydrogenase kinases (PDK1-4) and 2 different pyruvate dehydrogenase phosphatases (PDP 1 and 2), which are differentially expressed in mammalian tissues (Bowker-Kinley et al. 1998). Although PDHα has three phosphorylation sites, phosphorylation of site 1 (S293, in the immature rodent and human PDHα protein) reduces overall PDC activity by >97% (Patel and Korotchkina 2006). Since PDC regulation determines the rates of pyruvate oxidation, and therefore the relative ratio of glycolytic vs. oxidative glucose metabolism in cells, it is surprising that relatively little is known about the differential expression of PDC components and regulatory proteins in astrocytes vs. neurons. Moreover, the potential role of differential PDHα phosphorylation in supporting the astrocyte-neuron lactate shuttle remains unexplored.
In this study, we have studied the contribution of this mechanism to patterns of glucose metabolism seen in primary cell cultures of these cell types. We provide evidence supporting a role for differential PDC activity in distinguishing glucose metabolism patterns between astrocytes and neurons in culture and in potentially coordinating the compartmentalized metabolism of glucose among brain cells.
MATERIALS AND METHODS
Materials
Fetal bovine serum, DMEM, B27 supplement, neurobasal media, penicillin/streptomycin, mouse anti-PDHα (1:1000), Zoom® 2D protein solubilizer #1, NuPAGER LDS sample buffer, NuPAGER sample reducing agent, mouse anti-COX IV (1:2000), Prolong antifade with DAPI, and fluorescently labeled secondary antibodies were purchased from Invitrogen (Carlsbad, CA). BCA Protein Assay Kit (reducing agent compatible) was purchased from Pierce (Rockford, IL). Mouse anti-PDHβ (1:1000) and mouse anti-DLAT/DLD (E2/E3 subunits, 1:1000) antibodies and MitoProfile assay kits for PDC activity were purchased from Mitoscience (Eugene, OR). Rabbit anti-PDK1 (1:1000) and PDK3 (1:3000) were obtained from Stressgen (Ann Arbor, MI) and Abgent (San Diego, CA), respectively. Rabbit anti-PDP1, PDP2 and mouse anti-PDK2 and PDK4 were generated in laboratory of Dr. R.A. Harris (Huang et al. 2003; Jeoung et al. 2006b). Rabbit anti-glial fibrillary acid protein (GFAP) and mouse anti-microtubule associated protein-2 (MAP2) were purchased from Chemicon (Temecula, CA) and Sigma-Aldrich (St. Louis, MO) respectively.
Generation of phospho-specific antibody against site 1 of PDHα
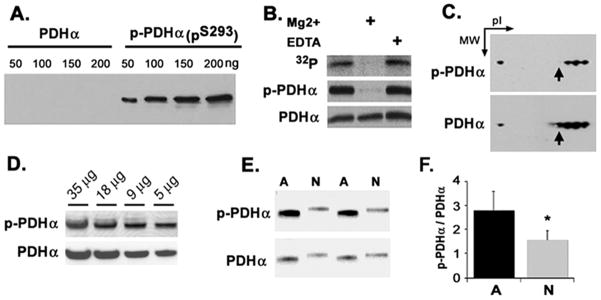
A pS293 phospho-specific antibody was generated in New Zealand white rabbits by injecting a peptide (YRYHGH(pS)MSDPG) (sequence of the precursor human and rodent protein) conjugated to the adjuvant KHL (Novus Biologicals). The resulting serum was negatively purified by preabsorption against unphosphorylated peptide. To evaluate the specificity of the rabbit anti- PDHαpS293 antibody, in vitro phosphorylation of brain mitochondria was accomplished by incubation with 6 μCi/mmol 32P-γ ATP alone, or with the addition of 10 mM MgSO4 for 30 min at 37°C. The reaction was terminated by the addition of sample buffer. The proteins were resolved by SDS-PAGE, transferred to nitrocellulose and subsequently evaluated for in vitro phosphorylation by autoradiography, or immunoblotting using antibodies against PDHαpS293 and PDHα.
Cell cultures
Primary cortical neuronal cultures were prepared from rat fetuses extracted by caesarian section from timed 17-day pregnant Sprague-Dawley female rats (Taconic; Albany, NY). Fetal cortices were dissected and meninges removed, and then tissue was minced and enzymatically digested with trypsin and DNase. Following digestion, cells were further triturated then passed through a cell strainer. Cells were plated onto poly-D-lysine-treated plates in DMEM containing 9% FBS and 1% penicillin-streptomycin at a density of 234,000 cells/cm2. After 24 h, the media was replaced with Neurobasal A media containing 2% 50X B27 supplement, 1% penicillin-streptomycin, and 0.5 mM glutamine. Half of the media was replaced every third day. Primary glial cultures were prepared from the cerebral cortices of neonatal pups and plated into tissue culture flasks as described (Armstrong 1998). Cells were grown in Dulbecco’s DMEM (Gibco Cat # 119565-092) containing 1 mM pyruvate and 10% fetal calf serum. Ten days after plating, the confluent flasks were subjected to a 1 h pre-shake (190 RPM in a rotary shaker). Dislodged cells (the microglia-rich fraction) were discarded and then fresh medium was added, followed by a second overnight shake. The next day, the O2A progenitors suspended in the supernatant were removed. The remaining adherent cells (> 95% GFAP-positive type 1 astrocytes) were trypsinized, re-plated onto plastic dishes or glass coverslips and used for further analysis. Trypan blue exclusion was used to test for cell viability. Rat hippocampal neuron/glia co-cultures (Fig. S1) were prepared and grown in Neurobasal/B27 medium as previously described (Schell et al. 2001).
Protein Isolation and Immunoblotting
After 7 or 10 days, for neurons or astrocytes respectively, the media was removed and the cells were rinsed with PBS. Proteins were extracted using Zoom 2D protein solubilizer #1 as described by the manufacturer. Homogenates containing 80 μg of total protein were prepared for immunoblotting by diluting samples with water, NuPAGE LDS sample buffer, and NuPAGE sample reducing agent to final volume of 20 μl. Samples were then heated to 70°C for 10 min to denature proteins. Neuron and astrocyte samples were subjected to electrophoreisis on 10% bis-tris polyacrylamide gels and then transferred to nitrocellulose. Membranes were blocked for 2 h in 10% normal serum (or 5% non-fat milk for LDH) in Tris-buffered saline (TBS) with 0.1% Tween-20 (TBS-T), then incubated with primary antibodies overnight at 4°C. Blots were rinsed in TBS-T, incubated with the appropriate peroxidase-conjugated secondary antibodies for 2 h. Blots were developed in ECL-plus and exposed to film. Films were digitized and then analyzed using the Gel Analyzer macro in ImageJ software (Wayne Rasband, NIH). Preparation and analysis of whole rat brain followed a similar procedure. Following rapid dissection, rat brain was immediately frozen in liquid nitrogen, then pulverized and extracted with Zoom 2D protein solubilizer #1, as described by the manufacturer. Protein concentrations were determined using the BCA protein assay kit.
Two-Dimensional Gel Electrophoresis
Brain mitochondria were isolated from adult rats by sucrose centrifugation as described previously (Clark and Nicklas 1970). Protein was extracted for electrophoreisis as described above. 2D-electrophoresis was performed on rat brain mitochondria using the Zoom bench top proteomics system according to the manufacturer’s protocol. Briefly, homogenates were loaded onto pH 3-10 IPG strips according to the manufacturer’s instructions. IPG strips were then separated using a step-voltage protocol (175 V, 15 min; 175-2000 V, 45 min; 2000 V, 45 min). IPG strips were then removed treated with NuPAGE LDS sample buffer with NuPAGE sample reducing agent, then NuPAGE LDS sample buffer with 125 mM iodoacetamide prior to separation in the second dimension. SDS-PAGE, transfer of proteins, and immunoblotting were performed as described above.
Immunocytochemistry
Cells grown on glass coverslips were fixed on ice with cold (−20°C) 70% acetone/30% methanol for 8 min then rinsed thoroughly in PBS. Coverslips were blocked by incubation in 2% fish skin gelatin in PBS at room temperature for 1 h, then incubated in 1% gelatin in PBS with primary antibodies at 4°C overnight. After a thorough rinsing in PBS, coverslips were incubated with appropriate fluorescent-conjugated secondary antibodies for 30 min in 1% gelatin, then rinsed and mounted using Prolong anti-fade with DAPI.
Biochemical Assays
Neuronal and astrocyte cultures were assayed for specific activity of the PDC using a commercially available MitoProfile microplate assay kit. This kit consists of an assay for PDC quantity (ELISA assay for PDC) and activity (spectrophotometric measurements of NADH production). The assays were performed according to the manufactures guidelines; the results of the both assays were then used to determine specific activity of the PDC (PDC activity/PDC quantity) and expressed as a mean of astrocyte specific activity. PDC activity was determined as described previously (Jeoung et al. 2006a).
Lactate dehydrogenase (LDH) assay and media lactate measurement
LDH measurements were performed with a Hitachi 747 analyzer (Roche Molecular Biochemicals Basel, Switzerland), based on conversion of pyruvate to lactate and simultaneous oxidation of NADH to NAD. The rate of decrease in NADH is directly proportional to the LDH activity and is determined spectrophotometrically at 340 nm. Results from this assay are expressed as activity (units/liter) normalized to total protein per sample (units/liter/mg total protein). Media lactate levels were assessed using a commercial CMA 600 microdialysis system. Lactate levels were measured at specified times following replacement of media with fresh media. This included three media washes lasting 10 sec each to remove residual lactate. Typical rates of observed lactate production using this approach with our cell cultures were ~600 nmol/60 min/106 astrocytes and ~150nmol/60 min/106 neurons. For dichloroacetate treatments of astrocytes, the drug was added at the time of media replacement.
Statistical Analysis
Two-tailed Student’s t-tests were used to determine pair-wise differences between neuron and astrocyte cultures. Analysis of variance was used to determine differences in instances with multiple comparisons.
RESULTS
Enriched Astrocyte and Neuronal Cultures Display Unique Metabolic Profiles
The two types of cell cultures displayed characteristic astrocytic or neuronal morphological features (Fig. 1). The purity of the cell cultures was assessed by the expression of the astrocyte specific intermediate filament GFAP and the neuronal specific microtubule stabilizing protein MAP2 in cell extracts (Fig. 1B). With prolonged exposure times, only a very weak GFAP signal could be detected in neuronal cultures. Based on microscopic counts of GFAP+ or MAP2+ cells (as in Fig. 1F), the respective cultures were both estimated to be >95% pure as shown previously (Grimaldi et al. 2003).
Fig. 1.
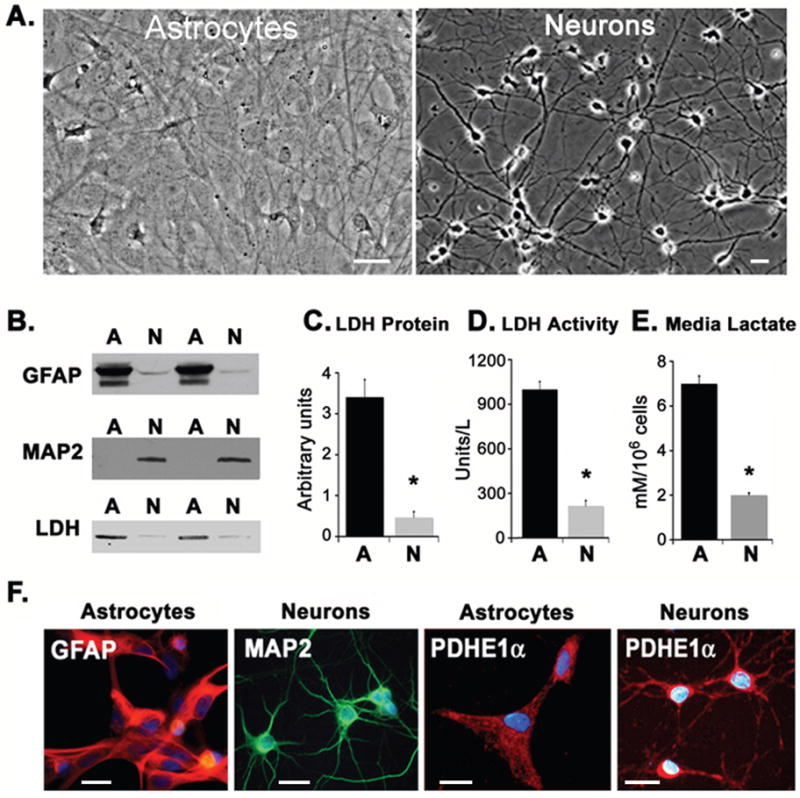
Characterization of primary cortical cultures. (A) Phase-contrast images illustrating the typical morphologies of cultured astrocytes (left) and neurons (right). (B) Western blots illustrating the relative expression of astrocyte-specific (GFAP, top) or neuron specific (MAP2, middle) markers or LDH (bottom) immunoreactivity. Duplicate samples are shown. (C) Densitometric analysis of LDH blots. Data are representative of quadruplicate measurements from three independent cultures. (D) LDH enzymatic activity expressed as units/liter for equivalent amounts of cell protein from astrocytes (dark bars) and neurons (light bars). (E) Lactate release into culture medium by astrocytes (dark bars) and neurons (light bars). Neuronal cultures showed significantly lower LDH levels and lactate production (asterisks). (F) In the left panels, cultures were double-stained with antibodies against GFAP (red) and MAP2 (green). Nuclei are labeled with DAPI (blue). Astrocyte cultures showed prominent immunorecativity for GFAP (red) but not MAP2, while neurons show immunoreactivity for MAP2 (green), but not GFAP (red). In the right panels, both types of cell cultures showed immunoreactivity for PDHα (red). Scale bars = 20μm.
Both astrocytic and neuronal cultures expressed LDH as demonstrated by Western blotting using a polyclonal antibody recognizing both isoforms (LDH-A and LDH-B, Fig. 1B). However, as shown by blot densitometry (Fig. 1C), LDH protein expression was significantly higher in astrocyte cultures than in neuronal cultures (P < 0 .0001). LDH enzyme activity, normalized to total protein, was also much higher in astrocytes (Fig. 1D; P < 0 .0001), consistent with a higher lactate production by astrocytes than by neurons (Fig. 1E).
Cultured astrocytes and Neurons Display Distinct Expression Profiles for the Pyruvate Dehydrogenase Complex, PDH Kinases and PDH Phosphatases
Diversion of pyruvate to lactate is facilitated by low pyruvate metabolism via PDC. Using a monoclonal antibody, we observed PDHα staining in both the cerebrocortical astrocyte and neuronal cultures (Fig. 1F). Mixed cultures of rat hippocampal astrocytes and neurons, grown in the same medium formulation as the cortical neurons, also showed prominent PDH staining in both cell types, with the highest PDHα levels localized to astrocytes (Fig. S1). Using Western blotting to probe equal amounts of extracted protein from the isolated cerebrocortical cell cultures, we determined that all subunits of the PDC could be detected in both neurons and astrocytes (Fig. 2A–C). A slight shift was observed in the mobility of PDHα during SDS-PAGE between astrocytes and neurons (Fig. 2A). This shift was observed using four different antibodies, and suggested the possible presence of different PDHα isoforms. However, when the PDC complex was immunoprecipitated from the two cell types using an antibody to the E2 subunit and was then probed by Western blot with the PDHα antibody, the difference in molecular weights disappeared (Fig. 2B). These data suggest that other abundant proteins in the crude cell extracts affect the apparent mobility of PDHα in the gel, causing an apparent difference in molecular weight. Astrocytes had significantly higher levels of immunoreactivity for all subunits of the PDC, but particularly for DLD (E3/E3bp) and PDHβ (Fig. 2A and 2C). Densitometric analysis of Western blots showed that astrocytes expressed significantly higher levels of PDHα (P = 0.034), PDHβ (P = 0.02), DLD (P < 0.001), and DLAT (P < 0.001) than neurons. In contrast, immunoreactivity for subunit IV of the mitochondrial cytochrome oxidase enzyme complex (COX IV) was equal in both culture preparations (P = 0.84) (Fig. 2C).
Fig. 2.
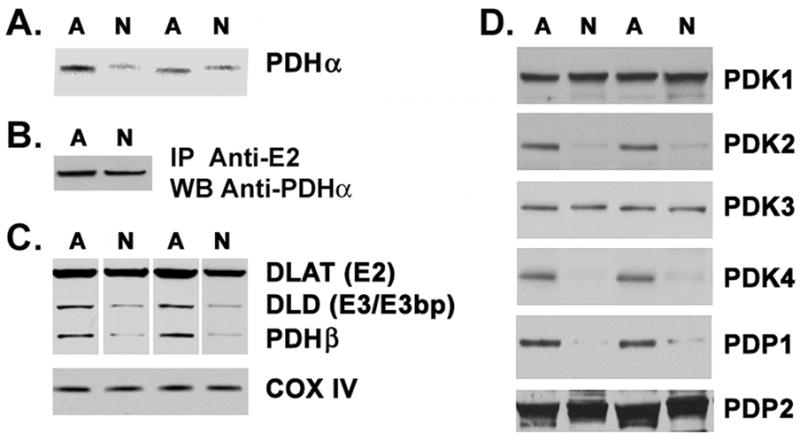
Pyruvate dehydrogenase complex (PDC) subunit expression in astrocytes and neurons. (A) Astrocyte and neuronal cell extracts (80μg protein) were analyzed by Western blot; duplicate experiments are shown. (B) Cell extracts were first immunoprecipitated with anti-E2 antisera then probed with anti-PDHα. (C) Western blots depicted in (A) were stripped and reprobed for PDHβ, DLAT (E2), DLD (3/E3bp), or COX IV. (D) Blots of cell extracts were probed for PDK1-4 and PDP1-2. Data presented here are representative of quadruplicate measurements from three independent cultures.
We also detected immunoreactivity for all known mammalian PDKs (PDK1-4) and PDPs (PDP1, 2) in both astrocyte and neuron cultures. However, unique expression levels of these proteins were noted in the two cell types (Fig. 2D). Blot densitometry showed astrocytes to have significantly higher immunoreactivity for PDK2 (P < 0 .0001) and PDK4 (P < 0 .0001). Neuronal cultures expressed significantly higher levels of PDK1 than astrocytes (P = 0.03), although the differential expression of PDK1 between astrocytes and neurons was less than that for PDK2 and PDK4. No significant difference in immunoreactivity for PDK3 (P = 0.93) was observed between the two cell types. Cultures of astrocytes and neurons also expressed both PDP1 and PDP2 (Fig. 2D). However, immunoreactivity of PDP1was significantly higher in astrocyte cultures (P < 0.0001), while the immunoreactivity of PDP2 was similar in both cultures (P = 0.23). Statistical analysis was performed on quadruplicate measurements from three independent cultures. The images depicted in Fig. 2 are representative duplicates from single experiments.
Cultured Astrocytes Display Higher PDHα Phosphorylation and Lower PDC Activity than Neurons
In order to trap and directly assess the phosphorylation status of the PDHα protein, we generated a phospho-specific antibody that specifically recognizes the phosphorylation of site 1 (serine 293). Using recombinant PDH, the antibody was found to recognize a protein containing phospho-serine 293 but had little to no cross-reactivity with the recombinant unphosphorlyated PDHα (Fig. 3A). To demonstrate the specificity of this antibody in tissue extracts we performed an in vitro PDHα phosphorylation assay in isolated rat whole brain mitochondria using 32P-γATP. Since the PDKs and PDPs are contained inside isolated intact mitochondria, the phosphorylation of PDHα can be easily manipulated in such preparations (Hopper et al. 2006). Autoradiographic analysis of SDS-PAGE separated proteins transferred to nitrocellulose membranes revealed the most prominent 32P-labeled band migrated at approximately 42 kDa. Treatment with Mg2+, which is required for PDP activity, greatly reduced labeling of this band (Fig. 3B, top panel). Subsequent immunoblotting of the same membrane with anti-PDHα or anti-PDHα demonstrated Mg2+-induced dephosphorylation of PDHα when the PDHα targeted antisera was employed. This reduction in immunoreactivity was not seen with PDHα targeted antisera (Fig. 3B). To further confirm the specificity of the PDHαpS293 antisera, we performed two-dimensional electrophoresis on rat brain mitochondrial protein extracts. Immunoblotting of separated proteins produced a train of spots, characteristic of phosphoproteins, at the molecular weight (42 kDa) and isoelectric point (pI) observed for PDHαpS293 (Hopper et al. 2006) (Fig. 3C). To determine the spotting differential of the PDHαpS293 antibody to the total PDHα antibody, we stripped the membrane and then re-probed it using a mouse monoclonal anti-PDHα antibody. Immunoblotting with anti-PDHα produced a similar train of spots at a similar MW and pI as the PDHαpS293 antibody, although one more spot was observed (Fig. 3C, see arrow).
Fig. 3.
Characterization of phospho-specific antibody to serine 293 of PDHα (PDHαpS293). (A) Recombinant human PDHα and PDHα containing PDHαpS293 were probed by Western blot using the anti- PDHαpS293 serum. (B) Rat brain mitochondria were incubated with 32P-ATP in the absence or presence of 10 mM Mg2+ or 1 mM EDTA for 30 min, before analysis by Western blot. The blot was exposed to film to generate an autoradiogram for the anti-PDHαpS293 serum and then stripped and re-probed for total PDHα. (C) Rat brain mitochondria were subjected to 2D-gel electrophoresis, membrane transfer, and probed by Western blot with anti- PDHαpS293 serum. The same blot was stripped and re-probed for total PDHα. (D) Rat brain extract was probed with anti- PDHαpS293 serum, then stripped and re-probed for total PDHα. Amount of protein loaded is indicated. (E) Astrocyte and neuronal cell extracts were probed by Western blot using anti PDHαpS293 serum; the same blot was stripped and re-probed for total PDHα. (F) Immunoblots of astrocyte and neuronal cell extracts were analyzed by densitometry and expressed as the ratio of phospho-PDHα to PDHα alpha. Data presented are representative of quadruplicate measurements from three independent cultures.
Using the PDHαpS293 antibody we found that rapidly processed whole brain homogenates contained significant levels of phosphorylated PDHα (Fig. 3D). We discovered that astrocyte cultures had significantly higher levels of PDHαpS293 (P< 0.001) immunoreactivity than neuronal cultures. Moreover, the ratio of PDHαpS293 to PDHα was much higher in astrocytes compared to neurons (P = 0.02) (Fig. 3E, F), suggesting that despite its high level of expression PDC might be less active in astrocytes than in neurons.
In order to determine whether the differential phosphorylation status of PDHα was linked to the differential PDK activity in the two cell types, we utilized dichloroacetate (DCA), an established inhibitor of PDKs when used at mM concentrations (Itoh et al. 2003). Using isolated whole brain mitochondria we were able to demonstrate a dose dependent inhibition of PDHα phosphorylation with DCA (Fig. 4A). Cultures astrocytes treated with 10 mM DCA showed an inhibition of PDHα phosphorylation (Figure 4B). DCA treatment also dose-dependently lowered lactate production by astrocytes, down to the level seen in neurons cultured without DCA (Fig. 4C). However, over its effective dose range for PDK inhibition, DCA produced toxicity in neurons but not astrocytes (Fig. 4D). In addition to being a weak inhibitor of PDK, DCA also inhibits cholesterol biosynthesis (Stacpoole and Greene 1992) and promotes oxidativestress (Hassoun and Ray 2003). The observed differential cell toxicity of DCA in cultured neurons vs. astrocytes precluded its further use for comparing reversible PDH phosphorylation between the cell types.
Fig. 4.
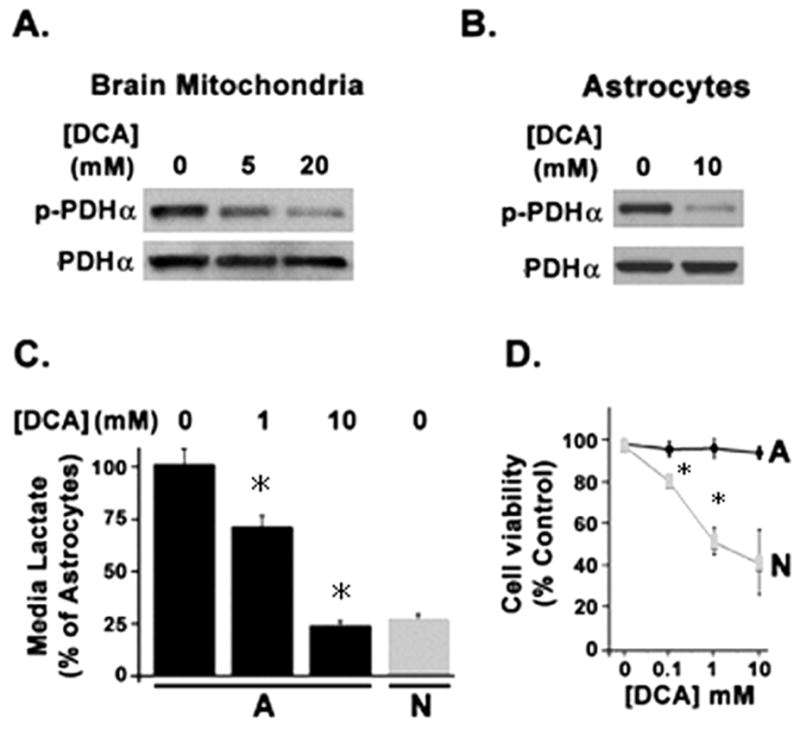
Effect of dichloroacetate (DCA) on PDH phosphorylation, lactate production, and cell viability. (A) Brain mitochondria incubated with indicated DCA concentrations were analyzed by Wetern blot using anti-phospho PDHα and anti-PDHα antisera. (B) Astrocytes incubated with 10 mM DCA for 1 h were analyzed similarly for PDHα phosphorylation. (C) Lactate release into the media over 1 h was measured in astrocytes treated with the indicated concentrations of DCA and compared to untreated neurons. Bar heights and error bars represent mean concentrations +/− SEM of media lactate determined in three experiments from four independent cultures. (*P < 0.05). (D) Neuronal and astrocytic cultures were incubated with indicated DCA concentrations for 1 h and assessed for cell viability.
In order to directly determine whether PDC activity was indeed higher in neurons despite greater overall expression of the complex proteins in astrocytes, we used a commercial assay that combines immunoprecipitation with quantification of PDC protein and enzyme activity. This assay revealed that neurons have more than 50% higher specific activity of the PDC than astrocytes (Fig. 5A). To directly relate PDHα phosphorylation status to the differential PDC activity observed between astrocyte and neuronal lysates, we assayed PDC activity with and without addition of recombinant active PDP-1 to force complete dephosphorylation of PDH α (Jeoung et al. 2006a). Neurons were again found to have high PDC activity that was stimulated ~25% after PDP-1 treatment. Astrocytes displayed much lower PDC activity compared to neurons but this activity was stimulated >300% after PDP-1 treatment (Fig. 5B & C). Thus, native PDC was operating at close to its maximal activity in neurons but far from its maximal activity in astrocytes. Together these data support a role for PDHα phosphorylation in determining the differential metabolic phenotype of cultured astrocytes and neurons.
Fig. 5.
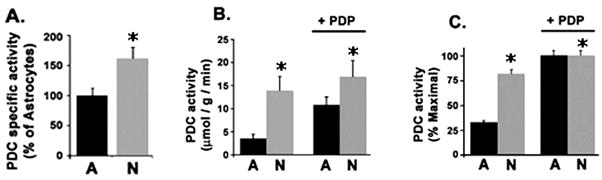
Effect of PDC dephosphorylation on PDC activity in astrocyte and neuronal extracts. (A) Specific activity of the PDC determined in astrocyte and neuronal lysates following PDC immunocapture. (B) PDC activity determined in astrocyte and neuronal extracts before and after addition of recombinant PDC phosphatase (PDP). (C) The absolute PDC activity shown in (B) is expressed as percent maximal activity for each cell type. Bar heights and error bars represent mean enzyme activity rates +/− SEM determined in experiments from four independent cultures of astrocytes or neurons. (*P < 0.001).
DISCUSSION
The aim of this study was to elucidate the role of the PDC in the differential metabolism of pyruvate/lactate in primary cultures of rat cerebral cortical astrocytes and neurons. We hypothesized that regulation of the PDC at the protein expression or activity contributed to the differential metabolic phenotype of neurons and astrocytes and to the directional shuttling of monocarboxylates between these cell types. We demonstrated that all of the subunits of the PDC are expressed in cultured astrocytes and neurons but that astrocytes express significantly higher immunoreactivity for all subunits compared to neurons (Fig. 2A–C). By contrast, immunoreactivity for COX IV, a component of the mitochondrial oxidative phosphorylation complex, is similar in both cell types in culture.
PDC activity is regulated by reversible phosphorylation of PDHα (Patel and Korotchkina 2006), and using isozyme specific antibodies we show that the PDH kinases and phosphatases are differentially expressed between astrocytes and neurons. Control of PDK expression levels allows cells and tissues to regulate PDC activity and therefore glucose oxidation rates (Lydell et al. 2002). The higher expression of PDK2 and PDK4 in astrocytes vs. neurons is thus consistent with the higher PDHα phosphorylation status, lower PDC activity, and higher lactate production displayed by cultured astrocytes. Higher LDH immunoreactivity was also observed in astrocytes using a polyclonal antibody to both LDH isozymes (LDH-A and LDH-B) (Fig. 1B), as well as higher LDH activity. This observation supports the pervading notion of cultured astrocytes being highly glycolytic cells.
PDP-1 was the predominant phosphatase expressed in astrocytes. PDP-1 activity strongly depends on the presence of calcium while PDP-2 is believed to be calcium independent (Karpova et al. 2003). Regulation of PDH activity in brain slices has previously been attributed to changes in calcium accumulation in neurons (Lynch et al. 1983). Our results suggest that astrocytes may have contributed to these prior observations. Since many neurotransmitters increase astrocytic calcium levels (Fiacco and McCarthy 2006), it is possible that astrocytic PDC activity is regulated by intra- and intercellular signals that control PDHα phosphorylation. Recent in vivo studies support this notion, as oxidative metabolism in glia is twice as great in the awake brain compared to the anesthetized brain, and the majority of increased oxidation is accounted for by increased PDH activity (Serres et al. 2008).
The increased expression levels of PDC regulatory enzymes in astrocytes are consistent with a tighter regulation of pyruvate (and lactate) metabolism in this cell type. Tight control of PDHα phosphorylation status in astrocytes may ensure pyruvate being blocked from entry into the Krebs cycle and being converted instead to lactate via LDH activity. Since transport of lactate (and pyruvate) through MCTs is bidirectional and dependent on concentration gradients of the monocarboxylates and H+ (Halestrap and Meredith 2004; Hertz and Dienel 2005), astrocytic PDC activity status may contribute significantly to the directional shuttling of monocarboxylates from astrocytes to neurons. The dephosphorylation of PDHα and lowering of astrocytic lactate production by DCA is consistent with this notion. Moreover, the strong activation of PDC in astrocytic extracts through forced dephosphorylation of PDHα suggests a novel potential mechanism for varying both the degree of astrocytic oxidative activity and the degree of astrocytic-neuronal metabolic coupling.
A major consideration for the appropriate interpretation of our results is the fact that our study employed primary cultures of neurons and astrocytes, and the expression of PDC components and PDC activity in cultured astrocytes and neurons does not necessarily reflect the in vivo situation. For example, even though we detected equivalent immunoreactivity for COX IV between cultured neurons and astrocytes, studies performed in vivo have reported much lower levels of COX immunostaining in glial cells vs. neurons (e.g., (Taskinalp et al. 2000). Moreover, regionally specific heterogeneity may exist in the metabolic phenotype of astrocytes in different parts of the brain (reviewed in(Hertz et al. 2007). In vivo, glial metabolism near synapses is largely performed by protoplasmic astrocytes, which ensheath synapses in gray matter and express low levels of GFAP. The relationship between the type 1 astrocyte cultures used in this study and peri-synaptic astrocytes in vivo is unclear. However, our study did reveal that cultured astrocytes have high levels of PDC components in their mitochondria, similar to protoplasmic astrocytes isolated acutely from brain (Lovatt et al. 2007).
It is also possible that the different culture medias used to generate pure preparations of cortical astrocytes vs. neurons affect the expression profiles of metabolic enzymes observed in our study and in prior studies (Bittar et al. 1996; Broer et al. 1997; Pierre and Pellerin 2005; Ramos et al. 2003). For example, the B27 supplement used for neuronal cultures contains insulin but is serum-free, while astrocytes are grown in routine cell culture medium with 10% serum. Previous work has shown that certain trophic factors can indeed promote astrocytes to take on a more oxidative phenotype (Escartin et al. 2007). However, we did observe a much higher expression of PDHα in astrocyte vs. neurons using hippocampal neuron-astrocyte co-cultures in which both cell types experienced identical serum-free media conditions (Fig S1). We did detect a high level of baseline PDHα phosphorylation in rat brain extract, but have not yet been able to distinguish whether this predominantly reflects neuronal or astrocytic contributions due to difficulties in using our antibodies for immunohistochemical detection in situ.
Given the challenges of determining cell-specific metabolic phenotypes in situ in the brain, a significant literature has accumulated on the differential metabolic properties of cultured neurons and astrocytes. Our finding reported here may help inform some of the interpretations of this literature. Changes in PDH phosphorylation may allow astrocytes to switch between oxidation of glucose to other fuels that bypass PDH (Hertz et al. 2007; Pierre and Pellerin 2005). Cultured neurons, on the other hand appear to maintain high PDC activity due to low PDHα phosphorylation levels, thus remaining poised for and committed to pyruvate oxidation.
Studies using nuclear magnetic resonance in whole animals provide compelling evidence that the degree of astroglial oxidative metabolism is regulated by synaptic activity (Oz et al. 2004; Serres et al. 2008). Specifically, pyruvate flux through PDC—and also through the anaplerotic pathway that uses pyruvate carboxylase—double in the glial compartment of awake rodents compared to anesthetized ones and comprises between 20–40% of total Krebs cycle activity in brain (Bluml et al. 2002; Cruz and Cerdan 1999; Gruetter et al. 2001; Oz et al. 2004; Serres et al. 2008; Zielke et al. 2007). Studies in humans suggest a similar metabolic arrangement (Bluml et al. 2002; Gruetter et al. 2001). Our observations suggest one mechanism by which astrocytes can regulate the balance between their anaerobic and oxidative metabolism (Hertz et al. 2007), via kinase and phosphatase control of PDC activity. Overall, our findings obtained with cultured cells are consistent with a potential role for astrocytes in controlling the fuel supply for neuronal metabolism (Kasischke et al. 2004; Pellerin and Magistretti 2004). This conclusion, also supported by recent studies in vivo, implies that brain cells possess significant control mechanisms for metabolic plasticity.
Supplementary Material
Supplementary Fig. S1. Immunofluorescent staining of PDHα (top panel) and GFAP (middle panel) along with fluorescent DAPI staining (blue, bottom panel) in mixed hippocampal cultures of neurons and astrocytes. Astrocyes (A) exhibited intense labeling for PDHα. Neurons (N) showed a lower level of staining in their somas, but also exhibited punctate PDHα label throughout their axonal (arrowheads in top panel) and dendritic processes. Bottom panels show merged fluorescent markers. Scale bar = 20μm.
Acknowledgments
This study was supported in part by NIH grants NS37814 (A.V) and CA113506 (M.J.S.), DK42885 (M.S.P.) and DK47844 (R.A.H). Additional support was provided through Department of Defense (DOD) grant MDA905-03-2-0001 (A.V). The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the view of the DOD or the Uniformed Services University.
References
- Armstrong RC. Isolation and characterization of immature oligodendrocyte lineage cells. Methods-a Companion to Methods in Enzymology. 1998;16(3):282. doi: 10.1006/meth.1998.0685. [DOI] [PubMed] [Google Scholar]
- Bittar PG, Charnay Y, Pellerin L, Bouras C, Magistretti PJ. Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. J Cereb Blood Flow Metab. 1996;16(6):1079–89. doi: 10.1097/00004647-199611000-00001. [DOI] [PubMed] [Google Scholar]
- Bluml S, Moreno-Torres A, Shic F, Nguy CH, Ross BD. Tricarboxylic acid cycle of glia in the in vivo human brain. NMR Biomed. 2002;15(1):1–5. doi: 10.1002/nbm.725. [DOI] [PubMed] [Google Scholar]
- Bouzier-Sore AK, Voisin P, Bouchaud V, Bezancon E, Franconi JM, Pellerin L. Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur J Neurosci. 2006;24(6):1687–94. doi: 10.1111/j.1460-9568.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329 ( Pt 1):191–6. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer S, Rahman B, Pellegri G, Pellerin L, Martin JL, Verleysdonk S, Hamprecht B, Magistretti PJ. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J Biol Chem. 1997;272(48):30096–102. doi: 10.1074/jbc.272.48.30096. [DOI] [PubMed] [Google Scholar]
- Cerdan S, Rodrigues TB, Sierra A, Benito M, Fonseca LL, Fonseca CP, Garcia-Martin ML. The redox switch/redox coupling hypothesis. Neurochem Int. 2006;48(6–7):523–30. doi: 10.1016/j.neuint.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Clark JB, Nicklas WJ. The metabolism of rat brain mitochondria. Preparation and characterization. J Biol Chem. 1970;245(18):4724–31. [PubMed] [Google Scholar]
- Cruz F, Cerdan S. Quantitative 13C NMR studies of metabolic compartmentation in the adult mammalian brain. NMR Biomed. 1999;12(7):451–62. doi: 10.1002/(sici)1099-1492(199911)12:7<451::aid-nbm571>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Escartin C, Pierre K, Colin A, Brouillet E, Delzescaux T, Guillermier M, Dhenain M, Deglon N, Hantraye P, Pellerin L, et al. Activation of astrocytes by CNTF induces metabolic plasticity and increases resistance to metabolic insults. J Neurosci. 2007;27(27):7094–104. doi: 10.1523/JNEUROSCI.0174-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD. Astrocyte calcium elevations: properties, propagation, and effects on brain signaling. Glia. 2006;54(7):676–90. doi: 10.1002/glia.20396. [DOI] [PubMed] [Google Scholar]
- Grimaldi M, Maratos M, Verma A. Transient receptor potential channel activation causes a novel form of [Ca 2+]I oscillations and is not involved in capacitative Ca 2+ entry in glial cells. J Neurosci. 2003;23(11):4737–45. doi: 10.1523/JNEUROSCI.23-11-04737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter R, Seaquist ER, Ugurbil K. A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am J Physiol Endocrinol Metab. 2001;281(1):E100–12. doi: 10.1152/ajpendo.2001.281.1.E100. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447(5):619–28. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- Harris RA, Huang B, Wu P. Control of pyruvate dehydrogenase kinase gene expression. Adv Enzyme Regul. 2001;41:269–88. doi: 10.1016/s0065-2571(00)00020-0. [DOI] [PubMed] [Google Scholar]
- Hassoun EA, Ray S. The induction of oxidative stress and cellular death by the drinking water disinfection by-products, dichloroacetate and trichloroacetate in J774.A1 cells. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135(2):119–28. doi: 10.1016/s1532-0456(03)00082-6. [DOI] [PubMed] [Google Scholar]
- Hertz L, Dienel GA. Lactate transport and transporters: general principles and functional roles in brain cells. J Neurosci Res. 2005;79(1–2):11–8. doi: 10.1002/jnr.20294. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27(2):219–49. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hopper RK, Carroll S, Aponte AM, Johnson DT, French S, Shen RF, Witzmann FA, Harris RA, Balaban RS. Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry. 2006;45(8):2524–36. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Wu P, Popov KM, Harris RA. Starvation and diabetes reduce the amount of pyruvate dehydrogenase phosphatase in rat heart and kidney. Diabetes. 2003;52(6):1371–6. doi: 10.2337/diabetes.52.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26(7):865–77. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Esaki T, Shimoji K, Cook M, Law MJ, Kaufman E, Sokoloff L. Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc Natl Acad Sci U S A. 2003;100(8):4879–84. doi: 10.1073/pnas.0831078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeoung NH, Sanghani PC, Zhai L, Harris RA. Assay of the pyruvate dehydrogenase complex by coupling with recombinant chicken liver arylamine N-acetyltransferase. Anal Biochem. 2006a;356(1):44–50. doi: 10.1016/j.ab.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Jeoung NH, Wu P, Joshi MA, Jaskiewicz J, Bock CB, Depaoli-Roach AA, Harris RA. Role of pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) in glucose homoeostasis during starvation. Biochem J. 2006b;397(3):417–25. doi: 10.1042/BJ20060125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova T, Danchuk S, Kolobova E, Popov KM. Characterization of the isozymes of pyruvate dehydrogenase phosphatase: implications for the regulation of pyruvate dehydrogenase activity. Biochim Biophys Acta. 2003;1652(2):126–35. doi: 10.1016/j.bbapap.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305(5680):99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci. 2002;22(5):1523–31. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, et al. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27(45):12255–66. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydell CP, Chan A, Wambolt RB, Sambandam N, Parsons H, Bondy GP, Rodrigues B, Popov KM, Harris RA, Brownsey RW, et al. Pyruvate dehydrogenase and the regulation of glucose oxidation in hypertrophied rat hearts. Cardiovasc Res. 2002;53(4):841–51. doi: 10.1016/s0008-6363(01)00560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Kessler M, Halpain S, Baudry M. Biochemical effects of high-frequency synaptic activity studied with in vitro slices. Fed Proc. 1983;42(12):2886–90. [PubMed] [Google Scholar]
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209(Pt 12):2304–11. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Muir D, Berl S, Clarke DD. Acetate and fluoroacetate as possible markers for glial metabolism in vivo. Brain Res. 1986;380(2):336–40. doi: 10.1016/0006-8993(86)90231-3. [DOI] [PubMed] [Google Scholar]
- Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci. 2004;24(50):11273–9. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34(Pt 2):217–22. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91(22):10625–9. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Neuroscience. Let there be (NADH) light. Science. 2004;305(5680):50–2. doi: 10.1126/science.1100428. [DOI] [PubMed] [Google Scholar]
- Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94(1):1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- Pysh JJ, Khan T. Variations in mitochondrial structure and content of neurons and neuroglia in rat brain: an electron microscopic study. Brain Res. 1972;36(1):1–18. doi: 10.1016/0006-8993(72)90762-7. [DOI] [PubMed] [Google Scholar]
- Ramos M, del Arco A, Pardo B, Martinez-Serrano A, Martinez-Morales JR, Kobayashi K, Yasuda T, Bogonez E, Bovolenta P, Saheki T, et al. Developmental changes in the Ca2+-regulated mitochondrial aspartate-glutamate carrier aralar1 in brain and prominent expression in the spinal cord. Brain Res Dev Brain Res. 2003;143(1):33–46. doi: 10.1016/s0165-3806(03)00097-x. [DOI] [PubMed] [Google Scholar]
- Schell MJ, Erneux C, Irvine RF. Inositol 1,4,5-trisphosphate 3-kinase A associates with F-actin and dendritic spines via its N terminus. J Biol Chem. 2001;276(40):37537–46. doi: 10.1074/jbc.M104101200. [DOI] [PubMed] [Google Scholar]
- Serres S, Raffard G, Franconi JM, Merle M. Close coupling between astrocytic and neuronal metabolisms to fulfill anaplerotic and energy needs in the rat brain. J Cereb Blood Flow Metab. 2008;28(4):712–24. doi: 10.1038/sj.jcbfm.9600568. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A. 1998;95(1):316–21. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole PW, Greene YJ. Dichloroacetate. Diabetes Care. 1992;15(6):785–91. doi: 10.2337/diacare.15.6.785. [DOI] [PubMed] [Google Scholar]
- Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab. 2003;284(5):E855–62. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- Suh SW, Aoyama K, Matsumori Y, Liu J, Swanson RA. Pyruvate administered after severe hypoglycemia reduces neuronal death and cognitive impairment. Diabetes. 2005;54(5):1452–8. doi: 10.2337/diabetes.54.5.1452. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Driscoll BF, Law MJ, Sokoloff L. Role of sodium and potassium ions in regulation of glucose metabolism in cultured astroglia. Proc Natl Acad Sci U S A. 1995;92(10):4616–20. doi: 10.1073/pnas.92.10.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskinalp O, Aktas RG, Cigali B, Kutlu AK. Immunohistochemical demonstration of cytochrome oxidase in different parts of the central nervous system: a comparative experimental study. Anat Histol Embryol. 2000;29(6):345–9. doi: 10.1046/j.1439-0264.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Brown AM, Westenbroek R, Pellerin L, Ransom BR. Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J Neurosci Res. 2005;81(5):644–52. doi: 10.1002/jnr.20573. [DOI] [PubMed] [Google Scholar]
- Tovar-Mendez A, Miernyk JA, Randall DD. Regulation of pyruvate dehydrogenase complex activity in plant cells. Eur J Biochem. 2003;270(6):1043–9. doi: 10.1046/j.1432-1033.2003.03469.x. [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16(3):877–85. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega C, Poitry-Yamate CL, Jirounek P, Tsacopoulos M, Coles JA. Lactate is released and taken up by isolated rabbit vagus nerve during aerobic metabolism. J Neurochem. 1998;71(1):330–7. doi: 10.1046/j.1471-4159.1998.71010330.x. [DOI] [PubMed] [Google Scholar]
- Walz W, Mukerji S. Lactate release from cultured astrocytes and neurons: a comparison. Glia. 1988;1(6):366–70. doi: 10.1002/glia.440010603. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ, Tildon JT. Effect of fluorocitrate on cerebral oxidation of lactate and glucose in freely moving rats. J Neurochem. 2007;101(1):9–16. doi: 10.1111/j.1471-4159.2006.04335.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. Immunofluorescent staining of PDHα (top panel) and GFAP (middle panel) along with fluorescent DAPI staining (blue, bottom panel) in mixed hippocampal cultures of neurons and astrocytes. Astrocyes (A) exhibited intense labeling for PDHα. Neurons (N) showed a lower level of staining in their somas, but also exhibited punctate PDHα label throughout their axonal (arrowheads in top panel) and dendritic processes. Bottom panels show merged fluorescent markers. Scale bar = 20μm.