Abstract
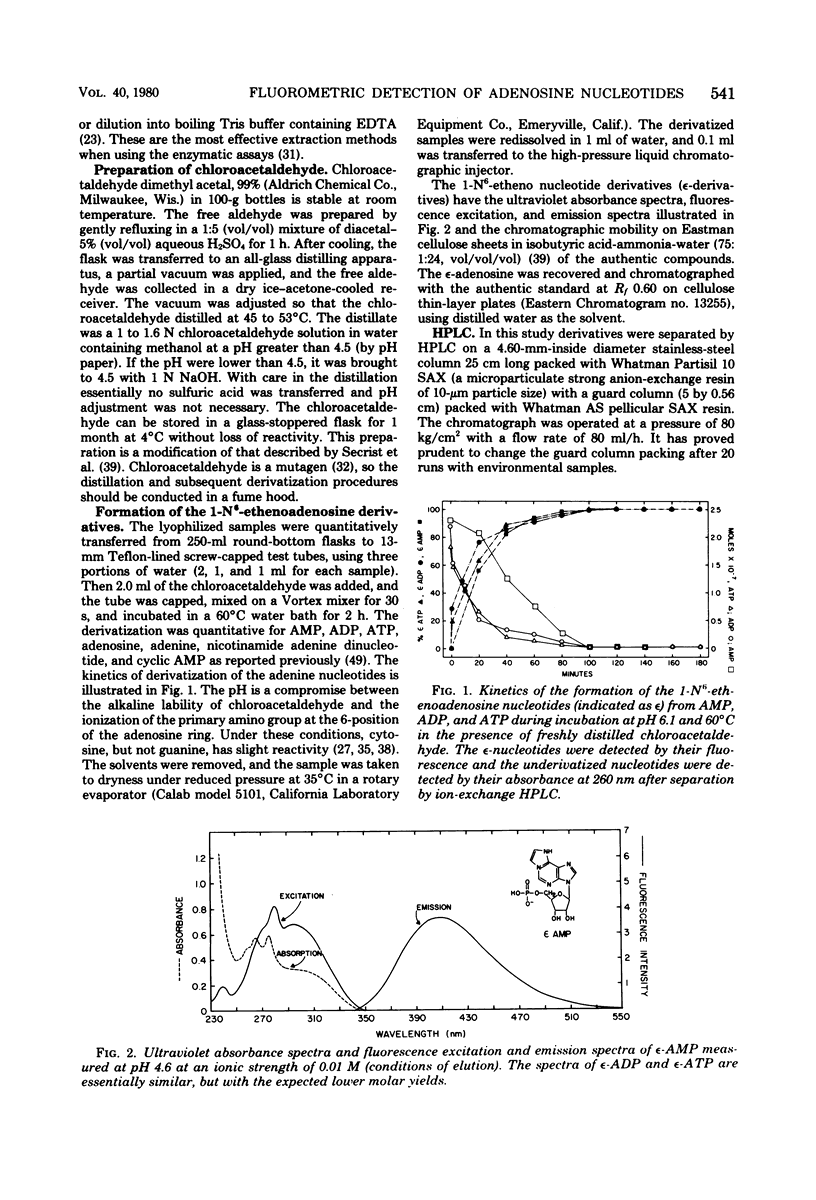
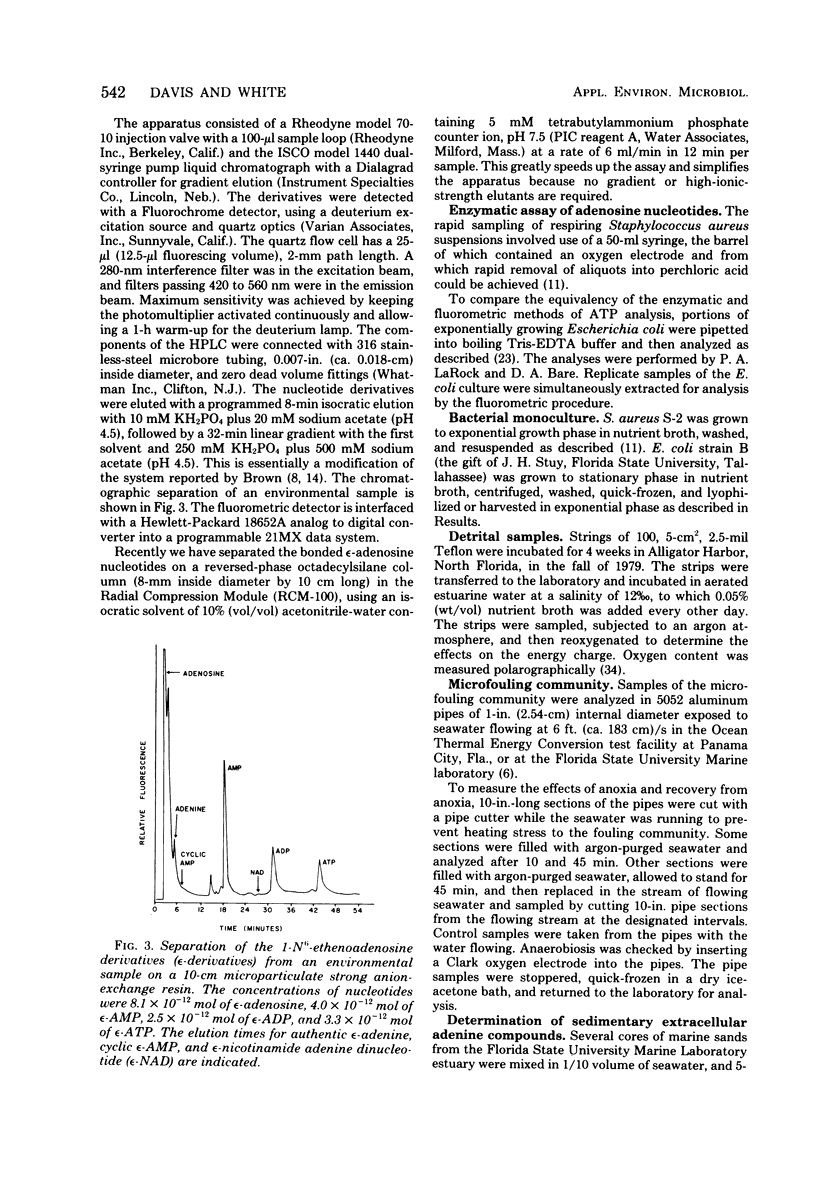
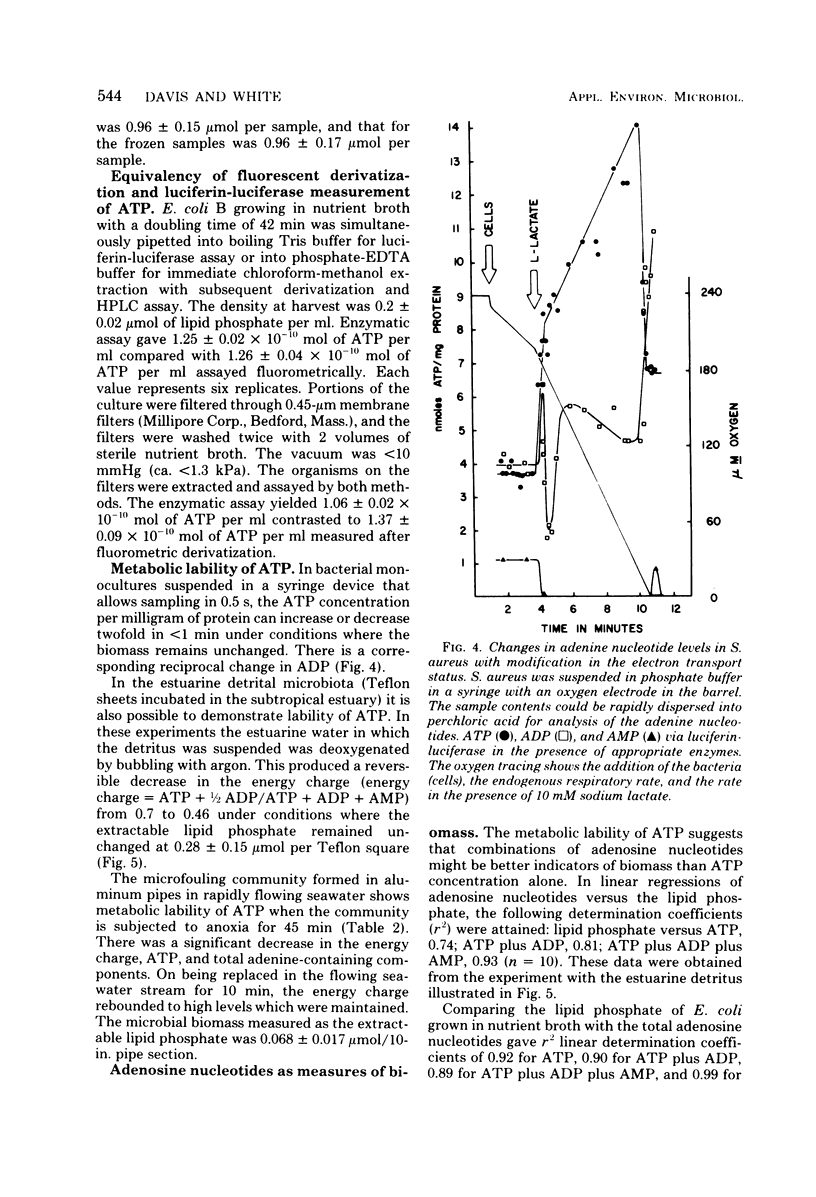
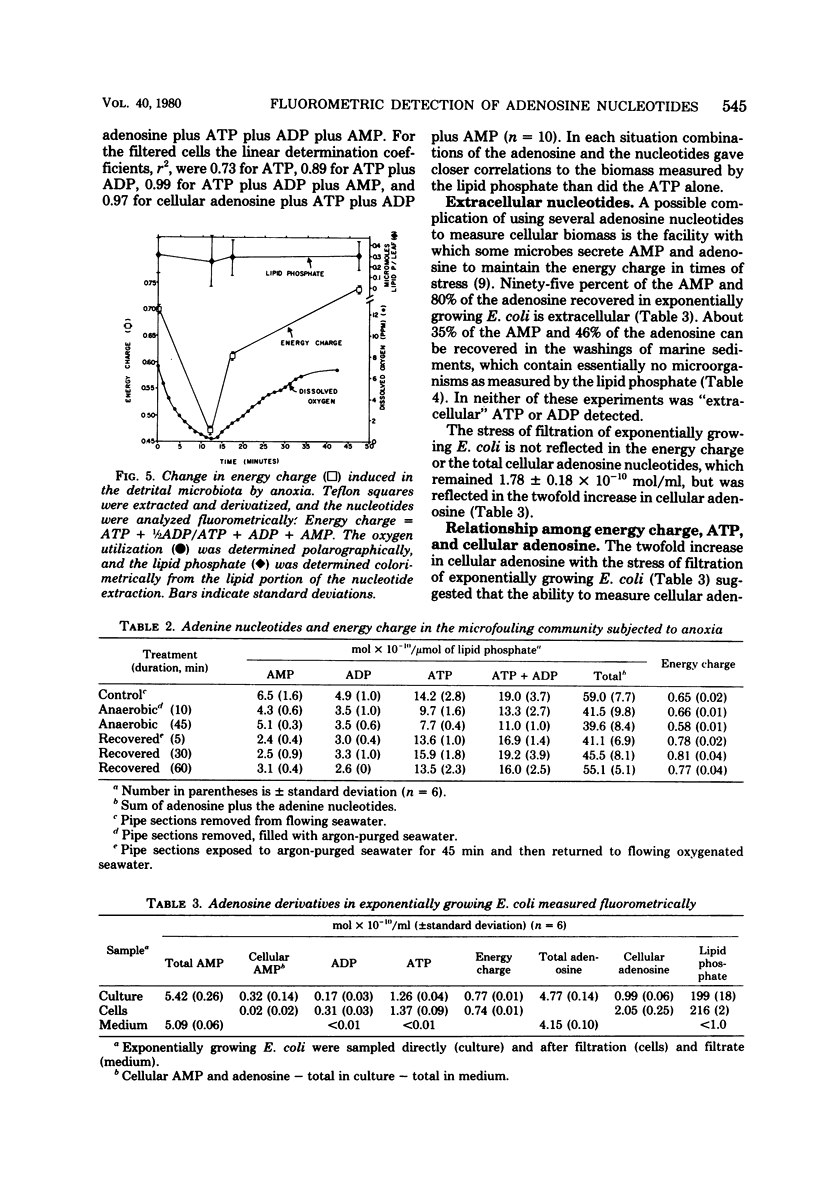
Adenosine, adenine, cyclic adenosine monophosphate (AMP), AMP, nicotinamide adenine dinucleotide, adenosine diphosphate, and adenosine triphosphate (ATP) were recovered quantitatively from aqueous portions of lipid extracts of microfouling, detrital, and sedimentary microbial communities. These could be detected quantitatively in the picomolar range by forming their 1-N6-etheno derivatives and analyzing by high-pressure liquid chromatography with fluorescence detection. Lipid extraction and subsequent analysis allowed the simultaneous measurement of the microbial community structure, total microbial biomass with the quantitative recovery of the adenine-containing cellular components, which were protected from enzymatic destruction. This extraction and fluorescent derivatization method showed equivalency with the luciferin-luciferase method for bacterial ATP measurements. Quick-freezing samples in the field with dry ice-acetone preserved the ATP and energy charge (a ratio of adenosine nucleotides) for analysis at remote laboratories. The metabolic lability of ATP in estuarine detrital and microfouling communities, as well as bacterial monocultures of constant biomass, showed ATP to be a precarious measure of biomass under some conditions. Combinations of adenosine and adenine nucleotides gave better correlations with microbial biomass measured as extractable lipid phosphate in the detrital and microfouling microbial communities than did ATP alone. Stresses such as anoxia or filtration are reflected in the rapid accumulation of intracellular adenosine and the excretion of adenosine and AMP into the surrounding milieu. Increases in AMP and adenosine may prove to be more sensitive indicators of metabolic status than the energy charge.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aftring R. P., Taylor B. F. Assessment of microbial fouling in an ocean thermal energy conversion experiment. Appl Environ Microbiol. 1979 Oct;38(4):734–739. doi: 10.1128/aem.38.4.734-739.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov S. I., Sosunova L. N. O metodakh vydeleniia ATF iz mikroorganizmov. Mikrobiologiia. 1975 Nov-Dec;44(6):1107–1111. [PubMed] [Google Scholar]
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Azam F., Hodson R. E. Dissolved ATP in the sea and its utilisation by marine bacteria. Nature. 1977 Jun 23;267(5613):696–698. doi: 10.1038/267696a0. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bobbie R. J., White D. C. Characterization of benthic microbial community structure by high-resolution gas chromatography of Fatty Acid methyl esters. Appl Environ Microbiol. 1980 Jun;39(6):1212–1222. doi: 10.1128/aem.39.6.1212-1222.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. R. The rapid separation of nucleotides in cell extracts using high-pressure liquid chromatography. J Chromatogr. 1970 Oct 21;52(2):257–272. doi: 10.1016/s0021-9673(01)96573-2. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhople A. M., Hanks J. H. Quantitative extraction of adenosine triphosphate from cultivable and host-grown microbes: calculation of adenosine triphosphate pools. Appl Microbiol. 1973 Sep;26(3):399–403. doi: 10.1128/am.26.3.399-403.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenbaum P. E., Keyser P. D., White D. C. Role of vitamin K2 in the organization and function of Staphylococcus aureua membranes. J Bacteriol. 1975 Feb;121(2):442–449. doi: 10.1128/jb.121.2.442-449.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenbaum P. E., White D. C. Role of lipid in the formation and function of the respiratory system of Staphylococcus aureus. Ann N Y Acad Sci. 1974 Jul 31;236(0):115–123. doi: 10.1111/j.1749-6632.1974.tb41486.x. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Maitra P. K. Control of respiration and metabolism in growing Klebsiella aerogenes. The role of adenine nucleotides. Biochem J. 1969 May;112(5):647–656. doi: 10.1042/bj1120647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwick R. A., Brown P. R. The performance of microparticle chemically-bonded anion-exchange resins in the analysis of nucleotides. J Chromatogr. 1975 Oct 29;112:650–662. doi: 10.1016/s0021-9673(00)99994-1. [DOI] [PubMed] [Google Scholar]
- Herron J. S., King J. D., White D. C. Recovery of Poly-beta-Hydroxybutyrate from Estuarine Microflora. Appl Environ Microbiol. 1978 Feb;35(2):251–257. doi: 10.1128/aem.35.2.251-257.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holms W. H., Hamilton I. D., Robertson A. G. The rate of turnover of the adenosine triphosphate pool of Escherichia coli growing aerobically in simple defined media. Arch Mikrobiol. 1972;83(2):95–109. doi: 10.1007/BF00425016. [DOI] [PubMed] [Google Scholar]
- Karl D. M., Holm-Hansen O. Effects of luciferin concentration on the quantitative assay of ATP using crude luciferase preparations. Anal Biochem. 1976 Sep;75(1):100–112. doi: 10.1016/0003-2697(76)90060-9. [DOI] [PubMed] [Google Scholar]
- Karl D. M. Occurrence and ecological significance of GTP in the ocean and in microbial cells. Appl Environ Microbiol. 1978 Aug;36(2):349–355. doi: 10.1128/aem.36.2.349-355.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. D., White D. C., Taylor C. W. Use of lipid composition and metabolism to examine structure and activity of estuarine detrital microflora. Appl Environ Microbiol. 1977 May;33(5):1177–1183. doi: 10.1128/aem.33.5.1177-1183.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles C. J., Smith L. Measurements of ATP levels of intact Azotobacter vinelandii under different conditions. Biochim Biophys Acta. 1970 Mar 3;197(2):152–160. doi: 10.1016/0005-2728(70)90026-5. [DOI] [PubMed] [Google Scholar]
- Leonard N. J., Tolman G. L. Fluorescent nucleosides and nucleotides. Ann N Y Acad Sci. 1975 Aug 8;255:43–58. doi: 10.1111/j.1749-6632.1975.tb29212.x. [DOI] [PubMed] [Google Scholar]
- Lundin A., Thore A. Comparison of methods for extraction of bacterial adenine nucleotides determined by firefly assay. Appl Microbiol. 1975 Nov;30(5):713–721. doi: 10.1128/am.30.5.713-721.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J., Simmon V., Streitwieser D., Ames B. N. Mutagenicity of chloroacetaldehyde, a possible metabolic product of 1,2-dichloroethane (ethylene dichloride), chloroethanol (ethylene chlorohydrin), vinyl chloride, and cyclophosphamide. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3190–3193. doi: 10.1073/pnas.72.8.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miović M. L., Gibson J. Nucleotide pools and adenylate energy charge in balanced and unbalanced growth of Chromatium. J Bacteriol. 1973 Apr;114(1):86–95. doi: 10.1128/jb.114.1.86-95.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschel R. C., Leonard N. J. Fluorescent modification of guanine. Reaction with substituted malondialdehydes. J Org Chem. 1976 Jan 23;41(2):294–300. doi: 10.1021/jo00864a023. [DOI] [PubMed] [Google Scholar]
- Nickels J. S., King J. D., White D. C. Poly-beta-Hydroxybutyrate Accumulation as a Measure of Unbalanced Growth of the Estuarine Detrital Microbiota. Appl Environ Microbiol. 1979 Mar;37(3):459–465. doi: 10.1128/aem.37.3.459-465.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattsangi P. D., Leonard N. J., Frihart C. R. 1,N2-ethenoguanine and N2,3-ethenoguanine. Synthesis and comparison of the electronic spectral properties of these linear and angular triheterocycles related to the Y bases. J Org Chem. 1977 Sep 30;42(20):3292–3296. doi: 10.1021/jo00440a020. [DOI] [PubMed] [Google Scholar]
- Secrist J. A., 3rd, Barrio J. R., Leonard N. J., Weber G. Fluorescent modification of adenosine-containing coenzymes. Biological activities and spectroscopic properties. Biochemistry. 1972 Sep 12;11(19):3499–3506. doi: 10.1021/bi00769a001. [DOI] [PubMed] [Google Scholar]
- Welsch F., Smith L. Kinetics of synthesis and utilization of adenosine triphosphate by intact cells of Rhodospirillum rubrum. Biochemistry. 1969 Aug;8(8):3403–3408. doi: 10.1021/bi00836a039. [DOI] [PubMed] [Google Scholar]
- Wiebe W. J., Bancroft K. Use of the adenylate energy charge ratio to measure growth state of natural microbial communities. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2112–2115. doi: 10.1073/pnas.72.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M., Tamura Z. Fluorimetric determination of adenine and adenosine and its nucleotides by high-performance liquid chromatography. J Chromatogr. 1976 Jul 21;123(1):220–224. doi: 10.1016/s0021-9673(00)81119-x. [DOI] [PubMed] [Google Scholar]


