Abstract
Context
Cognitive function in older adults is related to independent living and need for care. However, few studies have addressed whether improving cognitive functions might have short- or long-term effects on activities related to living independently.
Objective
To evaluate whether 3 cognitive training interventions improve mental abilities and daily functioning in older, independent-living adults.
Design
Randomized, controlled, single-blind trial with recruitment conducted from March 1998 to October 1999 and 2-year follow-up through December 2001.
Setting and Participants
Volunteer sample of 2832 persons aged 65 to 94 years recruited from senior housing, community centers, and hospital/clinics in 6 metropolitan areas in the United States.
Interventions
Participants were randomly assigned to 1 of 4 groups: 10-session group training for memory (verbal episodic memory; n=711), or reasoning (ability to solve problems that follow a serial pattern; n=705), or speed of processing (visual search and identification; n=712); or a no-contact control group (n=704). For the 3 treatment groups, 4-session booster training was offered to a 60% random sample 11 months later.
Main Outcome Measures
Cognitive function and cognitively demanding everyday functioning.
Results
Thirty participants were incorrectly randomized and were excluded from the analysis. Each intervention improved the targeted cognitive ability compared with baseline, durable to 2 years (P<.001 for all). Eighty-seven percent of speed-, 74% of reasoning-, and 26% of memory-trained participants demonstrated reliable cognitive improvement immediately after the intervention period. Booster training enhanced training gains in speed (P<.001) and reasoning (P<.001) interventions (speed booster, 92%; no booster, 68%; reasoning booster, 72%; no booster, 49%), which were maintained at 2-year follow-up (P<.001 for both). No training effects on everyday functioning were detected at 2 years.
Conclusions
Results support the effectiveness and durability of the cognitive training interventions in improving targeted cognitive abilities. Training effects were of a magnitude equivalent to the amount of decline expected in elderly persons without dementia over 7- to 14-year intervals. Because of minimal functional decline across all groups, longer follow-up is likely required to observe training effects on everyday function.
Nearly half of community-dwelling persons aged 60 years and older express concern about declining mental abilities.1 Although there is substantial evidence that many cognitive abilities and processes are related to measures of functional status, need for care, and quality of life, few studies have addressed whether improving cognitive functions might have short- or long-term effects on activities related to living independently. Interventions designed to delay or prevent the need for nursing homes, home care, and hospital stays can save health care costs, while also ensuring the independence and dignity of the aging population.
A growing body of research supports the protective effects of late-life intellectual stimulation on incident dementia.2,3 Recent research from both human and animal studies indicates that neural plasticity endures across the lifespan, and that cognitive stimulation in the environment is an important predictor of enhancement and maintenance of cognitive functioning, even in old age. Moreover, sustained engagement in cognitively stimulating activities has been found to impact neural structure in both older humans and rodents.4–6 Conversely, limited education has been found to be a risk factor for dementia.7 There is also a sizeable body of literature documenting that different types of cognitive training programs have large and durable effects on the cognitive functioning of older adults, even in advanced old age.8–15 At the same time, several important issues remain understudied. First, prior cognitive training studies with older adults have often paid relatively little attention to the use of appropriate control groups, the representativeness or heterogeneity of participants, the generalizability of training findings beyond particular laboratories, or adherence of participants to training protocols. For example, it has not been uncommon for such studies to analyze only compliant participants. Second, the broader implications of training on daily functioning in older adults, for the most part, have not been studied.
The primary objective of the ACTIVE (Advanced Cognitive Training for Independent and Vital Elderly) trial was to test the effectiveness and durability of 3 distinct cognitive interventions in improving the performance of elderly persons on basic measures of cognition and on measures of cognitively demanding daily activities (eg, food preparation, driving, medication use, financial management). These interventions previously had been found successful in improving cognitive abilities under laboratory or small-scale field conditions.8–16 We hypothesized that the effects of cognitive training on primary outcomes will be largely mediated through the basic cognitive abilities being trained. The detailed hypotheses may be summarized by 2 points: each training group will perform better than the other training and control groups on their respective primary and proximal outcomes, and those groups that received booster training will perform better than those that did not receive booster training on their primary and proximal outcomes.
METHODS
Participants
The recruitment goal for the ACTIVE trial was to enroll a diverse sample of older adults who, at enrollment, were living independently in good functional and cognitive status. Recruitment was conducted from March 1998 to October 1999; 2-year follow-up data were collected through December 2001. Details of the recruitment procedures have been published elsewhere.17 Participants aged 65 to 94 years were enrolled across 6 field sites using a variety of sampling frames and recruitment strategies (state driver’s license and identification card registries, medical clinic rosters, senior center and community organization rosters, senior housing sites, local churches, and rosters of assistance and service programs for low-income elderly persons). Oral assent was obtained for brief telephone screening, and written informed consent was obtained in person from each potential participant prior to administration of in-person screening measures.
Persons were excluded from participation if they were younger than 65 years at screening; if they had already experienced substantial cognitive decline (score of ≤22 on the Mini-Mental State Examination [MMSE]18); had a self-reported diagnosis of Alzheimer disease; had already experienced substantial functional decline (self-reported need for weight-bearing support or full caregiver performance of dressing, personal hygiene, or bathing 3 or more times in the previous 7 days); had medical conditions that would predispose them to imminent functional decline or death (eg, stroke within the past 12 months, certain cancers, or current chemotherapy or radiation treatment for cancer); had recent cognitive training; were unavailable during the testing and intervention phases of the study; or had severe losses in vision (self-reported difficulty in reading newsprint, or measured vision worse than 20/70 with best correction), hearing (interviewer-rated), or communicative ability (interviewer-rated) that would sufficiently impair performance to make participation impossible.
Study Design
The study protocol was approved by the institutional review boards at the University of Alabama at Birmingham; Wayne State University, Detroit, Mich; the Hebrew Rehabilitation Center for the Aged, Roslindale, Mass; the Johns Hopkins University School of Medicine, Baltimore, Md; Indiana University, Bloomington; Purdue University, Indianapolis, Ind; Pennsylvania State University, University Park; the University of Florida, Gainesville; and the New England Research Institutes, Watertown, Mass.
The ACTIVE trial was sponsored by the National Institute on Aging and the National Institute of Nursing Research, and was randomized, controlled, and single-blind, using a 4-group design, including a no-contact control group and 3 intervention groups (memory training, reasoning training, or speed-of-processing training). These 3 interventions were selected because they showed the most promise in smaller laboratory studies and had been related to instrumental activities of daily living (IADL).8,19–26 Each intervention group received a 10-session intervention, conducted by certified trainers, for 1 of 3 cognitive abilities—memory, inductive reasoning, or speed of processing. Assessors were blinded to participant intervention assignment. Training exposure and social contact were standardized across interventions so that each intervention served as a contact control for the other 2 interventions. Booster training was provided to a random sub sample in each intervention group. Measurement points consisted of baseline tests, an immediate posttest (following the intervention), and 1 and 2 annual posttests.
Interventions
The interventions were conducted in small group settings in ten 60- to 75-minute sessions over 5- to 6-week periods. These were behavioral interventions with no pharmacological component. In all 3 conditions, sessions 1 through 5 focused on strategy instruction and individual and group exercises to practice the strategy. Sessions 6 through 10 provided additional practice exercises but introduced no new strategies.
Memory training12,27–29 focused on verbal episodic memory. Participants were taught mnemonic strategies for remembering word lists and sequences of items, text material, and main ideas and details of stories. Participants received instruction in a strategy or mnemonic rule, exercises, individual and group feedback on performance, and a practice test. For example, participants were instructed how to organize word lists into meaningful categories and to form visual images and mental associations to recall words and texts. The exercises involved laboratory like memory tasks (eg, recalling a list of nouns, recalling a paragraph), as well as memory tasks related to cognitive activities of everyday life (eg, recalling a shopping list, recalling the details of a prescription label).
Reasoning training10,13 focused on the ability to solve problems that follow a serial pattern. Such problems involve identifying the pattern in a letter or number series or understanding the pattern in an everyday activity such as prescription drug dosing or travel schedules. Participants were taught strategies to identify a pattern and were given an opportunity to practice the strategies in both individual and group exercises. The exercises involved abstract reasoning tasks (eg, letter series) as well as reasoning problems related to activities of daily living.
Speed-of-processing training8,30 focused on visual search skills and the ability to identify and locate visual information quickly in a divided-attention format. Participants practiced increasingly complex speed tasks on a computer. Task difficulty was manipulated by decreasing the duration of the stimuli, adding either visual or auditory distraction, increasing the number of tasks to be performed concurrently, or presenting targets over a wider spatial expanse. Difficulty was increased each time a participant achieved criterion performance on a particular task.
Eleven months after the initial training was provided, booster training was offered to a randomly selected 60% of initially trained subjects in each of the 3 intervention groups. Booster training was delivered in four 75-minute sessions over a 2- to 3-week period.
Measures
The ACTIVE trial had multiple outcomes, both proximal (cognitive abilities) and primary (daily function) (Table 1). Composites were created to represent each domain. Each composite was the average of 2 or 3 test scores, equally weighted, and was designed as a measure of ability rather than performance on a specific test.
Table 1.
Outcome Measures
| Time Point | |||||
|---|---|---|---|---|---|
| Measure | Baseline | Immediate Posttest |
First and Second Annual Posttests |
Mode of Administration |
Reliability* |
| Proximal Outcome Composites | |||||
| Memory | |||||
| Hopkins Verbal Learning Test31 | X | X | X | Paper and pencil | 0.73 |
| Auditory Verbal Learning Test32 | X | X | X | Paper and pencil | 0.78 |
| Rivermead Behavioral Memory Test33 | X | X | X | Paper and pencil | 0.60 |
| Reasoning | |||||
| Word series34 | X | X | X | Paper and pencil | 0.84 |
| Letter series35 | X | X | X | Paper and pencil | 0.86 |
| Letter sets36 | X | X | X | Paper and pencil | 0.69 |
| Speed of processing | |||||
| Useful Field of View (tasks 2–4)23,25,37 | X | X | X | Computer | 0.80 |
| Primary Outcome Composites | |||||
| Everyday problem solving | |||||
| Everyday Problems Test38 | X | X | X | Paper and pencil | 0.87 |
| Observed Tasks of Daily Living21,39 | X | X | Paper and pencil | 0.75† | |
| Everyday speed | |||||
| Complex Reaction Time (2 tests)8 | X | X | X | Computer | 0.45 0.56 |
| Timed Instrumental Activities of Daily Living (IADL)40 | X | X | X | Behavioral observation | 0.64 |
| Activities of Daily Living and IADL functioning | |||||
| Minimum Data Set–Home Care (3 scores)41 | X | X | Survey | 0.80† | |
| Interview | 0.75† | ||||
| 0.30† | |||||
| Driving habits | |||||
| Difficulty, avoidance, (space)‡42 | X | X | Survey | 0.60 | |
| Interview | 0.80§ | ||||
Test-retest correlations except where noted.
Cronbach α.
“Space” indicates a driver’s extent of travel in a specified period of time.
α or κ from reference cited.
Proximal outcomes permitted a test of the impact of the 3 interventions on the appropriate cognitive abilities. Memory assessment focused on episodic verbal memory tasks. Reasoning assessment focused on tasks requiring identification of patterns in letter or word series problems. Speed-of-processing assessment focused on identifying the minimum stimulus duration at which participants could identify and localize information, with 75% accuracy, under varying levels of cognitive demand.
Primary outcomes were aspects of functional activities, both performance-based and self-reported. Everyday problem solving represented the ability to reason and correctly identify information in common everyday stimuli (eg, medication labels, charts, forms). This was measured via paper-and-pencil testing and behavioral simulations of everyday tasks. Everyday speed emphasized the speed with which participants interacted with real-world stimuli. Participants were asked to look up a specific telephone number, find food items on a crowded shelf of groceries, find ingredients on food labels, count out specified amounts of change, find specified information on medicine bottles, and respond appropriately to different traffic signs. Activities of daily living (ADL) and instrumented activities of daily living included self-ratings drawn from the Minimum Data Set—Home Care (MDS-HC).43 Driving habits included self-ratings of driving difficulty and avoidance of specific driving situations.
Tests were standardized by pooling scores at all time points and applying a Blom transformation,44 producing more normally distributed scores. Scores for tests at each time point were standardized to the baseline mean and SD. If 1 or more tests of a composite were missing, the composite score was calculated as the average of the non-missing tests.
Analysis
To evaluate the effects of ACTIVE training over 2 years, a repeated-measures, mixed-effects model was used.45 The dependent variables were the proximal and primary composites measured at 4 time points: baseline, immediate post-test, first annual evaluation (A1), and second annual evaluation (A2). At posttest only the cognitive variables, the Everyday Problems Test, and the primary speed composite were measured. The independent variables were restricted to the basic design features: fixed effects for training group (memory, reasoning, speed, control); time (3 or 4 points); booster training; field site; and replicate within site. Three interaction terms were chosen for their importance and interpretability: time × training, representing the net effect of the trial; time × booster, representing nonspecific effects of the additional social contact of attending booster training, regardless of content; and time × booster × training, representing the training-specific effects of each booster intervention. For this analysis, the repeated-measures model was fitted to the available data, ignoring missing data. Then, to determine if selective attrition influenced the trial results, missing data were imputed using multiple imputation procedures,46 and the analysis was repeated.
Hypotheses were tested by comparing outcome composite scores at later times (posttest, A1, and A2) to baseline scores and to control group scores, yielding net differences. The net effect of training at any time was defined as: (trained mean – control mean at later time) – (trained mean – control mean at baseline). Similarly, the net effect of each booster training was defined as: (booster mean – unboosted mean at later time) – (booster mean – unboosted mean at baseline). Results are expressed as effect sizes (ie, difference in means divided by intrasubject SD) to allow direct comparison of different outcomes. In addition, covariate-adjusted training effects were examined, with covariates of age, sex, cognitive status (MMSE score), years of education, and visual acuity. Given the substantial variation associated with field site and replicate, these 2 factors were also included as covariates in all analyses.
Secondary analyses investigated the percentage of participants who showed reliable improvement in each training group. A participant was classified as having improved reliably on a particular measure if his or her performance at a follow-up occasion exceeded baseline performance on that measure by 1 SEM.47 The formula for reliable change was computed as outlined by Dudek,47 and analyses were conducted using SAS v8.2 (SAS Institute Inc, Cary, NC). P<.05 was considered significant.
RESULTS
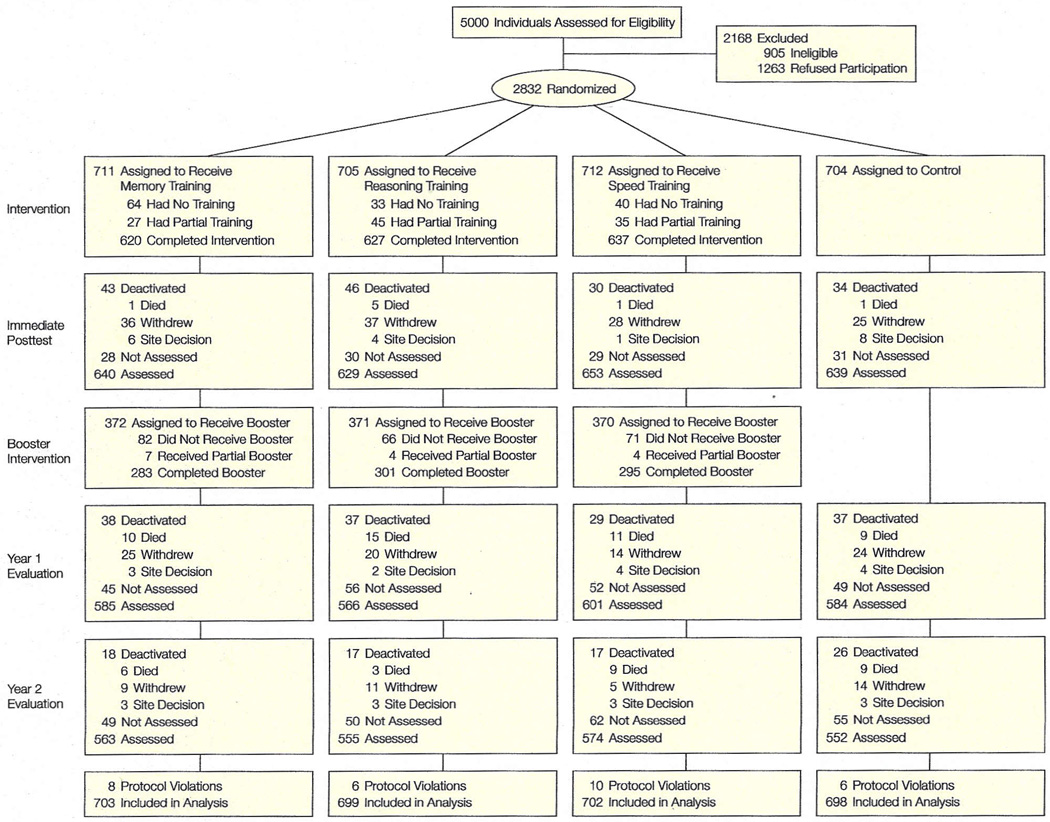
Five thousand individuals were contacted for participation (Figure 1). A total of 2832 persons were eligible, 905 (18.1%) were ineligible, and 1263 (25.3%) refused (either directly or passively by not coming to any appointments) prior to randomization. Reasons for ineligibility were: cognitive impairment on the MMSE (270 [29.8%]), vision impairment (192 [21.2%]), unavailability due to schedule (202 [22.3%]), too young (85 [9.4%]), medical conditions predisposing to imminent decline or short life expectancy (79 [8.7%]), significant ADL disability (48 [5.3%]), impaired communication (15 [1.7%]), diagnosed Alzheimer disease (7 [0.8%]), and prior participation in cognitive training trials (7 [0.8%]). Enrollment at the field sites ranged from 405 to 498 participants. Thirty eligible persons were randomized inappropriately, thus violating protocol, and were excluded from analyses. The analytic sample consists of 2802 participants randomized by the New England Research Institutes with a concealed system. Intention-to-treat analyses were used.
Figure 1. Flow of Participants Through the Trial.
“Withdrew” indicates subjects withdrew for reasons including scheduling conflicts, poor health, and lack of interest in continuing; “site decision,” that subjects were withdrawn by study sites because they repeatedly missed appointments or were uncooperative or disruptive during testing sessions.
Ineligible participants were comparable with eligible participants in age (mean, 77 years) and proportion of women (77%). Ineligible participants tended to have a higher percentage of nonwhite persons (48%) and lower cognitive function (mean MMSE score, 20.9). Potential participants who were eligible and randomized (n=2802) were comparable with the group that was eligible and not randomized (n=1263). Compared with the nonrandomized group, the randomized group was slightly younger (mean, 74 vs 75 years), more educated (13.5 vs 12.3 years), scored higher in cognitive function (MMSE score, 27.3 vs 26.8), and had fewer nonwhite participants (27% vs 40%). The baseline characteristics of the ACTIVE cohort and its comparability with the general population are provided in Table 2.
Table 2.
Baseline Characteristics of Participants (N = 2802)*
| Characteristic | Sample | General Population† |
P Value |
|
|---|---|---|---|---|
| Age, mean (SD) [range], y | 73.6 (5.9) [65–94] | NA | ||
| Age groups, y, %48 | ||||
| 65–74 | 60.1 | 57.6 | <.001 | |
| 75–84 | 35.0 | 32.5 | ||
| ≥85 | 4.9 | 9.9 | ||
| Women, %49 | 75.9 | 57.9 | <.001 | |
| Race, %49 | ||||
| White | 73.3 | NA | ||
| African American | 26.0 | 8.6 | <.001 | |
| Other/unknown | 0.7 | NA | ||
| High school graduate, %49 | 88.6 | 67.0 | <.001 | |
| Married, %49 | 35.9 | 56.6 | <.001 | |
| MMSE score, mean (SD) [range]50 | 27.3 (2.0) [23–30] | 26.4 (2.1) | <.001 | |
| SF–36 physical function, mean (SD) [range]51‡ |
68.8(24.1) [0–100] | 62 | <.001 | |
NA indicates not applicable; MMSE, Mini-Mental State Examination; and SF-36, Short Form Health Survey (36 item).
Data from references in “characteristic” column.
For general population, only percentage given.
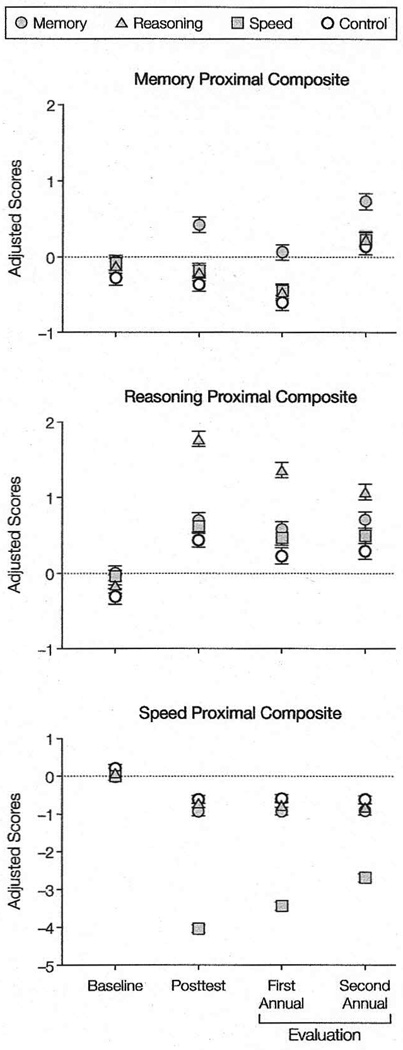
Results of the analyses are summarized in Table 3 and Table 4. Eighty-nine percent of participants completed treatment (≥8 training sessions), and 80% of the sample was retained at the 2-year follow-up, despite the advanced age of the cohort. The net effect of ACTIVE training on the proximal (cognitive) composites is displayed in the top portion of Table 3. Each training program produced an immediate effect on its corresponding cognitive ability. It is important to note that while these analyses were conducted on Blom-transformed variables, a near-identical pattern of findings was obtained with the untransformed variables. Temporal trends in the mean cognitive composite scores by intervention group are shown in Figure 2.
Table 3.
Training Effects on Proximal and Primary Outcomes*
| Memory Training | Reasoning Training | Speed Training | Control† | ||||
|---|---|---|---|---|---|---|---|
| Measure | Net Effect Size (P Value)‡ |
Showing Reliable Improvement, %§ |
Net Effect Size (P Value)‡ |
Showing Reliable Improvement, %§ |
Net Effect Size (P Value)‡ |
Showing Reliable Improvement, %§ |
Showing Reliable Improvement, %§ |
| Proximal Outcome Composites | |||||||
| Memory | |||||||
| Posttest | 0.257 (<.001) | 26 | −0.009 | 17 | −0.012 | 13 | 15 |
| A1 | 0.212 (<.001) | 22 | −0.011 | 11 | −0.021 | 12 | 14 |
| A2 | 0.174 (<.001) | 40 | −0.03 | 27 | −0.052 | 28 | 29 |
| Reasoning | |||||||
| Posttest | −0.018 | 34 | 0.480 (<.001) | 74 | −0.026 | 35 | 39 |
| A1 | 0.021 | 34 | 0.402 (<.001) | 63 | −0.003 | 29 | 31 |
| A2 | 0.045 | 36 | 0.257 (<.001) | 53 | −0.019 | 30 | 35 |
| Speed∥ | |||||||
| Posttest | −0.045 | 34 | 0.003 | 33 | −1.463(<.001) | 87 | 31 |
| A1 | −0.054 | 35 | −0.033 | 34 | −1.212 (<.001) | 81 | 32 |
| A2 | −0.034 | 36 | −0.043 | 35 | −0.867 (<.001) | 73 | 37 |
| Primary Outcome Composites | |||||||
| Everyday problem soMng | |||||||
| A1 | −0.045 | 19 | 0.03 | 23 | 0.008 | 20 | 21 |
| A2 | −0.073 | 21 | −0.027 | 25 | 0.031 | 26 | 23 |
| ADL and IADL functioning∥ | |||||||
| A1 | 0.02 | 17 | −0.125 | 18 | −0.05 | 14 | 16 |
| A2 | 0.017 | 17 | −0.056 | 16 | −0.07 | 17 | 17 |
| Everyday speed∥ | |||||||
| Posttest | −0.091 (.02) | 31 | 0.004 | 22 | −0.016 | 26 | 27 |
| A1 | −0.041 | 31 | 0.05 | 28 | 0.001 | 31 | 30 |
| A2 | −0.007 | 33 | 0.03 | 29 | −0.009 | 30 | 29 |
| Driving habits∥¶ | |||||||
| A1 | 0.017 | 19 | −0.052 | 19 | 0.068 | 16 | 19 |
| A2 | 0.085 | 16 | 0.079 | 16 | 0.077 | 16 | 18 |
Only significant P values reported. A1 indicates first annual evaluation; A2, second annual evaluation; ADL, activities of daily living; and IADL, instrumental activities of daily living.
Net effect of the control is 0 at all time points, since net effect of group is defined as (group mean-control mean at time point)-(group mean-control mean at baseline).
Net difference divided by intrasubject SD (see “Methods” section).
Calculated as the percentage of participants in each group who were ≥1 SEM above baseline.
Favorable response is in the negative direction.
For self-reported drivers only.
Table 4.
Net Effect of Booster Training on Proximal and Primary Outcomes*
| Memory Training | Reasoning Training | Speed Training | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Showing Reliable Improvement, %‡ |
Showing Reliable Improvement, %‡ |
Showing Reliable Improvement, %‡ |
Control† | |||||||
| Measure | Net Effect Size (P Value)§ |
No Booster |
Booster | Net Effect Size (P Value)§ |
No Booster |
Booster | Net Effect Size (P Value)§ |
No Booster |
Booster | Showing Reliable Improvement, %‡ |
| Proximal Outcome Composites | ||||||||||
| Memory | ||||||||||
| A1 | 0.044 | 23 | 21 | −0.043 | 13 | 9 | −0.004 | 13 | 10 | 14 |
| A2 | 0.060 | 39 | 40 | −0.012 | 27 | 28 | 0.042 | 27 | 29 | 29 |
| Reasoning | ||||||||||
| A1 | −0.009 | 35 | 32 | −0.304 (<.001) | 49 | 72 | 0.125 (.003) | 23 | 34 | 31 |
| A2 | −0.036 | 37 | 35 | 0.152 (<.001) | 47 | 57 | −0.039 | 33 | 28 | 35 |
| Speed∥ | ||||||||||
| A1 | −0.03 | 37 | 33 | −0.043 | 31 | 36 | −0.919 (<.001) | 68 | 92 | 32 |
| A2 | 0.02 | 35 | 38 | −0.065 | 33 | 36 | −0.347 (<001) | 65 | 79 | 37 |
| Primary Outcome Composites | ||||||||||
| Everyday problem solving | ||||||||||
| A1 | −0.007 | 18 | 20 | 0.001 | 24 | 23 | 0.019 | 21 | 20 | 21 |
| A2 | −0.033 | 19 | 23 | −0.037 | 25 | 25 | −0.06 | 27 | 25 | 23 |
| ADL and IADL functioning∥ | ||||||||||
| A1 | −0.088 | 17 | 17 | −0.206 | 17 | 19 | −0.246 (.04) | 13 | 16 | 16 |
| A2 | 0.096 | 18 | 16 | −0.196 | 15 | 17 | −0.217 | 18 | 18 | 17 |
| Everyday speed∥ | ||||||||||
| A1 | 0.041 | 31 | 30 | −0.068 | 26 | 29 | −0.149 (.01) | 28 | 34 | 30 |
| A2 | −0.033 | 33 | 33 | −0.019 | 26 | 30 | −0.091 | 27 | 33 | 29 |
| Driving habits∥¶ | ||||||||||
| A1 | −0.082 | 14 | 23 | −0.059 | 19 | 19 | 0.088 | 16 | 15 | 19 |
| A2 | −0.025 | 14 | 18 | −0.052 | 14 | 17 | −0.055 | 14 | 18 | 18 |
Only significant P values reported. A1 indicates first annual evaluation; A2, second annual evaluation; ADL, activities of daily living; and IADL, instrumental activities of daily living.
Net effect of the control is 0 at all time points, since net effect of group is deflned as (group mean–control mean at time point)–(group mean–control mean at baseline).
Net difference divided by intrasubject SD (see “Methods” section).
Calculated as the percentage of participants in each group who were ≥1 SEM above baseline.
Favorable response is in the negative direction.
For self-reported drivers only.
Figure 2. Cognitive Outcomes: Mean Scores Across Time by Group.
Data are Blom-transformed and also adjusted for time, booster, field site, and replicate. Error bars indicate SE.
The net effect of ACTIVE training on functional outcome composites is detailed in the lower section of Table 3. These effects were generally small on the effect-size scale—most were below 0.10—and did not differ significantly from zero at A1 or A2. It is important to note, however, that the vast majority of this sample remained functionally independent over the course of the 24-month observation period. For the crucial measures of ADL performance—measures that have been shown to predict movement into home care and institutional programs—a relatively low ADL decline rate (defined as ≥2 points on the summary measure) of 6% was observed at 12 months, with a modest increase to 8% at 24 months.
The impact of booster training at A1 and A2 is detailed in Table 4. Again, the strongest effects were seen in cognitive outcomes, where boosters for reasoning and speed training administered shortly before A1 produced significantly better performance. The impact of reasoning and speed booster training was greater at A1 than at A2. No effect was detected for memory booster on the memory proximal composite. Compared with those who did not receive booster training, participants randomized to speed booster performed significandy better at A1 on the functioning and everyday speed composites (P<.05), and marginally better at A2 (P<.10). Similarly, compared with those who did not receive booster training, participants randomized to reasoning booster performed marginally better on the functioning composite at A1 (P<.10).
The results of covariate-adjusted analyses were generally similar. While effect sizes were universally higher after adjusting for age, sex, education, visual function, and mental status, the overall pattern of results was the same and is not presented here. Similarly, analyses of imputed data sets did not differ in outcomes, suggesting that the trial results were not influenced by selective attrition.
Consistent with results of the primary analyses, secondary analyses indicated large immediate intervention gains on the cognitive outcomes. Eighty-seven percent of speed-trained, 74% of reasoning-trained, and 26% of memory-trained participants demonstrated reliable improvement on the pertinent cognitive composite immediately following intervention. While intervention participants showed reliable posttest gains, a comparable proportion of control participants also improved, and the proportion of control participants exhibiting reliable retest gain remained fairly constant across study intervals.
In terms of the proportion of the intervention group showing reliable gain in the trained domain, booster effects occurred for the speed conditions (boost, 92%; no boost, 68%; control, 32%) and the reasoning conditions (boost, 72%; no boost, 49%; control, 31%). While some dissipation of intervention effects occurred across time, cognitive effects were maintained from baseline to A2, particularly for boosted participants (79% [speed boost] vs37% [controls]; 57% [reasoning boost] vs 35% [controls]).
COMMENT
To date, ACTIVE is the largest trial (N=2802) of cognitive interventions for the improvement of older adults’ performance on specific cognitive and perceptual abilities. Although studies have successfully used laboratory-based interventions to improve cognitive performance in older adults,8,10,12,14,27,29,52 the ACTIVE trial improved on previous studies in that it used a multisite, randomized, controlled design; included a large, diverse sample; used common multisite intervention protocols; and examined primary outcomes as well as long-term transfer effects to everyday activities.
Overall, this large-scale study demonstrated that cognitive interventions helped normal elderly individuals to perform better on multiple measures of the specific cognitive ability for which they were trained. It did not, however, demonstrate the generalization of such interventions to everyday performance, at least in the initial 2 years. The effect sizes for the cognitive abilities at immediate posttest are for the most part consistent with previous research. Moreover, these effect sizes are comparable with or greater than the amount of longitudinal decline that has been reported in previous studies (Table 5), suggesting that these interventions have the potential to reverse age-related decline. Specifically, age-related decline for reasoning ability in samples of elderly persons without dementia has been found to be on the order of 0.22 SD over a 7-year interval (ages 67–74 years) and to increase to 0.42 SD over a 14-year interval (ages 67–81 years).53 Thus, immediate reasoning training effects (0.48 SD) were comparable with the amount of decline reported to occur in elderly persons without dementia over a 14-year interval. Likewise, decline in memory ability has been reported to be approximately 0.25 SD over a 6- or 7-year interval. Thus, memory training effects (0.25 SD) were comparable with the expected decline over a 7-year interval in elderly persons without dementia.53–55 Finally, decline for speed has been reported to be approximately 0.16 SD over 2 years.8 Immediate speed training effects (1.46 SD) were therefore 9 times greater than the expected decline over a 2-year interval in elderly persons without dementia.
Table 5.
Training Effects on Proximal Outcomes Relative to Expected Annualized Declines*
| Source | Age Range, y |
Magnitude of Change | Annualized Change, SD/y |
ACTIVE Immediate Training Effect, SD |
ACTIVE Training Effect at 2 y, SD |
|
|---|---|---|---|---|---|---|
| Memory | ||||||
| Schaie,53 1996 | 67–81 | 0.25 SD over 7 y (semantic lists) | 0.036 | 0.25 | 0.17 | |
| Small et al,54 1999 | ≥78 | 0.25 SD over 6 y (immediate word recall) | 0.041 | |||
| Zelinski and Bumright,55 1997 | 67–81 | 0.50 SD over 16 y (immediate word recall) | 0.031 | |||
| 1.00 SD over 16 y (immediate text recall) | 0.031 | |||||
| Reasoning | ||||||
| Schaie,53 1996 | 67–81 | 0.22 SD over 7 y | 0.030 | 0.48 | 0.26 | |
| 0.42 SD over 14 y | ||||||
| Speed | ||||||
| Ball and Owsley,8 2000 | ≥55 | 0.16 SD over 2 y | 0.08 | 1.46 | 0.87 | |
ACTIVE indicates Advanced Cognitive Training for Independent and Vital Elderly.
Although training impact on the proximal composites decreased over time, it remained statistically significant, attesting to the durability of the intervention training effects. This is an important finding, since prior interventions (especially memory) have not shown 2-year durability. Furthermore, a very high percentage of trained participants achieved reliable improvement on the cognitive abilities, and ceiling effects at baseline on the cognitive measures explain lack of reliable improvement for most others. Of further note, the tests of training effects were conservative compared with those used in prior cognitive aging research. That is, prior cognitive training research has not used intention-to-treat analyses, instead excluding participants who dropped out or were noncompliant. In addition, prior research has not used diverse samples in terms of education and ethnicity. Thus, relative to prior work, training effects on cognitive abilities in this study are strong.
Insufficient sample size was ruled out as an explanation for the small effect sizes to date on the functional outcomes. The study was sufficiently powered to detect an effect size of 0.20 at 95% power with a sample of 2832.56 Power calculations were based on 6 Bonferroni corrected, 2-sided comparisons with an overall a level of .05, a correlation of 0.7 between baseline and follow-up (based on pilot data), and an 80% completion rate.17 Based on these same assumptions, there was 90% power to detect booster training effects. Given that we retained more than 80% of the initial sample over the 2-year follow-up period and found no differential loss across treatment and control conditions, there should have been sufficient power to detect a significant effect of the cognitive training on the functional outcomes.
The absence of transfer to real-world outcomes is not particularly surprising. In addition to several decades of cognitive science research demonstrating the difficulty of obtaining such transfer, most of our subjects were not yet impaired in the domains of training. Indeed, there are several other potential explanations for the observed lack of transfer to daily function: the proportion of participants functioning at ceiling levels (ie, 43% had no room for improvement, as indicated by baseline performance within 1 SEM of the “best” value) on the daily functional composite, the evidence of strong practice or retest effects in the control group, and the control group’s lack of functional decline over the 2-year follow-up period.
With respect to ceiling effects in everyday functional abilities, this finding does not reflect poor measurement choices; rather, one would expect that most participants would show high levels of competence on these self- and household-care tasks if they continue to reside independently in the community, as was true at enrollment. Thus, improved cognitive function could not be expected to improve intact everyday abilities over a 2-year period.
Consistent with prior cognitive intervention research showing large re-test or practice effects,57 the approximately 5 hours of practice on cognitive tests at each assessment occasion resulted in retest effects for the control group. Approximately 25% of control participants showed reliable gain on cognitive and functional composites as a result of practice effects, and these re-test effects were evident across study intervals. Particularly notable were practice effects on the daily function composites. These large retest effects contributed to ceiling-level performance across groups that precluded demonstration of additional gain as a result of training.
In terms of the observed lack of functional decline in the control group, it is important to note that individuals with extant functional or cognitive decline were carefully screened out, and the study focused instead on intact individuals whose future decline rates were likely to mimic or be less than rates for the general elderly population. It was therefore unclear whether participants would show evidence of decline similar to established population parameters over the 12- and 24-month observation periods, or whether individuals in the sample would be more resilient and less subject to decline over such a short period of time. In specifying expected effect sizes for the functional outcome measures, the former position was adopted (ie, decline rates would follow established patterns). However, for the crucial everyday measures of IADL and ADL performance, the observed decline rates were significantly below established population norms. At 12 months, only 25% of participants experienced a 2-point or greater drop in the 36-point IADL scale, while by 24 months 28% had experienced this small increase in dependency. For the 30-point ADL performance scale, 6% were more dependent at 12 months and 8% at 24 months. Prior longitudinal research on cognitively demanding measures of everyday functioning indicates that age-related decline occurs later for these tasks than for the more basic abilities that were the focus of training. Reliable age-related decline on everyday problem-solving tasks has been shown not to occur until individuals are in their mid seventies, whereas declines on basic abilities such as reasoning and memory typically occur in their mid sixties.58
In summary, it is clear that proximal training effects occurred, that they continued (albeit at lower levels) through 24 months, and that a significant segment of trained individuals went forward through 2 years of life with better cognitive skills than did the controls. Due to lack of functional declines thus far, it is not yet clear whether differential functional decline across treatment groups will be observed in the future as this select cohort enters more fully into an age of functional loss.
Acknowledgment
The principal investigators thank Robin Barr of the National Institute on Aging (NIA) for his authorship of the request for applications and his continuing support and helpful comments throughout the conduct of the project. The program officers at the NIA and the National Institute of Nursing Research (NINR) and the principal investigators thank the members of the ACTIVE Advisory Committee and Data and Safety Monitoring Board for their careful oversight and insightful comments and suggestions (alphabetical order): Laurence G. Branch, PhD, Duke University Medical School; Sara T. Fry, PhD, RN, Boston College School of Nursing; John J. McArdle, PhD, University of Virginia; Timothy Salthouse, PhD, Georgia Institute of Technology; Barbara Tilley, PhD, Medical University of South Carolina; May L. Wykle, PhD, RN, Case Western Reserve University. The program officers at the NIA and NINR and the principal investigators also thank the statisticians at Data Coordinating Center for their role in study design and data analysis (listed in alphabetical order): Michael Doherty, MS, Linda Kasten, MA, Carol Link, PhD, and Elizabeth Wright, PhD, as well as those previously affiliated: Henry Feldman, PhD, Ken Kleinman, ScD, and George Reed, PhD.
Funding/Support: ACTIVE is supported by grants from the National Institute on Aging and the National Institute of Nursing Research to Hebrew Rehabilitation Center for the Aged (U01 NR04507), Indiana University School of Medicine (U01 NR04508), Johns Hopkins University (U01 AG14260), New England Research Institutes (U01 AG14282), Pennsylvania State University (U01 AG14263), University of Alabama at Birmingham (U01AG14289), and University of Florida (U01 AG014276).
Footnotes
Financial Disclosure: Dr Ball owns stock in Visual Awareness Inc. which owns the patent for Useful Field of View testing and training software.
Author Contributions: The New England Research Institutes served as the coordinating center for the study and handled all data analyses as determined by the consensus of the authors; each author of the study was principal investigator of his or her site, and all had access to the data.
Study concept and design: Ball, Jobe, Leveck, Marsiske, Mom’s, Rebok, Smith, Tennstedt, Unverzagt, Willis.
Acquisition of data: Ball, Marsiske, Morris, Rebok, Smith, Unverzagt, Willis.
Analysis and interpretation of data: Ball, Berch, Helmers, Marsiske, Morris, Rebok, Tennstedt, Unverzagt, Willis.
Drafting of the manuscript: Ball, Berch, Helmers, Marsiske, Morris, Rebok, Smith, Tennstedt.
Critical revision of the manuscript for important intellectual content: Ball, Jobe, Leveck, Marsiske, Rebok, Tennstedt, Unverzagt, Willis.
Statistical expertise: Marsiske, Tennstedt, Willis.
Obtained funding: Ball, Berch, Helmers, Jobe, Marsiske, Morris, Rebok, Smith, Tennstedt, Unverzagt, Willis.
Administrative, technical, or material support: Ball, Berch, Helmers, Marsiske, Morris, Smith, Tennstedt, Unverzagt.
Study supervision: Ball, Jobe, Leveck, Marsiske, Rebok, Smith, Tennstedt, Unverzagt, Willis.
Members of the ACTIVE Study Group: Participating Centers and Investigators (alphabetical by center): Hebrew Rehabilitation Center for the Aged: John N. Morris, PhD, Richard Jones, ScD, Suzanne Leveille, PhD, Paul Malloy, PhD, Adrienne Rosenberg, MA; Indiana University School of Medicine: David M. Smith, MD, Frederick W. Unverzagt, PhD, Kathy E. Johnson, PhD, Ralph W. Swindle, PhD, Morris Weinberger, PhD, Fredric D. Wolinsky, PhD, Elizabeth Wells, BA; Johns Hopkins University: George W. Rebok, PhD, Jason Brandt, PhD, Mary Kay Cresci, PhD, RN, Ada Davis, PhD, RN, Joseph Gallo, MD, MPH, Pearl German, PhD, Cynthia Steele, PhD, MPH, RN, Laura Talbot, PhD, EdD, RN, Kevin M. Alford, BS; New England Research Institutes (Data Coordinating Center): Sharon Tennstedt, PhD, Elizabeth Wright, PhD, Carol Link, PhD, Patricia Forde, BS; Pennsylvania State University: Sherry L. Willis, PhD, Frank Ahern, PhD, Gretchen Cornwall, PhD, Toni Miles, PhD, K. Warner Schaie, PhD, Keith Whitfield, PhD, Susan Youtz, PhD, Steve Zarit, PhD, Jeannie McKenzie, DrPH, Pam Davis, MEd; University of Alabama at Birmingham: Karlene Ball, PhD, Linda Duke, PhD, Jerri Edwards, PhD, Gerald McGwin, PhD, Cynthia Owsley, PhD, MSPH, Edward Rickert, PhD, Daniel Roenker, PhD, Jeff Roseman, MD, David Roth, PhD, Michael Sloane, PhD, David Vance, PhD, Virginia Wadley, PhD, Michelle McCrary, BA, Martha Frankel, MA; University of Florida (UF)/Wayne State University (WSU): Michael Marsiske, PhD (UF), Ann L. Horgas, PhD, RN (WSU), Manfred K. Diehl, PhD (UF), Jeffrey W. Dwyer, PhD (UF), R. Darin Ellis, PhD (WSU), Melissa M. Franks, PhD (WSU), Gail A. Jensen, PhD (WSU), Gisela Labouvie-Vief, PhD (WSU), Audrey B. Anthony, BA (WSU); NIH Program Officers (alphabetical by institute): National Institute of Nursing Research: Karin F. Helmers, PhD, Mary D. Leveck, PhD; National Institute on Aging: Daniel B. Berch, PhD, Jared B. Jobe, PhD.
REFERENCES
- 1.Lowenthal MF, Berkman PC, Buehler JA, Pierce RC, Robinson BC, Trier ML. Aging and Mental Disorder in San Francisco. San Francisco, Calif: Jossey-Bass; 1967. [Google Scholar]
- 2.Wilson RS, Mendes de Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 3.Scarmeas N, Levy G, Tang MX, Manly J, Stem Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churchill JD, Galvez R, Colcombe S, Swain RA, Greenough WT. Exercise, experience, and the aging brain. Neurobiol Aging. doi: 10.1016/s0197-4580(02)00028-3. In press. [DOI] [PubMed] [Google Scholar]
- 5.Krampe RT, Ericcson KA. Maintaining excellence: deliberate practice and elite performance in young and older pianists. J Exp Psychol Gen. 1996;125:331–359. doi: 10.1037//0096-3445.125.4.331. [DOI] [PubMed] [Google Scholar]
- 6.Recanzone GH. Cerebral cortical plasticity: perception and skill acquisition. In: Gazzaniga S, editor. The New Cognitive Neurosciences. Cambridge, Mass: The MIT Press; 2000. pp. 237–247. [Google Scholar]
- 7.Callahan CM, Hall KS, Hui SL, Musick BS, Unversagt FW, Hendrie HC. Relationship of age, education, and occupation with dementia among a community-based sample of African Americans. Arch Neurol. 1996;53:134–140. doi: 10.1001/archneur.1996.00550020038013. [DOI] [PubMed] [Google Scholar]
- 8.Ball K, Owsley C. Increasing mobility and reducing accidents of older drivers. In: Schaie KW, Pietrucha M, editors. Mobility and Transportation in the Elderly. New York, NY: Springer Publishing Co Inc; 2000. pp. 213–251. [Google Scholar]
- 9.Ball KK, Beard BL, Roenker DL, Miller RL, Griggs DS. Age and visual search: expanding the useful field of view. J Opt Soc Am A. 1988;5:2210–2219. doi: 10.1364/josaa.5.002210. [DOI] [PubMed] [Google Scholar]
- 10.Bartes PB, Willis SL. Plasticity and enhancement of intellectual functioning in old age: Penn State’s Adult Development and Enrichment Project (ADEPT) In: Craik FIM, Trehub S, editors. Aging and Cognitive Processes. New York, NY: Plenum Press; 1982. pp. 353–390. [Google Scholar]
- 11.Greenberg C, Powers SM. Memory improvement among adult learners. Educ Gerontol. 1987;12:385–394. [Google Scholar]
- 12.Rebok G, Balcerak U. Memory self-efficacy and performance differences in young and old adults: effects of mnemonic training. Dev Psychol. 1989;25:714–721. [Google Scholar]
- 13.Willis SL. Cognitive training and everyday competence. In: Schaie KW, editor. Annual Review of Gerontology and Geriatrics. Vol 7. New York, NY: Springer; 1987. [PubMed] [Google Scholar]
- 14.Willis SL, Nesselroade CS. Long-term effects of fluid ability training in old-old age. Dev Psychol. 1990;26:905–910. [Google Scholar]
- 15.Yesavage JA. Nonpharmacologic treatments for memory losses with normal aging. Am J Psychiatry. 1985;142:600–605. doi: 10.1176/ajp.142.5.600. [DOI] [PubMed] [Google Scholar]
- 16.Willis SL, Schaie KW. Training the elderly on the ability factors of spatial orientation and inductive reasoning. Psychol Aging. 1986;1:239–247. doi: 10.1037//0882-7974.1.3.239. [DOI] [PubMed] [Google Scholar]
- 17.Jobe JB, Smith DM, Ball K, et al. ACTIVE: a cognitive intervention trial to promote independence in older adults. Control Clin Trials. 2001;4:453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folstein MF, Flolstein SE, McHugh PR. “Minimental state ”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Ball K, Owsley C, Stalvey B, Roenker D, Sloane M, Graves M. Driving avoidance and functional impairment in older drivers. Accid Anal Prev. 1998;30:313–322. doi: 10.1016/s0001-4575(97)00102-4. [DOI] [PubMed] [Google Scholar]
- 20.Ball K, Owsley C, Sloane M, Roenker D, Bruni J. Visual attention problems as a predictor of vehicle crashes in older drivers. Invest Ophthalmol Vis Sci. 1993;34:3110–3123. [PubMed] [Google Scholar]
- 21.Diehl M, Willis SL, Schaie KW. Everyday problem solving in older adults: observational assessment and cognitive correlates. Psychol Aging. 1995;10:478–490. doi: 10.1037//0882-7974.10.3.478. [DOI] [PubMed] [Google Scholar]
- 22.Leirer VO, Morrow DG, Pariante GM, Sheikh JI. Elders’ nonadherence, its assessment, and computer assisted instruction for medication recall training. J Am Geriatr Soc. 1988;36:877–884. doi: 10.1111/j.1532-5415.1988.tb05779.x. [DOI] [PubMed] [Google Scholar]
- 23.Owsley C, Ball K, Sloane M, Roenker D, Bruni J. Visual/cognitive correlates of vehicle accidents in older drivers. Psychol Aging. 1991;6:403–415. doi: 10.1037//0882-7974.6.3.403. [DOI] [PubMed] [Google Scholar]
- 24.Owsley C, McGwin G, Ball K. Vision impairment, eye disease, and injurious motor vehicle crashes in the elderly. Ophthalmic Epidemiol. 1998;5:101–113. doi: 10.1076/opep.5.2.101.1574. [DOI] [PubMed] [Google Scholar]
- 25.Owsley C, Ball K, McGwin G, et al. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;279:1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- 26.Willis SL, Jay GM, Diehl M, Marsiske M. Longitudinal change and prediction of everyday task competence in the elderly. Res Aging. 1992;14:68–91. doi: 10.1177/0164027592141004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmusson DX, Rebok GW, Bylsma FW, Brandt J. Effects of three types of memory training in normal elderly. Aging Neuropsychol Cogn. 1999;6:56–66. [Google Scholar]
- 28.Lachman ME, Weaver SL, Bandura M, Elliot E, Lewkowicz C. Improving memory and control beliefs through cognitive restructuring and self generated strategies. J Gerontol. 1992;47:293–299. doi: 10.1093/geronj/47.5.p293. [DOI] [PubMed] [Google Scholar]
- 29.Kliegl R, Smith J, Baltes PB. On the locus and process of magnification of age differences during mnemonic training. Dev Psychol. 1990;26:894–904. [Google Scholar]
- 30.Ball K, Beard B, Roenker D, Miller R, Ball D. Visual search: age and practice. Invest Ophthalmol Vis Sci. 1988;29:448. [Google Scholar]
- 31.Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991;5:125–142. [Google Scholar]
- 32.Rey A. L’examen psychologique dans les cas d’encephalopathie tramatique. Archives de Psychologie. 1941;28:21. [Google Scholar]
- 33.Wilson B, Cockbum J, Baddeley A. The River-mead Behavioral Memory Test. Reading, England: Thames Valley Test Co; 1985. [Google Scholar]
- 34.Gonda J, Schaie K. Schaie-Thurstone Mental Abilities Test: Word Series Test. Palo Alto, Calif: Consulting Psychologists Press; 1985. [Google Scholar]
- 35.Thurstone L, Thurstone T. Examiner Manual for the SRA Primary Mental Abilities Test (Form 10–14) Chicago, III: Science Research Associates; 1949. [Google Scholar]
- 36.Ekstrom RB, French JW, Harman H, Derman D. Kit of Factor Referenced Cognitive Tests. Revised ed. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- 37.Ball K, Owsley C. The useful field of view test: a new technique for evaluating age-related declines in visual function. J Am Optom Assoc. 1993;64:71–79. [PubMed] [Google Scholar]
- 38.Willis S, Marsiske M. Manual for the Everyday Problems Test. University Park: Pennsylvania State University; 1993. [Google Scholar]
- 39.Diehl M, Marsiske M, Horgas A, Saczynski J. Psychometric properties of the Revised Observed Tasks of Daily Living (OTDL-R). Paper presented at: Annual Meeting of the Gerontological Society of America; November 20–23, 1998; Philadelphia, Pa. [Google Scholar]
- 40.Owsley C, Sloane M, McGwin G, Ball K. Timed Instrumental Activites of Daily Living (TIADL) tasks: relationship to cognitive function and everyday performance assessments in older adults. Gerontology. doi: 10.1159/000058360. In press. [DOI] [PubMed] [Google Scholar]
- 41.Morris J, Morris S. ADL assessment measures for use with frail elders. In: Teresi J, Lawton M, Holmes D, Ory M, editors. Measurement in Elderly Chronic Care Populations. New York, NY: Springer; 1997. [Google Scholar]
- 42.Owsley C, Stalvey B, Wells J, Sloane M. Older drivers and cataract: driving habits and crash risk. J Gerontol A Biol Sci Med Sci. 1999;54:M203–M211. doi: 10.1093/gerona/54.4.m203. [DOI] [PubMed] [Google Scholar]
- 43.Morris JN, Fries BE, Steel K, et al. Comprehensive clinical assessment in community setting: applicability of the MDS-HC. J Am Geriatr Soc. 1997;45:1017–1024. doi: 10.1111/j.1532-5415.1997.tb02975.x. [DOI] [PubMed] [Google Scholar]
- 44.Blom G. Statistical Estimates and Transformed Beta Variables. New York, NY: John Wiley & Sons; 1958. [Google Scholar]
- 45.Cnaan A, Laire NM, Slasor S. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16:2349–2380. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 46.Schafer JL. Analysis of Incomplete Multivariate Data. London: Chapman & Hall; 1997. [Google Scholar]
- 47.Dudek FJ. The continuing misinterpretation of the standard error of measurement. Psychol Bull. 1979;86:335–337. [Google Scholar]
- 48.cohen RA, Van Nostrand JF. Trends in the health of older Americans: United States 1994. Vital Health Stat. 1995;3:1–328. [PubMed] [Google Scholar]
- 49.Federal Interagency Forum on Aging-Related Statistics. Older Americans 2000: Key Indicators of Well Being. Washington, DC: Us Government Printing Office; 2000. [Google Scholar]
- 50.Crum RM, Anthony JC, Basset SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 51.McHorney CA, Kosinski M, Ware JE. Comparisons of the costs and quality of norms for the SF-36 health survey collected by mail versus telephone interview: results from a national survey. Med Care. 1994;32:551–567. doi: 10.1097/00005650-199406000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Edwards JD, Wadley VG, Myers RS, Roenker DL, Cissell GM, Ball KK. The transfer of a speed of processing intervention to near and far cognitive functions. Gerontology. doi: 10.1159/000065259. In press. [DOI] [PubMed] [Google Scholar]
- 53.Schaie KW. Intellectual Development in Adulthood: The Seattle Longitudinal Study. New York, NY: Cambridge University Press; 1996. [Google Scholar]
- 54.Small BJ, Dixon RA, Hultsch DF, Hertzog C. Longitudinal changes in quantitative and qualitative indicators of word and story recall in young-old and old-old adults. J Gerontol B Psycho Sci Soc Sci. 1999;54:P107–P115. doi: 10.1093/geronb/54b.2.p107. [DOI] [PubMed] [Google Scholar]
- 55.Zelinski E, Burnright KP. Sixteen-year longitudinal and time lag changes in memory and cognition in older adults. Psychol Aging. 1997;12:503–513. doi: 10.1037//0882-7974.12.3.503. [DOI] [PubMed] [Google Scholar]
- 56.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Mahwah, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 57.Hofland BF, Willis SL, Baltes PB. Fluid intelligence performance in the elderly: intraindividual variability and conditions of assessment. J Educ Psychol. 1981;73:573–586. [Google Scholar]
- 58.Willis S, Marsiske M. Life-span perspective on practical intelligence. In: Tupper D, Cicerone K, editors. The Neuropsychology of Everyday Life. Boston, Mass: Kluwer Academic Publishers; 1990. pp. 183–198. [Google Scholar]