Abstract
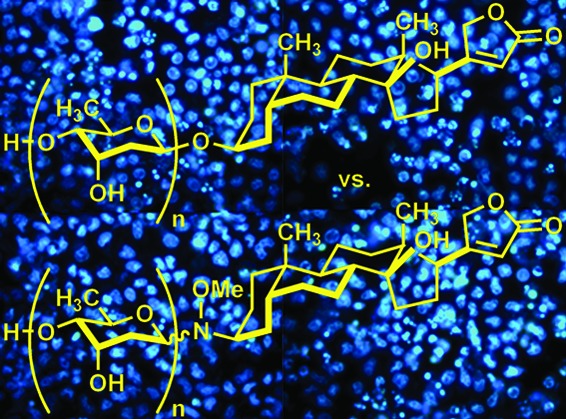
Digitoxin is a cardiac glycoside currently being investigated for potential use in oncology; however, an investigation of anticancer activity as a function of oligosaccharide chain length has not yet been performed. We generated mono-, di-, and tri-O-digitoxoside derivatives of digitoxin and compared their activities to the corresponding MeON-neoglycosides. Both classes of cardenolide derivatives display comparable oligosaccharide chain length-dependent cytotoxicity toward human cancer cell lines. Further investigation revealed that both classes of compounds induce caspase-9-mediated apoptosis in non-small cell lung cancer cells (NCI-H460). Because O-glycosides and MeON-neoglycosides share a similar mode of action, the convenience of MeON-neoglycosylation could be exploited in future SAR work to rapidly survey large numbers of carbohydrates to prioritize selected O-glycoside candidates for traditional synthesis.
Keywords: Apoptosis, caspase, digitoxin, cancer, cardiac glycoside
Digitoxin (Figure 1, 3), a naturally occurring cardiac glycoside isolated from digitalis, has been used to treat congestive heart failure and cardiac arrhythmias since the late 1700s. The canonically recognized target of digitoxin is Na+/K+-ATPase, a heterodimeric membrane protein responsible for maintaining the Na+/K+ balance across the plasma membranes of most eukaryotic cells.2 Partial Na+/K+-ATPase inhibition leads to an increase in intracellular Ca2+ concentration within myocardial cells, resulting in an increase in heart contractility. More recently, digitoxin has been shown to display significant anticancer effects, and some researchers have begun to investigate its potential use in oncology.5−8 Digitoxin inhibits cancer cell growth in vitro,9−10 and for many cell lines, it does so at concentrations commonly observed in the plasma of cardiac patients.9 Cardiac glycosides have been shown to reduce metastasis by inducing anoikis in tumor cell lines14 and to induce apoptosis in a number of malignant cell lines, often at nontoxic doses.10,18 Digitoxin's anticancer effects may in part be related to the drug's cardiotonic activity (i.e., by increasing Ca2+ concentrations). For example, sustained cardiac glycoside-induced increases in Ca2+ can trigger apoptosis in cancer cells through caspase activation.19 However, cardiac glycosides can also activate apoptosis pathways before ion currents are detected,20 consistent with other studies that indicate that cardenolide-mediated signaling and cardiotonic activity are independent.20−26
Figure 1.
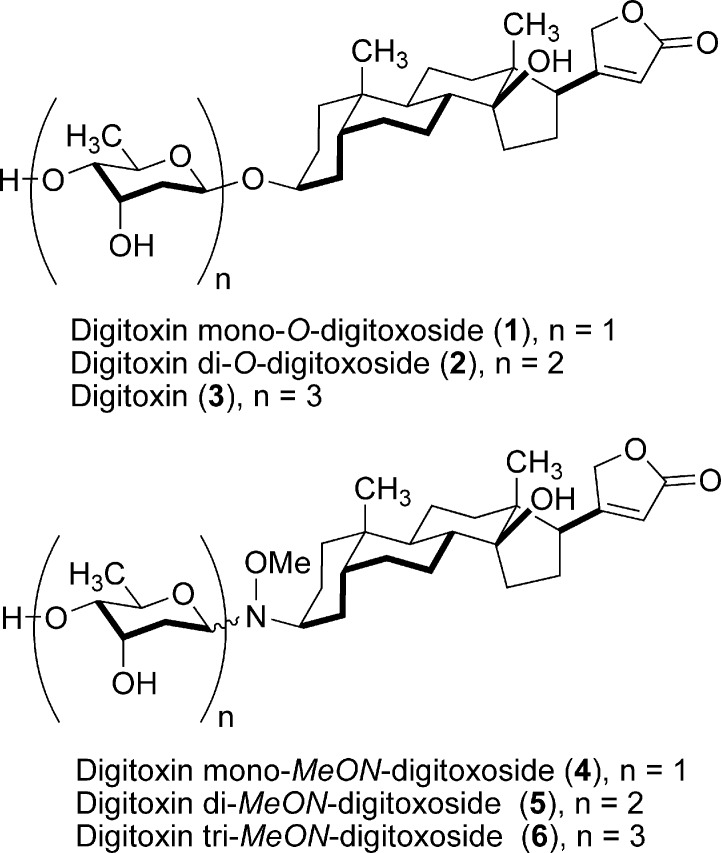
Digitoxin mono-, di-, and tri-O-digitoxosides 1−3 and mono-, di-, and tri-MeON-digitoxosides 4−6.
The carbohydrate moiety of digitoxin is critical to Na+/K+-ATPase inhibition; cardiac glycosides are invariably better Na+/K+-ATPase inhibitors than the corresponding aglycons.24,27 Because Na+/K+-ATPase represents a promising anticancer target, groups have begun to determine how cardiac glycoside carbohydrates influence anticancer properties.9,24,27,32 The difficulties inherent to chemical glycosylation have limited the progress made at elucidating the relationship between carbohydrate structure and anticancer activity in natural digitoxin O-glycosides. MeON-neoglycosylation, a chemoselective glycosylation methodology that employs unprotected, unactivated reducing sugars,33 has enabled the rapid glycorandomization of digitoxin analogues to survey the influence of differential glycosylation.24 Subtle sugar modifications dramatically influenced the cytotoxicities of digitoxin MeON-neoglycosides, and several analogues displayed enhanced potency and tumor selectivity relative to the parent natural product.24 A combination of NMR experiments, ab initio calculations, and crystallographic studies have revealed that non-natural MeON-neoglycosides display similar conformational properties to their natural O-glycoside counterparts.24,38 It remains unclear, however, whether or not MeON-neoglycosides and O-glycosides display comparable biological activities toward human cancer cells. Also, while a number of structure−activity relationship (SAR) studies of digitoxin have been conducted, to our knowledge, anticancer activity as a function of oligosaccharide chain length has not been investigated.
Thus, we initiated a study to compare directly the biological effect of O-glycosidic versus MeON-neoglycosidic linkages in mono-, di-, and tridigitoxoside analogues of digitoxin (Figure 1). Our results suggest that digitoxin O-glycosides and MeON-neoglycosides may share a common mechanism of action in human ovarian adenocarcinoma cells (SK-OV-3), human non-small cell lung carcinoma cells (NCI-H460), and human intestinal epithelial cancer cells (HT-29). While MeON-neoglycosides are less active, both classes of compounds induce apoptosis in NCI-H460 cells via the caspase-9-mediated intrinsic pathway, and for both classes of compounds, pro-apoptotic activity is inversely correlated with oligosaccharide chain length. On the basis of these findings, future carbohydrate SAR work might be expedited by conducting rapid MeON-neoglycosylation-based screens of carbohydrate structures to provide a means for identifying corresponding O-glycoside structures worthy of synthesis and further investigation as anticancer agents.
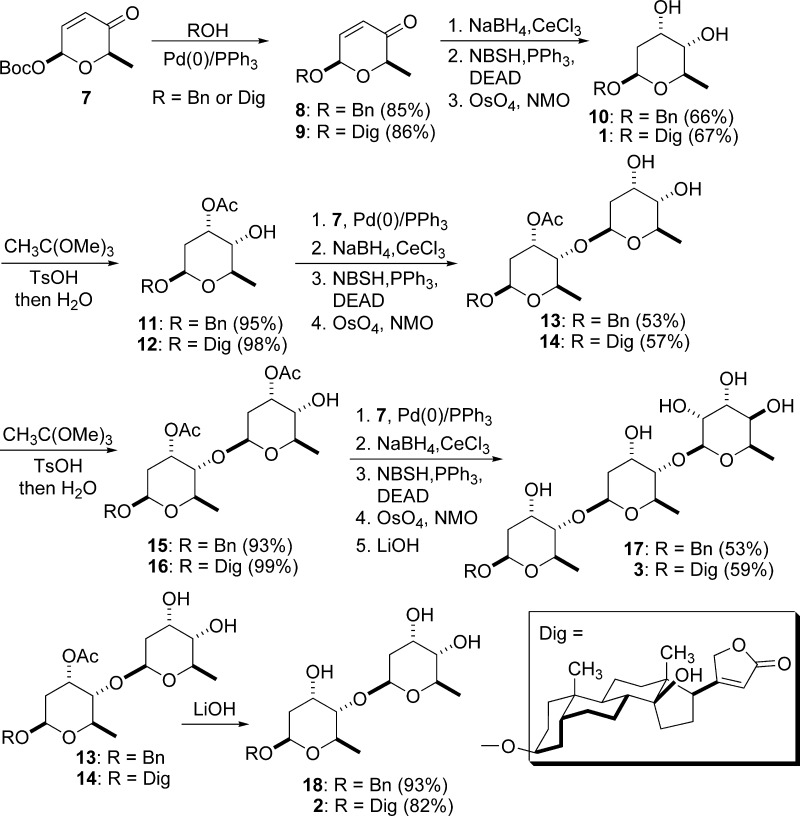
Previously, we have demonstrated the utility of our “de novo” carbohydrate synthetic methodology for the synthesis of natural and unnatural oligosaccharides.39−44 We synthesized digitoxin (3) and its mono- (1) and didigitoxoside (2) analogues as shown in Scheme 1.45−48 Briefly, palladium-catalyzed glycosylation of pyranone 7 with benzyl alcohol or digitoxigenin provided the pyranone 8 or 9, which then underwent Luche reduction, reductive rearrangement of allylic alcohols, and dihydroxylation to provide exclusively the diol 10 or digitoxin monosaccharide 1. A regioselective protection of diols 10 or 1 was achieved by the formation of an orthoester and regioselective ring opening to give alcohol 11 or 12, respectively. By applying the same reaction sequence above to alcohol 11 or 12, the disaccharide 13 or 14 was prepared. The regioselective protection of diols 13 or 14 gave the alcohol 15 or 16, which then underwent palladium-catalyzed glycosylation, Luche reduction, 1,3-allylic rearrangement, dihydroxylation, and acetate hydrolysis to provide the digitoxin trisaccharide 17 or 3. The digitoxin disaccharide 18 or 2 was prepared by basic hydrolysis of the disaccharide 13 or 14.
Scheme 1. Synthesis of Digitoxin Mono-, Di-, and Tri-O-digitoxosides 1−3.
NBSH, o-nitrobenzenesulfonylhydrazide; DEAD, diethyl azodicarboxylate; and NMO, N-methylmorpholine-N-oxide.
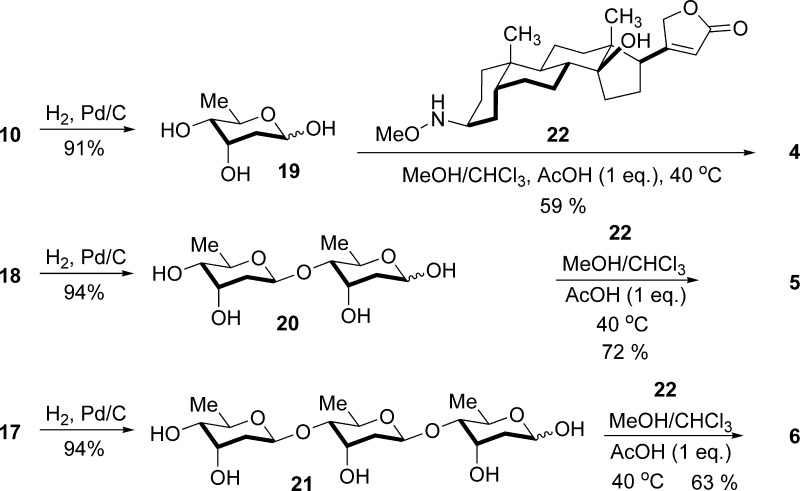
To generate digitoxin MeON-neoglycosides, we synthesized free, reducing digitoxose mono-, di-, and tridigitoxosides (19, 20, and 21, respectively, Scheme 2) as well as secondary oxyamine 22.24 Secondary oxyamines can be chemoselectively glycosylated to provide hydrolytically stable glycosides without sugar activation or protection.33 While our initial attempts to generate 4−6 using published conditions24 failed, in the presence of AcOH and MeOH, aglycon 22 provided the corresponding MeON-neoglycosides 4−6 in moderate yields when treated with 19−21. MeON-neoglycosides 4−6 exist predominantly in the β-pyranose form in solution.52
Scheme 2. Synthesis of Digitoxin Mono-, Di-, and Tri-MeON-digitoxosides 1−3.
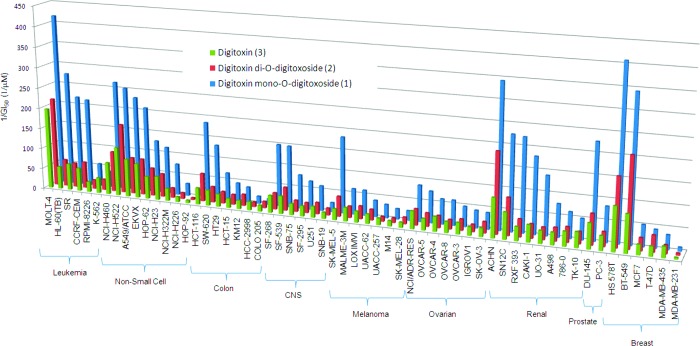
Because to our knowledge an investigation of anticancer activity as a function of oligosaccharide chain length has not yet been performed in digitoxin O-glycosides, we began by assessing the growth inhibitory effects of 1−3 in the National Cancer Institute's (NCI) panel of 60 human cancer cell lines (Figure 2). GI50 values ranged from 2 to 223 nM; such a range in susceptibilities is not unexpected since cancer cell lines express different levels of Na+/K+-ATPase isozymes.53,54 In most cases, for a given cell line, mono-O-digitoxoside 1 was a more potent inhibitor of tumor cell growth by a factor of 2−7 than the corresponding di- or tri-O-digitoxosides (2 and 3). Interestingly, these findings are consistent with a comparative molecular similarity index analysis for Na+/K+-ATPase inhibition, which predicted that for digitoxin the digitoxose residue closest to the aglycon (the α-sugar) was essential for Na+/K+-ATPase inhibition, whereas further sugar substitution was detrimental.27 These findings are also consistent with the fact that Thorson and co-workers have identified a number of digitoxin MeON-neoglycosidic monodigitoxoside derivatives that display improved cytotoxic properties relative to the parent trisaccharide natural product.24 Since MeON-neoglycosides display similar conformational properties to their O-glycoside counterparts,24,38 we became interested in learning whether the activity of MeON-neoglycosides 4−6 would mirror the oligosaccharide chain-length dependent activity of O-glycosides 1−3.
Figure 2.
Summary of GI50 data from growth inhibition assays. Reciprocal GI50 values are displayed for clarity.
First, we compared the activity of O-glycosides 1−3 and corresponding MeON-neoglycosides 4−6 in cytotoxicity assays on a small subset of human cancer cell lines from the NCI panel (Figure 3). Consistent with the growth inhibition assay results shown in Figure 2, mono-O-digitoxoside 1 was at least as potent a cytotoxin as the di- or tri-O-digitoxosides (2 and 3) in all cell lines. Interestingly, the corresponding neoglycosides 4−6 displayed a qualitatively similar potency trend (mono- > or ≈ di- and tridigitoxoside) for three of the four cell lines examined (SK-OV-3, NCI-H460, and HT-29). Despite the fact that neoglycosides 4−6 were less potent than the corresponding O-glycosides 1−3, the commonalities in oligosaccharide chain length-dependent potency trends suggest that O-glycosides and MeON-neoglycosides share a similar mechanism of action.
Figure 3.
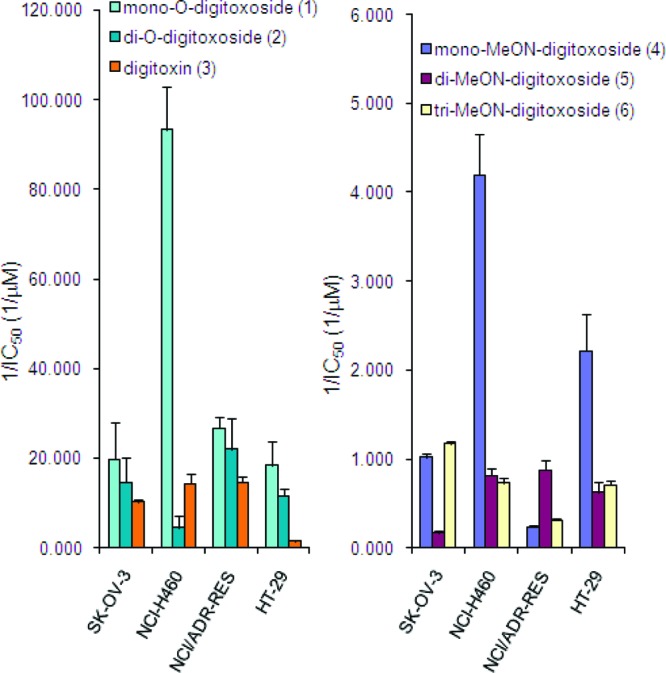
Summary of IC50 data from cytotoxicity assays. Reciprocal IC50 values are displayed for clarity; standard errors are depicted with error bars.
While the specific mechanism for cardenolide-induced cytotoxicity remains controversial, a growing body of literature demonstrates that digitoxin can induce apoptosis in malignant cell lines. However, few studies have been performed in non-small cell lung cancer cells, despite the fact that lung cancers have among the lowest 5 year survival rates. Moreover, the ability of digitoxin MeON-neoglycoside derivatives to induce apoptosis has not been assessed in any cancer cell line. Thus, to investigate further the possibility of a shared mechanism of action, we measured the ability of O-digitoxosides 1−3 and MeON-digitoxosides 4−6 to induce apoptosis in NCI-H460 (non-small cell lung cancer) cells.
Both classes of compounds induced apoptosis in NCI-H460 cells (Figure 4). This is the first report of MeON-neoglycoside-induced apoptosis. Consistent with our cytotoxicity results (Figure 3), we observed that for both compound classes the monodigitoxosides were more potent inducers of apoptosis than the corresponding di- or tri-digitoxosides. We also observed a higher percentage of apoptotic cells with O-glycosides 1−3 than with MeON-neoglycosides 4−6, again mirroring the trend observed in cytotoxicity assays. Because apoptosis is mediated primarily through the action of the caspase family of proteins,56 we assessed whether inhibiting these pathways using pharmacological inhibitors would protect NCI-H460 cells from undergoing apoptosis. We used the pan-caspase inhibitor ZVAD-FMK to inhibit both intrinsic and extrinsic pathways of apoptosis. Indeed, preincubation with ZVAD-FMK completely abrogated apoptosis induced by 1−6 even at the highest dose tested (Figure 4, 100 nM + ZV). These results reveal that for both classes of compounds the mode of drug action is dependent upon caspases.
Figure 4.
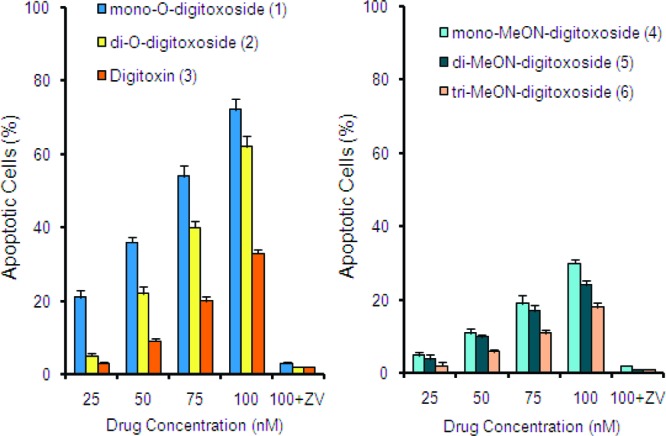
O-Digitoxosides 1−3 and MeON-digitoxosides 4−6 induce apoptosis in NCI-H460 non-small cell lung cancer cells. Apoptotic cells were quantified by Hoechst dye staining and microscopy. ZV = pan-caspase inhibitor ZVAD-FMK.
In the extrinsic pathway of apoptosis, the activation of pro-apoptotic receptors on the cell surface leads to caspase-8 activation and cell death. In the intrinsic pathway of apoptosis, intracellular signals lead to the release of pro-apoptotic proteins that activate caspase-9, eventually causing apoptosis.57 Because both O-digitoxosides 1−3 and MeON-digitoxosides 4−6 induced apoptotic cell death, we investigated the role of specific caspases involved in the process. Using species-specific fluorophore substrates, we assayed for both caspase-8 (extrinsic pathway) and caspase-9 (intrinsic pathway) to delineate the predominant mechanism of action of drugs 1 and 4 (Figure 5). Our results indicate that both O-digitoxoside 1 and MeON-digitoxoside 4 predominantly activated caspase-9, with very little change in caspase-8 activity. These results clearly implicate the intrinsic pathway of apoptosis in the mechanism of action for these compounds. However, the specific mechanism by which 1 and 4 activate the intrinsic pathway of apoptosis remains to be elucidated. It is possible that other proteins, such as Bcl-2, may be involved.61−62
Figure 5.
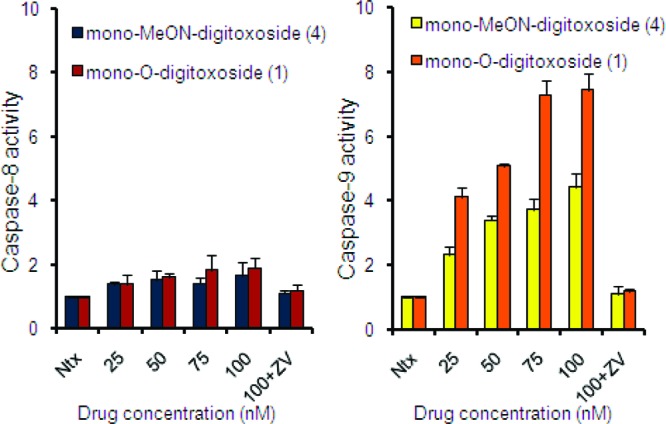
O-Digitoxoside 1 and MeON-digitoxoside 4 activate caspase-9, not caspase-8, in NCI-H460 non-small cell lung cancer cells. Cell lysates were incubated in fluorometric substrates for either caspase-8 (IETD-AFC) or caspase-9 (LEHD-AFC); samples were then read using a fluorescence plate reader. Ntx = no treatment. ZV = pan-caspase inhibitor ZVAD-FMK.
In summary, we have investigated for the first time the anticancer activity of digitoxin derivatives as a function of oligosaccharide chain length; we have also directly compared the biological activities of O-glycoside and MeON-neoglycoside drugs. O-Digitoxosides 1−3 and MeON-digitoxosides 4−6 both display comparable oligosaccharide chain length-dependent cytotoxicity toward human cancer cells. Further investigation revealed that both classes of compounds induce apoptosis in NCI-H460 cells; for both classes, apoptosis is driven primarily through the caspase-9-mediated intrinsic pathway of cell death. This marks the first study where a MeON-neoglycoside has been observed to induce apoptosis. Our results pave the way for the development of novel digitoxin analogues with greater antitumor potential than naturally occurring derivatives; more broadly, our results suggest a general strategy for carbohydrate SAR work. Although MeON-digitoxosides 4−6 were less potent than the corresponding O-digitoxosides in our study, MeON-neoglycosides can be made extremely efficiently.24 Given the structural similarities between O-glycosides and MeON-neoglycosides,24,38 as well as the parallel biological activities indicated above, the convenience of MeON-neoglycosylation could be exploited to rapidly survey large numbers of carbohydrates to prioritize selected O-glycoside candidates for traditional synthesis.
Acknowledgments
We thank Noël Peters (Keck-UWCCC Small Molecule Screening Facility) for performing cytotoxicity assays and Prof. Douglas Latch (Seattle University) for LC-MS assistance.
Supporting Information Available
Assay protocols and synthetic procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
J.M.L. was supported by the Camille and Henry Dreyfus Foundation, the Research Corporation, the MJ Murdock Charitable Trust, and Seattle University. G.A.O. was supported by the NIH (GM088839 and 090259) and the NSF (CHE-0749451).
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Kaplan J. H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 2002, 71, 511–535. [DOI] [PubMed] [Google Scholar]
- Haux J. Digitalis; impinges on more than just the (ion-) pump. Med. Hypotheses 2002, 59, 781–782. [DOI] [PubMed] [Google Scholar]
- Stenkvist B. Cardenolides and cancer. Anti-Cancer Drugs 2001, 12, 635–636. [DOI] [PubMed] [Google Scholar]
- Newman R. A.; Yang P.; Pawlus A. D.; Block K. I. Cardiac Glycosides as Novel Cancer Therapeutic Agents. Mol. Interventions 2008, 8, 36–49. [DOI] [PubMed] [Google Scholar]
- Lopez-Lazaro M.; Pastor N.; Azrak S. S.; Ayuso M. J.; Austin C. A.; Cortes F. Digitoxin inhibits the growth of cancer cell lines at concentrations commonly found in cardiac patients. J. Nat. Prod. 2005, 68, 1642–1645, and references cited therein. [DOI] [PubMed] [Google Scholar]
- Yeh J.-Y.; Huang W. J.; Kan S.-F.; Wang P. S. Inhibitory effects of digitalis on the proliferation of androgen dependent and independent prostate cancer cells. J. Urol. 2001, 166, 1937–1942. [PubMed] [Google Scholar]
- Simpson C. D.; Mawji I. A.; Anyiwe K.; Williams M. A.; Wang X.; Venugopal A. L.; Gronda M.; Hurren R.; Cheng S.; Serra S.; Zavareh R. B.; Datti A.; Wrana J. L.; Ezzat S.; Schimmer A. D. Inhibition of the Sodium Potassium Adenosine Triphosphatase Pump Sensitizes Cancer Cells to Anoikis and Prevents Distant Tumor Formation. Cancer Res. 2009, 69, 2739–2747. [DOI] [PubMed] [Google Scholar]
- Haux J.; Lam M.; Marthinsen A. B. L.; Strickert T.; Lundgren S. Digitoxin, in non toxic concentrations, induces apoptotic cell death in Jurkat T cells in vitro. Z. Onckol/J. Oncol. 1999, 1, 14–20. [Google Scholar]
- McConkey D. J.; Lin Y.; Nutt L. K.; Ozel H. Z.; Newman R. A. Cardiac Glycosides Stimulate Ca2+ Increases and Apoptosis in Androgen-independent, Metastatic Human Prostate Adenocarcinoma Cells. Cancer Res. 2000, 60, 3807–3812. [PubMed] [Google Scholar]
- Liu J.; Tian J.; Haas M.; Shapiro J. I.; Askari A.; Xie Z. Ouabain Interaction with Cardiac Na+/K+-ATPase Initiates Signal Cascades Independent of Changes in Intracellular Na+ and Ca2+ Concentrations. J. Biol. Chem. 2000, 275, 27838–27844. [DOI] [PubMed] [Google Scholar]
- Haas M.; Askari A.; Xie Z. Involvement of Src and Epidermal Growth Factor Receptor in the Signal-transducing Function of Na+/K+-ATPase. J. Biol. Chem. 2000, 275, 27832–27837. [DOI] [PubMed] [Google Scholar]
- Xie Z.; Askari A. Na(+)/K(+)-ATPase as a signal transducer. Eur. J. Biochem. 2002, 269, 2434–2439. [DOI] [PubMed] [Google Scholar]
- Saunders R.; Scheiner-Bobis G. Ouabain stimulates endothelin release and expression in human endothelial cells without inhibiting the sodium pump. Eur. J. Biochem. 2004, 271, 1054–1062. [DOI] [PubMed] [Google Scholar]
- Langenhan J. L.; Peters N. R.; Guzei I. A.; Hoffman F. M.; Thorson J. S. Enhancing the anticancer properties of cardiac glycosides by neoglycorandomization. Proc. Natl. Acad. Sci. USA 2005, 102, 12305–12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M.; Wang H.; Tian J.; Xie Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J. Biol. Chem. 2002, 277, 18694–18702. [DOI] [PubMed] [Google Scholar]
- Kometiani P.; Liu L.; Askari A. Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol. Pharmacol. 2005, 67, 929–936. [DOI] [PubMed] [Google Scholar]
- Paula S.; Tabet M. R.; Ball W. J. Jr. Interactions between cardiac glycosides and sodium/potassium-ATPase: three-dimensional structure-activity relationship models for ligand binding to the E2-Pi form of the enzyme versus activity inhibition. Biochemistry 2005, 44, 498–510. [DOI] [PubMed] [Google Scholar]
- Langenhan J. M.; Engle J. M.; Slevin L. K.; Fay L. R.; Lucker R. W.; Smith K. R.; Endo M. M. Modifying the glycosidic linkage in digitoxin analogs provides selective cytotoxins. Bioorg. Med. Chem. Lett. 2008, 18, 670–673. [DOI] [PubMed] [Google Scholar]
- Peri F.; Dumy P.; Mutter M. Chemo- and stereoselective glycosylation of hydroxylamino derivatives: A versatile approach to glycoconjugates. Tetrahedron 1998, 54, 12269–12278. [Google Scholar]
- Peri F.; Jimenez-Barbero J.; Garcia-Aparicio V.; Tvaroska I.; Nicotra F. Synthesis and Conformational Analysis of Novel N(OCH3)-linked Disaccharide Analogues. Chem.—Eur. J. 2004, 10, 1433–1444. [DOI] [PubMed] [Google Scholar]
- Yu X; O'Doherty G. A. De novo asymmetric synthesis and biological evaluation of the trisaccharide portion of PI-080 and vineomycin B2. Org. Lett. 2008, 10, 4529–4532. [DOI] [PubMed] [Google Scholar]
- Zhou M.; O'Doherty G. A. De novo synthesis of the trisaccharide subunit of landomycins A and E. Org. Lett. 2008, 10, 2283–2286. [DOI] [PubMed] [Google Scholar]
- Babu R. S.; Guppi S. R.; O'Doherty G. A. Synthetic Studies Towards Mannopeptimycin-E: Synthesis of a O-Linked Tyrosine 1,4-a,a-Manno,Manno-Pyanosyl-Pyranoside. Org. Lett. 2006, 8, 1605–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu R. S.; Zhou M.; O'Doherty G. A. De-Novo Synthesis of Oligosaccharides Using a Palladium Catalyzed Glycosylation Reaction. J. Am. Chem. Soc. 2004, 126, 3428–3429. [DOI] [PubMed] [Google Scholar]
- Zhou M.; O'Doherty G. A. A stereoselective synthesis of digitoxin and digitoxigen mono- and bisdigitoxoside from digitoxigenin via a palladium-catalyzed glycosylation. Org. Lett. 2006, 8, 4339–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M.; O'Doherty G. A. De Novo Asymmetric Syntheses of Digioxose and Digitoxin. J. Org. Chem. 2007, 73, 2485–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M.; O'Doherty G. A. The De Novo Synthesis of Oligosaccharides: Application to the Medicinal Chemical Study of Digitoxin. Curr. Top. Med. Chem. 2008, 8, 114–125. [DOI] [PubMed] [Google Scholar]
- For another synthesis of digitoxin and mono-/bis-digitoxoside derivatives, see; Wiesner K.; Tsai T. Y. R.; Jin H. On Cardioactive Steroids. XVI. Stereoselective β-Glycosylation of Digitoxose: The Synthesis of Digitoxin. Helv. Chim. Acta. 1985, 68, 300–314. [Google Scholar]
- The equilibrium distribution of α- and β-pyranose and furanose isomers is dependent on sugar identity; usually, the β-pyranose form predominates. See ref (24).
- Mijatovic T.; Roland I.; Van Quaquebeke E.; Nilsson B.; Mathieu A.; Van Vynckt F.; Darro F.; Blanco G.; Facchini V.; Kiss R. The alpha1 subunit of the sodium pump could represent a novel target to combat non-small cell lung cancers. J. Pathol. 2007, 212, 170–179. [DOI] [PubMed] [Google Scholar]
- Mobasheri A.; Fox R.; Evans I.; Cullingham F.; Martin-Vasallo P.; Foster C. S. Epithelial Na, K-ATPase expression is down-regulated in canine prostate cancer; a possible consequence of metabolic transformation in the process of prostate malignancy. Cancer Cell Int. 2003, 3, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lazaro M. Digitoxin as an anticancer agent with selectivity for cancer cells: Possible mechanisms involved. Expert Opin. Ther. Targets 2007, 11, 1043–1053. [DOI] [PubMed] [Google Scholar]
- Ghavami S.; Hashemi M.; Ande S. R.; Yeganeh B.; Ziao W.; Eshraghi M.; Bus C. J.; Kadkhoda K.; Wiechec E.; Halayko A. J.; Los M. Apoptosis and cancer: mutations within caspase genes. J. Med. Genet. 2009, 46, 497–510. [DOI] [PubMed] [Google Scholar]
- Iyer A. K.; Azad N.; Wang L.; Rojanasakul Y. Role of S-nitrosylation in apoptosis resistance and carcinogenesis. Nitric Oxide 2008, 19, 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessene G.; Czabotar P. E.; Colman P. M. BCL-2 family antagonists for cancer therapy. Nat. Rev. Drug Discovery 2008, 7, 989–1000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.