Abstract
Cell-based therapy holds great promise for cancer treatment. The ability to non-invasively track the delivery of various therapeutic cells (e.g. T cells and stem cells) to the tumor site, and/or subsequent differentiation/proliferation of these cells, would allow better understanding of the mechanisms of cancer development and intervention. This brief review will summarize the various methods for non-invasive cell tracking in cancer and cancer therapy. In general, there are two approaches for cell tracking: direct (cells are labeled with certain tags that can be detected directly with suitable imaging equipment) and indirect cell labeling (which typically uses a reporter gene approach). The techniques for tracking various cell types (e.g. immune cells, stem cells, and cancer cells) in cancer are described, which include fluorescence, bioluminescence, positron emission tomography (PET), single-photon emission computed tomography (SPECT), and magnetic resonance imaging (MRI). Non-invasive tracking of immune and stem cells were primarily intended for (potential) cancer therapy applications while tracking of cancer cells could further our understanding of cancer development and tumor metastasis. Safety is a major concern for future clinical applications and the ideal imaging modality for tracking therapeutic cells in cancer patients requires the imaging tags to be non-toxic, biocompatible, and highly specific. Each imaging modality has its advantages and disadvantages and they are more complementary than competitive. MRI, radionuclide-based imaging techniques, and reporter gene-based approaches will each have their own niches towards the same ultimate goal: personalized medicine for cancer patients.
Keywords: Cancer, cell tracking, cancer therapy, stem cells, positron emission tomography, molecular imaging, optical imaging, magnetic resonance imaging
INTRODUCTION
Over the last several decades, much effort has been dedicated to the treatment of cancer and one of the most promising approaches is targeted delivery of therapeutic agents. Construction of delivery vehicles that can home to the tumor (targeted and/or non-targeted) before delivering a therapeutic payload is critical for effective cancer therapy with minimal systemic toxicity. Besides certain nanoparticles and liposomes which have been extensively explored for cancer therapy applications [1-4], cell-based therapy also holds great promise [5,6]. Tracking the delivery of these cells (e.g. T cells and stem cells) to the tumor site is a prerequisite to effective cancer therapy [7]. This brief review will summarize the various methods for non-invasive cell tracking in cancer and cancer therapy.
Non-invasive imaging techniques are indispensable tools for such cell tracking. Molecular imaging, as defined by the Society of Nuclear Medicine, is “the visualization, characterization and measurement of biological processes at the molecular and cellular levels in humans and other living systems” [8]. Molecular imaging techniques typically include molecular magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), optical bioluminescence, optical fluorescence, targeted ultrasound, single photon emission computed tomography (SPECT), and positron emission tomography (PET) [9,10]. Continued development and wider availability of scanners dedicated to small animal imaging studies, which can provide a similar in vivo imaging capability in mice, primates, and human, can enable smooth transfer of knowledge and molecular measurements between species thereby facilitating clinical translation of novel imaging agents and/or techniques.
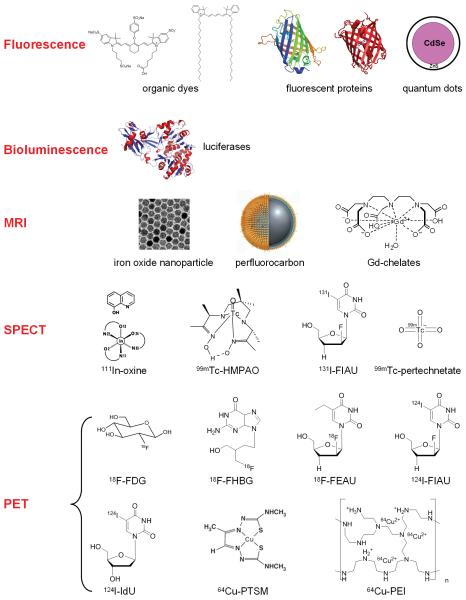
In general, there are two approaches for cell tracking: direct and indirect cell labeling [11]. Direct labeling is relatively easy, inexpensive, and well-established, where the cells are labeled with certain tags that can be detected directly with suitable imaging equipment. Indirect labeling typically uses a reporter gene approach which can allow for visualization of live cells (transfected with the reporter gene construct before injection) in vivo, after administration of a reporter probe that can be detected by certain imaging techniques. A broad array of image tags has been explored for cell tracking using a variety of imaging modalities (Fig. 1). The three major cell types that have been studied for cell tracking in cancer development and cancer therapy are immune cells, stem cells, and cancer cells.
Fig. (1).
Many imaging labels and techniques have been used to label cells and track them in vivo. Some involve direct labeling of the cells while others require genetic modification of the cells.
TRACKING OF IMMUNE CELLS
Immune cells, such as T cells, natural killer (NK) cells, B cells, and dendritic cells, play important roles in cancer immunotherapy [12,13]. Understanding the molecular basis of immune cell trafficking and biodistribution of these immune cells is critical for developing more efficacious strategies of cancer immunotherapy. Many early reports have investigated the tracking of radiolabeled lymphocytes in cancer patients [14-21], most of which used 111In-oxine as the radiolabel that has been approved by the Food and Drug Administration (FDA). In this section, we will describe various recent approaches for tracking these cells to the tumor sites. The cells used in these studies include T cells, NK cells, and a number of other immune cells (e.g. various types of killer cells which can respond to certain proteins or cells).
Tracking T Cells with Fluorescence
T cells have been extensively studied for cancer immunotherapy. Genetic modification of T cells holds great promise in serving as a platform to detect tumor antigens, improve anti-cancer efficacy, and neutralize the immune evasion of tumors [22]. Fluorescence imaging techniques have been used to track T cells in several studies. For in vivo applications, utilizing the near-infrared (NIR, 700-900 nm) window is advantageous since the absorbance spectra for all biomolecules reach minima in this region which provides a clear window for optical imaging [23]. The migration of T cells to tumors has been investigated with NIR organic dyes as the fluorescent labels, such as IRDye800CW [24] and VivoTag 680 (Fig. 2A) [25]. Both dyes bear an amine-reactive N-hydroxysuccinimide group for cell labeling, which was found to be biocompatible and suitable for monitoring cells at multiple resolutions in real time in their native environments by NIR fluorescence imaging.
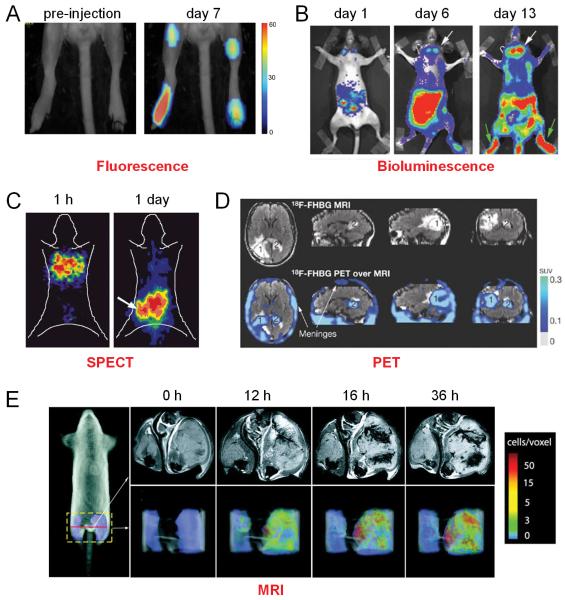
Fig. (2).
Tracking T cells in vivo with various techniques such as fluorescence, bioluminescence, SPECT, PET, and MRI. A. Fluorescence imaging delineates the accumulation of cytotoxic T lymphocytes in the tumor and lymph nodes. B. Bioluminescence imaging of firefly luciferase expression in T cells at days 1, 6, and 13 after T cell adoptive transfer in mice harboring brain tumors. White arrows point to T cells at the tumor and green arrows point to the bone marrow. C. Biodistribution of intravenously injected, 131I-FIAU-labeled T cells over time in mice bearing human lymphoma xenografts. D. MRI and 18F-FHBG PET of a patient who received infusions of autologous T cells that expressed interleukin 13 zetakine and HSV1-tk genes. A surgically resected tumor (1) and a new, non-resected tumor (2) are shown. The infused cells had localized at the site of tumor 1 and trafficked to tumor 2. SUV: standardized uptake value. E. Time course of CLIO-labeled T cell homing to the B16-OVA tumor. Adapted from [25,28,33,40,42].
Tracking T Cells with Bioluminescence
Although bioluminescence imaging (BLI) has been widely used for T cell trafficking in other diseases such as inflammation [26,27], its use in monitoring T cell trafficking to tumors has not been well studied. Due to the presumed “immune privilege” of the central nervous system, it is commonly believed that T cells have difficulty reaching tumors located in the brain. In one study, the biodistribution and anti-tumor activity of adoptively transferred T cells specific for an endogenous tumor-associated antigen (gp100), expressed by tumors implanted in the brain, was investigated [28]. BLI of luciferase expression in the antigen-specific T cells demonstrated the accumulation of transduced T cells in the bone marrow and the brain tumor (Fig. 2B), which suggested that peripheral tolerance to endogenous tumor-associated antigens can be overcome to treat tumors in the brain.
Tracking T Cells with PET/SPECT
Nuclear imaging techniques (i.e. PET and SPECT) have much better clinical potential than optical imaging in that they have superb tissue penetration capability and they are highly quantitative [9,29]. PET/SPECT imaging has been frequently used for T cell tracking. In one study, a 64Cu-labeled antibody was used to track transferred T cells (expressing the antigen recognizable by the antibody) with PET in living mice [30]. It was found that not all tumor-specific T cells localized to the tumors. Some also homed to the major lymphoid organs. In another report, T cells were labeled with 5-124I-iodo-2′-deoxyuridine (124I-IdU) to monitor their homing to tumors with PET imaging [31]. Significantly higher accumulation of 124I in the targeted tumors than the control tumors was observed.
One study compared the efficiency, stability, and toxicity of radiolabeling activated lymphocytes with three different agents: 99mTc-hexamethylpropylene amine oxime (99mTc-HMPAO), 111In-oxine, and 18F-2-fluoro-2-deoxy-d-glucose (18F-FDG, the most widely used PET tracer in the clinic) [32]. It was found that the mean labeling efficiencies of 111In-oxine and 18F-FDG were higher than that of 99mTc-HMPAO. Although none of the three agents induced any significant alteration in cell viability or immunophenotype, both 111In-oxine and 18F-FDG induced a loss of cytotoxic activity of lymphocytes against ovarian carcinoma cells.
Several groups have investigated the use of herpes simplex virus type 1 thymidine kinase (HSV1-tk) gene and its mutants as a reporter gene for various biomedical applications, including cell tracking. In addition, fusion of the HSV1-tk gene with other reporter genes such as fluorescent proteins and/or bioluminescent enzymes can enable multimodality imaging of the transfected cells, which provided not only a convenient way for cross-validation but also good translational potential. In some cases, the cells could be labeled with a PET or SPECT probe, 2′-fluoro-2′-deoxy-1-beta-D-arabinofuranosyl-5-124I-iodouracil (124I-FIAU) and 2′-fluoro-2′-deoxy-1-beta-D-arabinofuranosyl-5-131I-iodouracil (131I-FIAU) respectively [33], and then injected and tracked over time in live tumor-bearing mice (Fig. 2C). In most cases, the expression of the HSV1-tk gene or its mutants in the transfected T cells can be visualized by PET imaging after injection of a reporter probe, such as 2′-18F-fluoro-2′-deoxy-1-beta-d-arabinofuranosyl-5-ethyluracil (18F-FEAU) [34,35] or 9-[4-18F-fluoro-3-(hydroxymethyl)butyl]guanine (18F-FHBG) [36-40]. These cell tracking studies revealed important insights to cancer immunotherapy. For example, one study found that naive T cells did not localize to the tumor site, indicating that preimmunization was required [38]. Such observation would have been extremely difficult to reach if imaging were not used. Another study reported that the minimum detection threshold of T cells engineered to express the HSV1-sr39tk gene was approximately 7 × 105 T cells in the spleen and 1 × 104 T cells in the lymph nodes [37].
One recent case report described T cell trafficking to gliomas with 18F-FHBG PET [40]. A patient diagnosed with grade IV glioblastoma multiforme was enrolled in a trial of adoptive cellular immunotherapy. The trial involved infusion of ex vivo expanded autologous cytolytic CD8+ T cells (CTLs), genetically engineered to express both the interleukin 13 zetakine gene (which encodes a receptor protein that targets these CTLs to tumor cells) and the HSV1-tk gene. Whole-body and brain PET scan with 18F-FHBG was able to non-invasively detect the CTLs that express HSV1-tk (Fig. 2D). This report represents a key step toward the potential use of reporter gene techniques in clinical patient management, in particular cancer immunotherapy.
Tracking T Cells with MRI
MRI, with exquisite soft tissue contrast, is another widely used imaging modality for cell tracking in vivo [41]. In one early study, a highly derivatized cross-linked iron oxide (CLIO) nanoparticle was used to label CDLs for in vivo tracking of the injected cells at near single-cell resolution with MRI [42]. In a melanoma model, MRI demonstrated the extensive three-dimensional spatial heterogeneity of antigen-specific T-cell recruitment to the tumors, as well as a temporal regulation of T-cell recruitment within the tumor (Fig. 2E). It was suggested that serial administrations of CDLs appeared to home to different intratumoral locations, which may provide a more effective treatment regimen than a single bolus administration.
Tracking NK Cells
NK cells are a type of cytotoxic lymphocytes that constitute a major component of the innate immune system. They play a major role in the rejection of tumors and cells infected by viruses. Mounting experimental data have suggested that manipulating the balance between inhibitory and activating NK receptor signals, the sensitivity of target cells to NK cell-mediated apoptosis, and NK cell cross-talk with dendritic cells, may hold promise for efficacious cancer therapy [43]. To date, tracking of NK cells has been explored with many different imaging modalities, including BLI, fluorescence, MRI, and PET.
In an early study, BLI was used to monitor the homing of effector NK cells, transfected with both green fluorescent protein (GFP) and luciferase reporter genes, to the sites of tumor growth followed by tumor eradication [44]. The entire course of malignancy including engraftment, expansion, metastasis, response to therapy, and unique patterns of relapse was non-invasively detected by BLI. Subsequently, human NK cells were also labeled with clinically applicable iron oxide contrast agents [45] or 18F-FDG [46] and the accumulation of these labeled cells in tumors could be monitored in vivo with MRI and PET, respectively. Recently, tumor-targeted NK cells were labeled with a NIR dye, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine (DiD), to track their distribution in human prostate cancer xenografts with fluorescence imaging [47]. In vivo studies demonstrated a significant increase in tumor fluorescence at one day post-injection of tumor-targeted NK cells but not the parental NK cells.
Tracking Other Immune Cells
Besides the T cells and NK cells described above, a number of other immune cells have also been studied in animal tumor models. For example, the feasibility of in vivo cell tracking with MRI in anti-cancer cell therapy was demonstrated [48]. Ovalbumin-specific splenocytes were labeled with superparamagnetic iron oxide (SPIO) nanoparticles and injected into mice inoculated with ovalbumin-expressing tumors. Non-invasive MRI tracking of the injected splenocytes showed significant signal in the spleen at one day after injection, and in the tumor at two and three days after injection. Another report suggested that circulating endothelial precursor cells (CEPs), isolated from CD34+/CD133− cells of peripheral blood, could be promising candidates for treating ischemic diseases and tumor targeting/imaging [49]. In this study, the CEPs were fluorescently labeled and visualized by immunofluorescence microscopy but not in vivo imaging.
In vivo tracking of several other types of killer cells has been studied. Unlike the NK cells, these killer cells respond to certain biological factors (e.g. lymphokine or cytokine) or cells (e.g. macrophages). Lymphokine-activated killer (LAK) cells, a type of white blood cells that are stimulated in a laboratory to kill tumor cells, have been explored for cancer immunotherapy [50]. Tracking of GFP-labeled LAK cells in mice carrying B16 melanoma metastases has been reported [51]. Another study evaluated the in vivo distribution of macrophage-activated killer (MAK) cells, which were labeled with 18F-FDG or 111In-oxime, in patients with peritoneal relapse of epithelial ovarian carcinoma [52]. However, the leakage of 18F-FDG from the labeled cells significantly hampered accurate tracking of these cells to the tumor. Cytokine-induced killer (CIK) cells, derived from human peripheral blood or mouse splenocytes [53], have been combined with oncolytic viral therapy to achieve directed delivery to tumors in both immunodeficient and immunocompetent mice [54]. Pre-infection of CIK cells with modified vaccinia virus resulted in a prolonged eclipse phase with the virus remaining hidden until interaction with the tumor. Both BLI and fluorescence imaging (these reporter genes were inserted in the virus genome) revealed that the cells retained their ability to traffic to and to infiltrate the tumor effectively before releasing the virus.
TRACKING OF STEM CELLS
Immune cells have been extensively studied for cancer therapy, and the above mentioned studies only represent a small fraction of such endeavor which incorporated non-invasive cell tracking. Many other eukaryotic cell types have also been proposed and/or investigated for tumor targeting and tumor therapy, and one of the most vibrant research fields over the last decade is stem cells. For example, mesenchymal stem cells (MSCs) can migrate to and proliferate within sites of inflammation and tumors as part of the tissue remodeling process, which has been exploited as a tumor-targeting strategy for cell-based cancer therapy [55-57]. However, once injected intravenously, the stem cells migrate away from the initial injection site toward tumor beds and become difficult to visualize and track in vivo. Much research effort has been dedicated to labeling these cells with either reporter genes or various contrast agents to enable non-invasive cell tracking, as well as potentially quantifying the fate of the administered stem cells in vivo.
With BLI
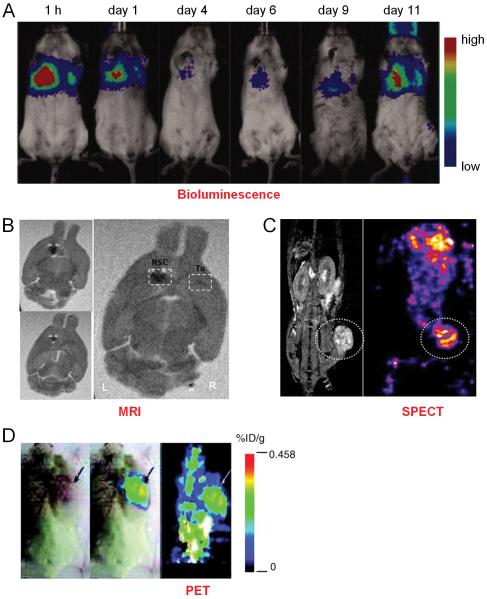
A recent study was undertaken to track the distribution and differentiation of MSCs in tumor-bearing mice [58]. The tumor cells and the MSCs were labeled with different reporter genes (both fluorescence and bioluminescence) and studied in two tumor models: subcutaneous breast cancer and breast cancer lung metastasis. Interestingly, it was found that when injected intravenously, the MSCs survived, proliferated, and differentiated in tumor sites but not elsewhere. Non-invasive BLI and histologic studies further revealed that the MSCs can selectively localize, survive, and proliferate in both subcutaneous tumor and lung metastasis (Fig. 3A). The MSCs that migrated to the lung tumor differentiated into osteoblasts, whereas the MSCs in the subcutaneous tumor differentiated into adipocytes.
Fig. (3).
Tracking stem cells in vivo with various techniques such as bioluminescence, MRI, SPECT, and PET. A. Trafficking the fate of luciferase-transfected mesenchymal stem cells (MSCs) in 4T1 tumor-bearing mice (both cells were co-injected intravenously). Bioluminescence imaging showed that luciferase activity dropped to the lowest level at day 4, then increased gradually and peaked at day 11. B. MRI visualization of Fe-Pro-labeled neural stem cells (NSCs) targeting human glioma in an orthotopic mouse model. Dark signals (white dotted boxes) indicated the labeled cells. Tu: tumor. C. Accumulation of hNIS-transfected AC133+ progenitor cells (APCs) around the implanted tumor as detected by 99mTc SPECT scan. The MRI image on the left provided anatomical information of the animal. D. PET images of tumor-bearing mice which were intravenously injected with genetically modified MSCs, and imaged with 18F-FHBG PET. %ID/g: percentage injected dose per gram of tissue. Adapted from [58,65,69,71].
With MRI
Over the last decade, labeling cells with ferumoxides or SPIO nanoparticles to track their migration has become routine practice in cellular MRI. Non-invasive imaging of a few or even single cells, labeled with SPIO nanoparticles, has been reported [41]. For non-phagocytic cells (e.g. stem cells) that do not spontaneously ingest ferumoxides, several tricks can be used to ensure high intracellular uptake and sufficient magnetic labeling, one of which is the use of cationic transfection agents (e.g. protamine sulfate) to coat the anionic ferumoxides particles [59,60]. Another method of labeling stem cells with magnetic nanoparticles is electroporation [61], which can be advantageous in certain scenarios since no transfection agent is needed thus off-label approval in transfection is not required for clinical use.
Almost a decade ago, magnetodendrimers were developed and explored as magnetic tags to label mammalian cells, including MSCs and human neural stem cells (NSCs), through non-specific membrane adsorption and subsequent intracellular localization in endosomes[62]. Subsequently, stem cells labeling with SPIO nanoparticles using commercially available transfection agents were investigated, which demonstrated the feasibility of this approach in both experimental and clinical settings [59].
A few studies investigated the tumor tropism of SPIO nanoparticle-labeled cells in murine glioma models with MRI, such as neural progenitor cells [63], bone marrow stromal cells [63], and MSCs [64]. It was found that systemically transplanted MSCs migrated toward gliomas with high specificity in a temporal-spatial pattern [64]. Seven days after MSC transplantation, the labeled cells were distributed throughout the tumor while after 14 days, most of the MSCs were found at the border between the tumor and normal parenchyma. The inherent tumor tropism of ferumoxide-protamine sulfate complex (Fe-Pro) labeled NSCs to primary and invasive tumor foci was also explored in a murine glioma model [65]. These Fe-Pro labeled NSCs were found to retain their proliferative status, tumor tropism, and stem cell character, while allowing in vivo MRI tracking at 7 Tesla (Fig. 3B). Using the same labeling strategy (i.e. Fe-Pro), the migration and incorporation of intravenously injected human CD34+/AC133+ endothelial progenitor cells (EPCs) into the neovasculature in a flank tumor model was also observed in another study [66].
Recently, complex formation between negatively charged fluorescent monodisperse SPIO nanoparticles and positively charged peptides, as well as the use of such complex formation to improve the MRI properties of labeled stem cells, was reported [67]. Stem cells labeled with this method exhibited a strong fluorescent signal and enhanced T2*-weighted MRI both in vitro and in vivo in a flank tumor model. Besides iron oxide nanoparticles, labeling cells with perfluorocarbon (PFC) nanoparticles for 19F MRI has also been explored [68]. It was found that CD34+CD133+CD31+ stem/progenitor cells readily internalized these PFC nanoparticles, without the aid of adjunctive labeling techniques, and remained functional in vivo. Further, the PFC-labeled cells exhibited distinct 19F signals which could be readily detected after both local and intravenous injection.
With Radionuclide-Based Imaging Techniques
Although SPECT imaging has been widely used for tracking radiolabeled lymphocytes, tracking stem cells to tumor sites with SPECT has been understudied. In one report, SPIO nanoparticle-labeled AC133+ progenitor cells (APCs) were used to carry the human sodium iodide symporter (hNIS) gene to the sites of implanted breast cancer in a mouse model [69]. In vivo real-time tracking of these cells was performed by MRI and expression of the hNIS gene was determined by SPECT imaging after injection of 99mTc-pertechnetate (Fig. 3C). This study suggested that genetically transformed, magnetically labeled APCs could be used as both delivery vehicles and cellular probes for detecting the migration/homing of cells.
Although labeling with 18F-FDG can enable short term tracking of the cells [70], PET imaging of stem cell trafficking to the tumor generally involves the incorporation of the HSV1-tk gene into the stem cells. To assess the efficacy of human MSCs for targeting microscopic tumors and suicide gene or cytokine gene therapy, immunodeficient mice were transplanted with human cancer cells and subsequently injected with a small number of MSCs expressing both the HSV1-tk and enhanced GFP (EGFP) reporter genes [71]. PET imaging with 18F-FHBG suggested that these MSCs can target microscopic tumors, subsequently proliferate/differentiate, and form a significant portion of the tumor stroma (Fig. 3D).
TRACKING OF CANCER CELLS
The abovementioned examples of using imaging techniques as tools for non-invasive tracking of immune and stems cells were primarily intended for (potential) cancer therapy applications. On the other hand, tracking of cancer cells and studying their distribution/activity in vivo can also further our understanding of cancer development and tumor metastasis. Similar as tracking immune cells and stem cells, the major imaging techniques used for cancer cell trafficking also include fluorescence, MRI, SPECT, and PET.
With Fluorescence Imaging
One early study investigated dual-color fluorescence imaging using red fluorescent protein (RFP)-expressing tumors transplanted in GFP-expressing transgenic mice [72]. Exquisite details of tumor-stroma interactions (e.g. tumor cell trafficking, tumor-induced angiogenesis, invasion, and metastasis), tumor-infiltrating lymphocytes, stromal fibroblasts and macrophages, were elucidated [72,73]. In certain cases, such color-coding of cancer cells growing in vivo can allow distinction of different cell types, including host from tumor, with single-cell resolution [73]. The development of transgenic mice which express fluorescent proteins has revolutionized cancer research and enabled the study/imaging of many important biological processes (not limited to cancer) that previously could only be studied in vitro.
Subsequently, dual-color fluorescent cells with one color fluorescent protein in the nucleus and another color fluorescent protein in the cytoplasm were genetically engineered [74]. With RFP expressed in the cytoplasm and GFP expressed in the nucleus, these dual-colored cells enabled the sub-cellular dynamics of cancer cell trafficking to be imaged in living animals. Highly elongated cancer cells and nuclei in narrow capillaries were visualized where both the nuclei and cytoplasm underwent extreme deformation during extravasation. Real-time trafficking of these cancer cells in lymphatic vessels was also demonstrated in subsequent studies (Fig. 4A) [75,76]. In general, whole-body imaging with fluorescent proteins is a powerful technology to follow the dynamics of cancer development and metastasis [77], which significantly furthers the understanding of cancer spread at the subcellular level in living mouse by enabling both macro and micro imaging technology thereby providing the basis for a new field of in vivo cancer cell biology.
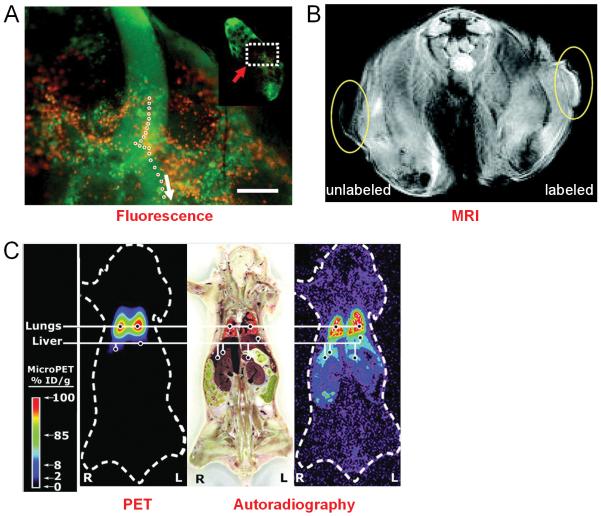
Fig. (4).
Tracking cancer cells in vivo with various imaging labels and techniques. A. Dual-color imaging of lymphatic structures and trafficking of cancer cells. Single-cell motion at the junction of afferent lymph duct and subcapsular sinus of the lymph node is delineated. White arrow indicates the direction of cancer cell trafficking and the inset is a low magnification image of the whole lymph node. B. MRI section of a mouse 7 days after inoculation with unlabeled tumor cells and gadolinium-rhodamine nanoparticle labeled tumor cells. C. Small animal PET imaging of 64Cu-PTSM-labeled C6 cells after intravenous injection into a mouse. Concordance between location of activity in the PET image and autoradiography section demonstrated that cells were trapped initially in the lungs. Adapted from [75,82,84].
Quantum dots (QDs) are inorganic fluorescent semiconductor nanoparticles with superior optical properties compared with organic dyes such as high quantum yields, high molar extinction coefficients, strong resistance to photobleaching and chemical degradation, narrow emission spectra, and large effective Stokes shifts [78,79]. QDs have been used as cell markers to study extravasation of intravenously injected, QD-labeled tumor cells in small animal models [80]. It was demonstrated that QD-labeled tumor cells permit in vivo imaging despite tissue autofluorescence. More importantly, these QD-labeled cells could also be used to analyze the distribution of tumor cells in organs and tissues and to track different populations of cells. By using multi-photon laser excitation, five different populations of cells could be simultaneously identified.
With MRI
SPIO nanoparticles have also been used to label and track tumor cells, besides immune cells and stem cells. In one study, MRI at 1.5 T allowed the detection of SPIO nanoparticle-labeled human cancer cells both in vitro and in vivo [81]. One disadvantage of iron oxide nanoparticles is that they give negative contrast, which can be difficult to interpret in many cases. On the other hand, gadolinium-based contrast agents give positive contrast in T1-weighted images. In one study, gadolinium-rhodamine nanoparticles were explored for labeling and tracking cancer cells in vivo [82]. Tumor cells could be efficiently labeled with these nanoparticles and when inoculated subcutaneously into the flanks of mice, they could be imaged with both MRI and optical imaging (Fig. 4B). It was suggested that this approach could be modified with different fluorophores and targeting agents for studying the trafficking of various cell types in the future.
With PET
More than a decade ago, tracking of 18F-FDG-labeled cells was investigated to elucidate the behavior of various metastatic tumor cells in the blood flow [83]. Although both cells accumulated in the lungs immediately after injection, the elimination of liver-metastatic RAW117 cells from the lungs was found to be faster than that of the lung-metastatic B16BL6 melanoma cells. This study suggested that the trafficking of metastatic tumor cells could greatly influence the organ specificity of cancer metastasis.
Subsequently, 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) (64Cu-PTSM) was used to label tumor cells, in comparison with 18F-FDG, for non-invasive PET imaging studies of cell trafficking in mice [84]. The labeling efficiency was directly proportional to 64Cu-PTSM concentration and influenced negatively by serum. Although label uptake per cell was greater with 64Cu-PTSM than with 18F-FDG, both 64Cu-PTSM- and 18F-FDG-labeled cells showed efflux of cell activity into the supernatant. Small animal PET imaging revealed that intravenously injected radiolabeled C6 cells trafficked to the lungs and liver (Fig. 3C).
One of the major advantages of 64Cu-PTSM over 18F-FDG is the significantly longer decay half-life (12.7 h versus 110 min), which can enable long term cell tracking up to a few days. Recently, 64Cu-labeled polyethylenimine (64Cu-PEI) was also evaluated for cell trafficking and tumor imaging and compared with 64Cu-PTSM [85]. Although it was demonstrated that 64Cu-PEI could be used for both cell trafficking and tumor imaging, and that PEGylation reduced the cellular toxicity of 64Cu-PEI, the cell labeling efficiency of 64Cu-PEI was significantly lower than that of 64Cu-PTSM.
CONCLUSION AND FUTURE PERSPECTIVES
A wide variety of labels and imaging techniques have been explored for labeling and tracking cells in cancer and cancer therapy. Although the cell types can vary which mainly include immune cells, stem cells, and cancer cells, the labeling strategies are essentially the same. Direct labeling of cells with image tags is easier than indirect labeling in most cases and the safety profiles of direct cell labeling techniques are generally quite good. However, the disadvantage of direct cell labeling is that the label itself is detected rather than the live cells of interest. The labels may leak out of the cells when the cells are alive or be taken up by other cells when the labeled cells die. Therefore, care must be taken when interpreting the experimental results and rigorous validation is certainly needed to obtain more robust and reliable data. With reporter gene techniques (i.e. indirect cell labeling), only live cells are detected thus they can provide more insights about the cell migration, differentiation, and proliferation in vivo.
Each imaging modality has its advantages and disadvantages in terms of sensitivity, tissue penetration, spatial resolution, and clinical potential (Table 1). Optical imaging is mostly applicable to preclinical studies where light penetration is less of an issue than in cancer patients. BLI can not be used in human studies while tracking of labeled cells with MRI, SPECT, and PET may all potentially be performed in patients. Combination of various imaging modalities can give complementary information. As a matter of fact, many of the reporter gene-based cell labeling studies incorporated multiple reporter genes. For example, fluorescent genes (e.g. GFP and RFP) can facilitate cell sorting to isolate the cells of interest, BLI (with luciferases) can enable in vivo long term monitoring of the cells in a quantitative manner in small animal models, and PET can allow for more clinically relevant, highly sensitive detection of the injected cells or the daughter cells. With these tools in hand, scientists can investigate the various aspects of cancer development and cancer therapy in a manner that was previously impossible. Future development and validation of various cell labeling/tracking techniques will further strengthen the arsenal for cell-based imaging and therapy of cancer.
Table 1.
Comparison of the imaging techniques that have been used for non-invasive cell tracking in cancer
| Modality | Resolution | Sensitivity | Tissue Penetration | Quantitation capability | Cost | Clinical potential |
|---|---|---|---|---|---|---|
| Fluorescence | medium | medium | poor | poor | $ | low |
| Bioluminescence | low | high | poor | good | $$ | none |
| MRI | high | low | good | poor | $$$ | high |
| SPECT | medium | high | good | good | $$ | high |
| PET | low | high | good | good | $$$ | high |
Cancer immunotherapy is already widely used in the clinic and cell labeling/tracking studies in this area have been well documented. Stem cells hold enormous promise for cancer therapy, however labeling and tracking stem cells has primarily been at the preclinical stage asstem cell research is still a relatively new field. These two major types of cells are primarily explored for the ultimate goal of efficacious cancer therapy with minimal side effects. Labeling/tracking cancer cells is mainly studied for the understanding of cancer development/spread and this area has not been well studied. One of the major reasons might be that many of the currently used preclinical tumor models are not highly relevant to human cancer in the clinical situation. In the future, combination of conventional cancer biology studies, more clinically relevant tumor models, and non-invasive imaging may give more insights into the many processes of cancer development and provide new avenues for novel therapeutic strategies against cancer.
Safety of cell labeling is always a big concern in potential clinical studies since introduction of foreign substances (e.g. image tags or genes) may cause unpredictable alterations in cells. Based on the available literature data, iron oxide labeling of cells appears to be safe and is in active clinical development. For indirect cell labeling, the most intensively studied PET reported gene, HSV1-tk, is also a suicide gene which adds an extra layer of control to ensure safety. With pilot human studies already reported [40], it is expected that reporter genes will gradually gain popularity in future clinical studies. The ideal imaging modality for tracking therapeutic cells (e.g. stem cells and immune cells) in cancer patients requires the imaging tags to be non-toxic, biocompatible, and highly specific to reduce perturbation to the target cells. Much future effort will be required before this can become a reality and clinical routine.
Lastly, the requirement for cell tracking techniques depends on the clinical scenario. In some cases, only short term tracking would be needed while in other cases, long term survival and proliferation would also need to be monitored. MRI, radionuclide-based imaging techniques, and reporter gene-based approaches will each have their own niches. Rather than identifying and optimizing one technique that is applicable for all clinical scenarios, it is probably more appropriate to optimize how a certain imaging technique/modality can be best used to serve the purpose of a specific situation. The various imaging modalities are more complementary than competitive with the same ultimate goal: personalized medicine for cancer patients.
ACKNOWLEDGMENTS
The authors are grateful for the financial support from Wisconsin Partnership Program, UW Carbone Cancer Center, NCRR 1UL1RR025011, and Susan G. Komen for the Cure.
ABBREVIATIONS
- APC
AC133+ progenitor cells
- BLI
Bioluminescence imaging
- CEP
Circulating endothelial precursor cell
- CIK cell
Cytokine-induced killer cell
- CLIO
Cross-linked iron oxide
- CTL
Cytolytic CD8+ T cell
- 64Cu-PEI
64Cu-labeled polyethylenimine
- 64Cu-PTSM
64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone)
- DiD
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine
- EGFP
Enhanced GFP
- EPC
Endothelial progenitor cell
- FDA
The Food and Drug Administration
- Fe-Pro
Ferumoxide-protamine sulfate complex
- 18F-FDG
18F-2-fluoro-2-deoxy-d-glucose
- 18F-FEAU
2′-18F-fluoro-2′-deoxy-1-beta-d-arabinofuranosyl-5-ethyluracil
- 18F-FHBG
9-[4-18F-fluoro-3-(hydroxymethyl)butyl]guanine
- GFP
Green fluorescent protein
- hNIS
Human sodium iodide symporter
- HSV1-tk
Herpes simplex virus type 1 thymidine kinase
- 124I-FIAU
2′-fluoro-2′-deoxy-1-beta-D-arabinofuranosyl-5-124I-iodouracil
- 131I-FIAU
2′-fluoro-2′-deoxy-1-beta-D-arabinofuranosyl-5-131I-iodouracil
- 124I-IdU
5-124I-iodo-2′-deoxyuridine
- LAK cell
Lymphokine-activated killer cell
- MAK cell
Macrophage-activated killer cell
- MRI
Magnetic resonance imaging
- MRS
Magnetic resonance spectroscopy
- MSC
Mesenchymal stem cell
- NIR
Near-infrared
- NK cell
Natural killer cell
- NSC
Neural stem cell
- PET
Positron emission tomography
- PFC
Perfluorocarbon
- QD
Quantum dot
- RFP
Red fluorescent protein
- SPECT
Single-photon emission computed tomography
- SPIO
Superparamagnetic iron oxide
- 99mTc-HMPAO
99mTc-hexamethylpropylene amine oxime
REFERENCES
- [1].Hong H, Zhang Y, Sun J, Cai W. Molecular imaging and therapy of cancer with radiolabeled nanoparticles. Nano Today. 2009;4:399–413. doi: 10.1016/j.nantod.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sumer B, Gao J. Theranostic nanomedicine for cancer. Nanomed. 2008;3:137–140. doi: 10.2217/17435889.3.2.137. [DOI] [PubMed] [Google Scholar]
- [3].Lammers T, Hennink WE, Storm G. Tumour-targeted nanomedicines: principles and practice. Br. J. Cancer. 2008;99:392–397. doi: 10.1038/sj.bjc.6604483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Huwyler J, Drewe J, Krahenbuhl S. Tumor targeting using liposomal antineoplastic drugs. Int. J. Nanomedicine. 2008;3:21–29. [PMC free article] [PubMed] [Google Scholar]
- [5].Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat. Rev. Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat. Rev. Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- [7].Thorne SH. Strategies to achieve systemic delivery of therapeutic cells and microbes to tumors. Expert Opin. Biol. Ther. 2007;7:41–51. doi: 10.1517/14712598.7.1.41. [DOI] [PubMed] [Google Scholar]
- [8].Mankoff DA. A definition of molecular imaging. J. Nucl. Med. 2007;48:18N, 21N. [PubMed] [Google Scholar]
- [9].Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- [10].Cai W, Chen X. Nanoplatforms for targeted molecular imaging in living subjects. Small. 2007;3:1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- [11].Ponomarev V. Nuclear imaging of cancer cell therapies. J. Nucl. Med. 2009;50:1013–1016. doi: 10.2967/jnumed.109.064055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fazle Akbar SM, Abe M, Yoshida O, Murakami H, Onji M. Dendritic cell-based therapy as a multidisciplinary approach to cancer treatment: present limitations and future scopes. Curr. Med. Chem. 2006;13:3113–3119. doi: 10.2174/092986706778742882. [DOI] [PubMed] [Google Scholar]
- [13].Gao JQ, Okada N, Mayumi T, Nakagawa S. Immune cell recruitment and cell-based system for cancer therapy. Pharm. Res. 2008;25:752–768. doi: 10.1007/s11095-007-9443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wagstaff J, Gibson C, Thatcher N, Crowther D. The migratory properties of indium-111 oxine labelled lymphocytes in patients with chronic lymphocytic leukaemia. Br. J. Haematol. 1981;49:283–291. doi: 10.1111/j.1365-2141.1981.tb07225.x. [DOI] [PubMed] [Google Scholar]
- [15].Wagstaff J, Gibson C, Thatcher N, Ford WL, Sharma H, Benson W, Crowther D. A method for following human lymphocyte traffic using indium-111 oxine labelling. Clin. Exp. Immunol. 1981;43:435–442. [PMC free article] [PubMed] [Google Scholar]
- [16].Dummer R, Schafer E, Eilles C, Borner W, Burg G. Lymphokine-activated killer-cell traffic in metastatic melanoma. Lancet. 1991;338:456–457. doi: 10.1016/0140-6736(91)91089-d. [DOI] [PubMed] [Google Scholar]
- [17].Dummer R, Becker JC, Eilles C, Schafer E, Borner W, Burg G. T cells migrate to tumour sites after extracorporeal interleukin 2 stimulation and reinfusion in a patient with metastatic melanoma. Br. J. Dermatol. 1993;128:399–403. doi: 10.1111/j.1365-2133.1993.tb00198.x. [DOI] [PubMed] [Google Scholar]
- [18].Fisher B, Packard BS, Read EJ, Carrasquillo JA, Carter CS, Topalian SL, Yang JC, Yolles P, Larson SM, Rosenberg SA. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J. Clin. Oncol. 1989;7:250–261. doi: 10.1200/JCO.1989.7.2.250. [DOI] [PubMed] [Google Scholar]
- [19].Morita T, Yonese Y, Minato N. In vivo distribution of recombinant interleukin-2-activated autologous lymphocytes administered by intra-arterial infusion in patients with renal cell carcinoma. J. Natl. Cancer. Inst. 1987;78:441–450. [PubMed] [Google Scholar]
- [20].Muller C, Zielinski CC, Linkesch W, Ludwig H, Sinzinger H. In vivo tracing of indium-111 oxine-labeled human peripheral blood mononuclear cells in patients with lymphatic malignancies. J. Nucl. Med. 1989;30:1005–1011. [PubMed] [Google Scholar]
- [21].Schafer E, Dummer R, Eilles C, Borner W, Martin R, Rendl J, Burg G. Imaging pattern of radiolabelled lymphokine-activated killer cells in patients with metastatic malignant melanoma. Eur. J. Nucl. Med. 1991;18:106–110. doi: 10.1007/BF00950755. [DOI] [PubMed] [Google Scholar]
- [22].Riviere I, Sadelain M, Brentjens RJ. Novel strategies for cancer therapy: the potential of genetically modified T lymphocytes. Curr. Hematol. Rep. 2004;3:290–297. [PubMed] [Google Scholar]
- [23].Cai W, Shin DW, Chen K, Gheysens O, Cao Q, Wang SX, Gambhir SS, Chen X. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6:669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- [24].Foster AE, Kwon S, Ke S, Lu A, Eldin K, Sevick-Muraca E, Rooney CM. In vivo fluorescent optical imaging of cytotoxic T lymphocyte migration using IRDye800CW near-infrared dye. Appl. Opt. 2008;47:5944–5952. doi: 10.1364/ao.47.005944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Swirski FK, Berger CR, Figueiredo JL, Mempel TR, von Andrian UH, Pittet MJ, Weissleder R. A near-infrared cell tracker reagent for multiscopic in vivo imaging and quantification of leukocyte immune responses. PLoS ONE. 2007;2:e1075. doi: 10.1371/journal.pone.0001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mandl S, Schimmelpfennig C, Edinger M, Negrin RS, Contag CH. Understanding immune cell trafficking patterns via in vivo bioluminescence imaging. J. Cell. Biochem. Suppl. 2002;39:239–248. doi: 10.1002/jcb.10454. [DOI] [PubMed] [Google Scholar]
- [27].Lee MH, Lee WH, Van Y, Contag CH, Liu CP. Image-guided analyses reveal that non-CD4 splenocytes contribute to CD4+ T cell-mediated inflammation leading to islet destruction by altering their local function and not systemic trafficking patterns. Mol. Imaging. 2007;6:369–383. [PubMed] [Google Scholar]
- [28].Prins RM, Shu CJ, Radu CG, Vo DD, Khan-Farooqi H, Soto H, Yang MY, Lin MS, Shelly S, Witte ON, Ribas A, Liau LM. Anti-tumor activity and trafficking of self, tumor-specific T cells against tumors located in the brain. Cancer Immunol. Immunother. 2008;57:1279–1289. doi: 10.1007/s00262-008-0461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cai W, Niu G, Chen X. Imaging of integrins as biomarkers for tumor angiogenesis. Curr. Pharm. Des. 2008;14:2943–2973. doi: 10.2174/138161208786404308. [DOI] [PubMed] [Google Scholar]
- [30].Matsui K, Wang Z, McCarthy TJ, Allen PM, Reichert DE. Quantitation and visualization of tumor-specific T cells in the secondary lymphoid organs during and after tumor elimination by PET. Nucl. Med. Biol. 2004;31:1021–1031. doi: 10.1016/j.nucmedbio.2004.06.002. [DOI] [PubMed] [Google Scholar]
- [31].Agger R, Petersen MS, Petersen CC, Hansen SB, Stodkilde-Jorgensen H, Skands U, Blankenstein T, Andersen TE, Hulgaard EF, Jorgensen JT, Marqversen J, Gundersen HJ, Hokland ME. T cell homing to tumors detected by 3D-coordinated positron emission tomography and magnetic resonance imaging. J. Immunother. 2007;30:29–39. doi: 10.1097/01.cji.0000211326.38149.7e. [DOI] [PubMed] [Google Scholar]
- [32].Botti C, Negri DR, Seregni E, Ramakrishna V, Arienti F, Maffioli L, Lombardo C, Bogni A, Pascali C, Crippa F, Massaron S, Remonti F, Nerini-Molteni S, Canevari S, Bombardieri E. Comparison of three different methods for radiolabelling human activated T lymphocytes. Eur. J. Nucl. Med. 1997;24:497–504. doi: 10.1007/BF01267680. [DOI] [PubMed] [Google Scholar]
- [33].Koehne G, Doubrovin M, Doubrovina E, Zanzonico P, Gallardo HF, Ivanova A, Balatoni J, Teruya-Feldstein J, Heller G, May C, Ponomarev V, Ruan S, Finn R, Blasberg RG, Bornmann W, Riviere I, Sadelain M, O’Reilly RJ, Larson SM, Tjuvajev JG. Serial in vivo imaging of the targeted migration of human HSV-TK-transduced antigen-specific lymphocytes. Nat. Biotechnol. 2003;21:405–413. doi: 10.1038/nbt805. [DOI] [PubMed] [Google Scholar]
- [34].Dobrenkov K, Olszewska M, Likar Y, Shenker L, Gunset G, Cai S, Pillarsetty N, Hricak H, Sadelain M, Ponomarev V. Monitoring the efficacy of adoptively transferred prostate cancer-targeted human T lymphocytes with PET and bioluminescence imaging. J. Nucl. Med. 2008;49:1162–1170. doi: 10.2967/jnumed.107.047324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dotti G, Tian M, Savoldo B, Najjar A, Cooper LJ, Jackson J, Smith A, Mawlawi O, Uthamanthil R, Borne A, Brammer D, Paolillo V, Alauddin M, Gonzalez C, Steiner D, Decker WK, Marini F, Kornblau S, Bollard CM, Shpall EJ, Gelovani JG. Repetitive noninvasive monitoring of HSV1-tk-expressing T cells intravenously infused into nonhuman primates using positron emission tomography and computed tomography with 18F-FEAU. Mol. Imaging. 2009;8:230–237. [PMC free article] [PubMed] [Google Scholar]
- [36].Kim YJ, Dubey P, Ray P, Gambhir SS, Witte ON. Multimodality imaging of lymphocytic migration using lentiviral-based transduction of a tri-fusion reporter gene. Mol. Imaging Biol. 2004;6:331–340. doi: 10.1016/j.mibio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- [37].Shu CJ, Radu CG, Shelly SM, Vo DD, Prins R, Ribas A, Phelps ME, Witte ON. Quantitative PET reporter gene imaging of CD8+ T cells specific for a melanoma-expressed self-antigen. Int. Immunol. 2009;21:155–165. doi: 10.1093/intimm/dxn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dubey P, Su H, Adonai N, Du S, Rosato A, Braun J, Gambhir SS, Witte ON. Quantitative imaging of the T cell antitumor response by positron-emission tomography. Proc. Natl. Acad. Sci. USA. 2003;100:1232–1237. doi: 10.1073/pnas.0337418100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Su H, Forbes A, Gambhir SS, Braun J. Quantitation of cell number by a positron emission tomography reporter gene strategy. Mol. Imaging Biol. 2004;6:139–148. doi: 10.1016/j.mibio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- [40].Yaghoubi SS, Jensen MC, Satyamurthy N, Budhiraja S, Paik D, Czernin J, Gambhir SS. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat. Clin. Pract. Oncol. 2009;6:53–58. doi: 10.1038/ncponc1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Long CM, Bulte JW. In vivo tracking of cellular therapeutics using magnetic resonance imaging. Expert Opin. Biol. Ther. 2009;9:293–306. doi: 10.1517/14712590802715723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kircher MF, Allport JR, Graves EE, Love V, Josephson L, Lichtman AH, Weissleder R. In vivo high resolution three-dimensional imaging of antigen-specific cytotoxic T-lymphocyte trafficking to tumors. Cancer Res. 2003;63:6838–6846. [PubMed] [Google Scholar]
- [43].Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat. Immunol. 2008;9:486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- [44].Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH, Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101:640–648. doi: 10.1182/blood-2002-06-1751. [DOI] [PubMed] [Google Scholar]
- [45].Daldrup-Link HE, Meier R, Rudelius M, Piontek G, Piert M, Metz S, Settles M, Uherek C, Wels W, Schlegel J, Rummeny EJ. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. Eur. Radiol. 2005;15:4–13. doi: 10.1007/s00330-004-2526-7. [DOI] [PubMed] [Google Scholar]
- [46].Meier R, Piert M, Piontek G, Rudelius M, Oostendorp RA, Senekowitsch-Schmidtke R, Henning TD, Wels WS, Uherek C, Rummeny EJ, Daldrup-Link HE. Tracking of [18F]FDG-labeled natural killer cells to HER2/neu-positive tumors. Nucl. Med. Biol. 2008;35:579–588. doi: 10.1016/j.nucmedbio.2008.02.006. [DOI] [PubMed] [Google Scholar]
- [47].Tavri S, Jha P, Meier R, Henning TD, Muller T, Hostetter D, Knopp C, Johansson M, Reinhart V, Boddington S, Sista A, Wels WS, Daldrup-Link HE. Optical imaging of cellular immunotherapy against prostate cancer. Mol. Imaging. 2009;8:15–26. [PubMed] [Google Scholar]
- [48].Smirnov P, Lavergne E, Gazeau F, Lewin M, Boissonnas A, Doan BT, Gillet B, Combadiere C, Combadiere B, Clement O. In vivo cellular imaging of lymphocyte trafficking by MRI: a tumor model approach to cell-based anticancer therapy. Magn. Reson. Med. 2006;56:498–508. doi: 10.1002/mrm.20996. [DOI] [PubMed] [Google Scholar]
- [49].Untergasser G, Koeck R, Wolf D, Rumpold H, Ott H, Debbage P, Koppelstaetter C, Gunsilius E. CD34+/CD133− circulating endothelial precursor cells (CEP): characterization, senescence and in vivo application. Exp. Gerontol. 2006;41:600–608. doi: 10.1016/j.exger.2006.03.019. [DOI] [PubMed] [Google Scholar]
- [50].Morse MA, Clay TM, Lyerly HK. Current status of adoptive immunotherapy of malignancies. Expert Opin. Biol. Ther. 2002;2:237–247. doi: 10.1517/14712598.2.3.237. [DOI] [PubMed] [Google Scholar]
- [51].Takashima K, Fujiwara H, Inada S, Atsuji K, Araki Y, Kubota T, Yamagishi H. Tracking of green fluorescent protein (GFP)-labeled LAK cells in mice carrying B16 melanoma metastases. Anticancer Res. 2006;26:3327–3332. [PubMed] [Google Scholar]
- [52].Ritchie D, Mileshkin L, Wall D, Bartholeyns J, Thompson M, Coverdale J, Lau E, Wong J, Eu P, Hicks RJ, Prince HM. In vivo tracking of macrophage activated killer cells to sites of metastatic ovarian carcinoma. Cancer Immunol. Immunother. 2007;56:155–163. doi: 10.1007/s00262-006-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Baker J, Verneris MR, Ito M, Shizuru JA, Negrin RS. Expansion of cytolytic CD8+ natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon gamma production. Blood. 2001;97:2923–2931. doi: 10.1182/blood.v97.10.2923. [DOI] [PubMed] [Google Scholar]
- [54].Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- [55].Ren C, Kumar S, Chanda D, Kallman L, Chen J, Mountz JD, Ponnazhagan S. Cancer gene therapy using mesenchymal stem cells expressing interferon-beta in a mouse prostate cancer lung metastasis model. Gene Ther. 2008;15:1446–1453. doi: 10.1038/gt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Bexell D, Gunnarsson S, Tormin A, Darabi A, Gisselsson D, Roybon L, Scheding S, Bengzon J. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol. Ther. 2009;17:183–190. doi: 10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ren C, Kumar S, Chanda D, Chen J, Mountz JD, Ponnazhagan S. Therapeutic potential of mesenchymal stem cells producing interferon-alpha in a mouse melanoma lung metastasis model. Stem Cells. 2008;26:2332–2338. doi: 10.1634/stemcells.2008-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wang H, Cao F, De A, Cao Y, Contag C, Gambhir SS, Wu JC, Chen X. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem Cells. 2009;27:1548–1558. doi: 10.1002/stem.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Frank JA, Miller BR, Arbab AS, Zywicke HA, Jordan EK, Lewis BK, Bryant LH, Jr., Bulte JW. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480–487. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- [60].Arbab AS, Yocum GT, Kalish H, Jordan EK, Anderson SA, Khakoo AY, Read EJ, Frank JA. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004;104:1217–1223. doi: 10.1182/blood-2004-02-0655. [DOI] [PubMed] [Google Scholar]
- [61].Walczak P, Kedziorek DA, Gilad AA, Lin S, Bulte JW. Instant MR labeling of stem cells using magnetoelectroporation. Magn. Reson. Med. 2005;54:769–774. doi: 10.1002/mrm.20701. [DOI] [PubMed] [Google Scholar]
- [62].Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, Zywicke H, Miller B, van Gelderen P, Moskowitz BM, Duncan ID, Frank JA. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat. Biotechnol. 2001;19:1141–1147. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- [63].Zhang Z, Jiang Q, Jiang F, Ding G, Zhang R, Wang L, Zhang L, Robin AM, Katakowski M, Chopp M. In vivo magnetic resonance imaging tracks adult neural progenitor cell targeting of brain tumor. Neuroimage. 2004;23:281–287. doi: 10.1016/j.neuroimage.2004.05.019. [DOI] [PubMed] [Google Scholar]
- [64].Wu X, Hu J, Zhou L, Mao Y, Yang B, Gao L, Xie R, Xu F, Zhang D, Liu J, Zhu J. In vivo tracking of superparamagnetic iron oxide nanoparticle-labeled mesenchymal stem cell tropism to malignant gliomas using magnetic resonance imaging. Laboratory investigation. J. Neurosurg. 2008;108:320–329. doi: 10.3171/JNS/2008/108/2/0320. [DOI] [PubMed] [Google Scholar]
- [65].Thu MS, Najbauer J, Kendall SE, Harutyunyan I, Sangalang N, Gutova M, Metz MZ, Garcia E, Frank RT, Kim SU, Moats RA, Aboody KS. Iron labeling and pre-clinical MRI visualization of therapeutic human neural stem cells in a murine glioma model. PLoS ONE. 2009;4:e7218. doi: 10.1371/journal.pone.0007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Arbab AS, Pandit SD, Anderson SA, Yocum GT, Bur M, Frenkel V, Khuu HM, Read EJ, Frank JA. Magnetic resonance imaging and confocal microscopy studies of magnetically labeled endothelial progenitor cells trafficking to sites of tumor angiogenesis. Stem Cells. 2006;24:671–678. doi: 10.1634/stemcells.2005-0017. [DOI] [PubMed] [Google Scholar]
- [67].Lee JH, Smith MA, Liu W, Gold EM, Lewis B, Song HT, Frank JA. Enhanced stem cell tracking via electrostatically assembled fluorescent SPION-peptide complexes. Nanotechnology. 2009;20:355102. doi: 10.1088/0957-4484/20/35/355102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Partlow KC, Chen J, Brant JA, Neubauer AM, Meyerrose TE, Creer MH, Nolta JA, Caruthers SD, Lanza GM, Wickline SA. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. FASEB J. 2007;21:1647–1654. doi: 10.1096/fj.06-6505com. [DOI] [PubMed] [Google Scholar]
- [69].Rad AM, Iskander AS, Janic B, Knight RA, Arbab AS, Soltanian-Zadeh H. AC133+ progenitor cells as gene delivery vehicle and cellular probe in subcutaneous tumor models: a preliminary study. BMC Biotechnol. 2009;9:28. doi: 10.1186/1472-6750-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Tamura M, Unno K, Yonezawa S, Hattori K, Nakashima E, Tsukada H, Nakajima M, Oku N. In vivo trafficking of endothelial progenitor cells their possible involvement in the tumor neovascularization. Life Sci. 2004;75:575–584. doi: 10.1016/j.lfs.2003.12.025. [DOI] [PubMed] [Google Scholar]
- [71].Hung SC, Deng WP, Yang WK, Liu RS, Lee CC, Su TC, Lin RJ, Yang DM, Chang CW, Chen WH, Wei HJ, Gelovani JG. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin. Cancer Res. 2005;11:7749–7756. doi: 10.1158/1078-0432.CCR-05-0876. [DOI] [PubMed] [Google Scholar]
- [72].Hoffman RM. In vivo imaging with fluorescent proteins: the new cell biology. Acta. Histochem. 2004;106:77–87. doi: 10.1016/j.acthis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- [73].Hoffman RM. Advantages of multi-color fluorescent proteins for whole-body and in vivo cellular imaging. J. Biomed. Opt. 2005;10:41202. doi: 10.1117/1.1992485. [DOI] [PubMed] [Google Scholar]
- [74].Jiang P, Yamauchi K, Yang M, Tsuji K, Xu M, Maitra A, Bouvet M, Hoffman RM. Tumor cells genetically labeled with GFP in the nucleus and RFP in the cytoplasm for imaging cellular dynamics. Cell Cycle. 2006;5:1198–1201. doi: 10.4161/cc.5.11.2795. [DOI] [PubMed] [Google Scholar]
- [75].Hayashi K, Jiang P, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Moossa AR, Bouvet M, Hoffman RM. Real-time imaging of tumor-cell shedding and trafficking in lymphatic channels. Cancer Res. 2007;67:8223–8228. doi: 10.1158/0008-5472.CAN-07-1237. [DOI] [PubMed] [Google Scholar]
- [76].McElroy M, Hayashi K, Garmy-Susini B, Kaushal S, Varner JA, Moossa AR, Hoffman RM, Bouvet M. Fluorescent LYVE-1 antibody to image dynamically lymphatic trafficking of cancer cells in vivo. J. Surg. Res. 2009;151:68–73. doi: 10.1016/j.jss.2007.12.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hoffman RM. Imaging cancer dynamics in vivo at the tumor and cellular level with fluorescent proteins. Clin. Exp. Metastasis. 2009;26:345–55. doi: 10.1007/s10585-008-9205-z. [DOI] [PubMed] [Google Scholar]
- [78].Cai W, Hsu AR, Li ZB, Chen X. Are quantum dots ready for in vivo imaging in human subjects? Nanoscale Res. Lett. 2007;2:265–281. doi: 10.1007/s11671-007-9061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Cai W, Chen X. Preparation of peptide conjugated quantum dots for tumour vasculature targeted imaging. Nat. Protoc. 2008;3:89–96. doi: 10.1038/nprot.2007.478. [DOI] [PubMed] [Google Scholar]
- [80].Voura EB, Jaiswal JK, Mattoussi H, Simon SM. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat. Med. 2004;10:993–998. doi: 10.1038/nm1096. [DOI] [PubMed] [Google Scholar]
- [81].Neumaier CE, Baio G, Ferrini S, Corte G, Daga A. MR and iron magnetic nanoparticles. Imaging opportunities in preclinical and translational research. Tumori. 2008;94:226–233. doi: 10.1177/030089160809400215. [DOI] [PubMed] [Google Scholar]
- [82].Vuu K, Xie J, McDonald MA, Bernardo M, Hunter F, Zhang Y, Li K, Bednarski M, Guccione S. Gadolinium-rhodamine nanoparticles for cell labeling and tracking via magnetic resonance and optical imaging. Bioconjug. Chem. 2005;16:995–999. doi: 10.1021/bc050085z. [DOI] [PubMed] [Google Scholar]
- [83].Koike C, Watanabe M, Oku N, Tsukada H, Irimura T, Okada S. Tumor cells with organ-specific metastatic ability show distinctive trafficking in vivo: analyses by positron emission tomography and bioimaging. Cancer Res. 1997;57:3612–3619. [PubMed] [Google Scholar]
- [84].Adonai N, Nguyen KN, Walsh J, Iyer M, Toyokuni T, Phelps ME, McCarthy T, McCarthy DW, Gambhir SS. Ex vivo cell labeling with 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) for imaging cell trafficking in mice with positron-emission tomography. Proc. Natl. Acad. Sci. USA. 2002;99:3030–3035. doi: 10.1073/pnas.052709599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Li ZB, Chen K, Wu Z, Wang H, Niu G, Chen X. 64Cu-labeled PEGylated polyethylenimine for cell trafficking and tumor imaging. Mol. Imaging Biol. 2009 doi: 10.1007/s11307-009-0228-x. ePub. [DOI] [PubMed] [Google Scholar]