Abstract
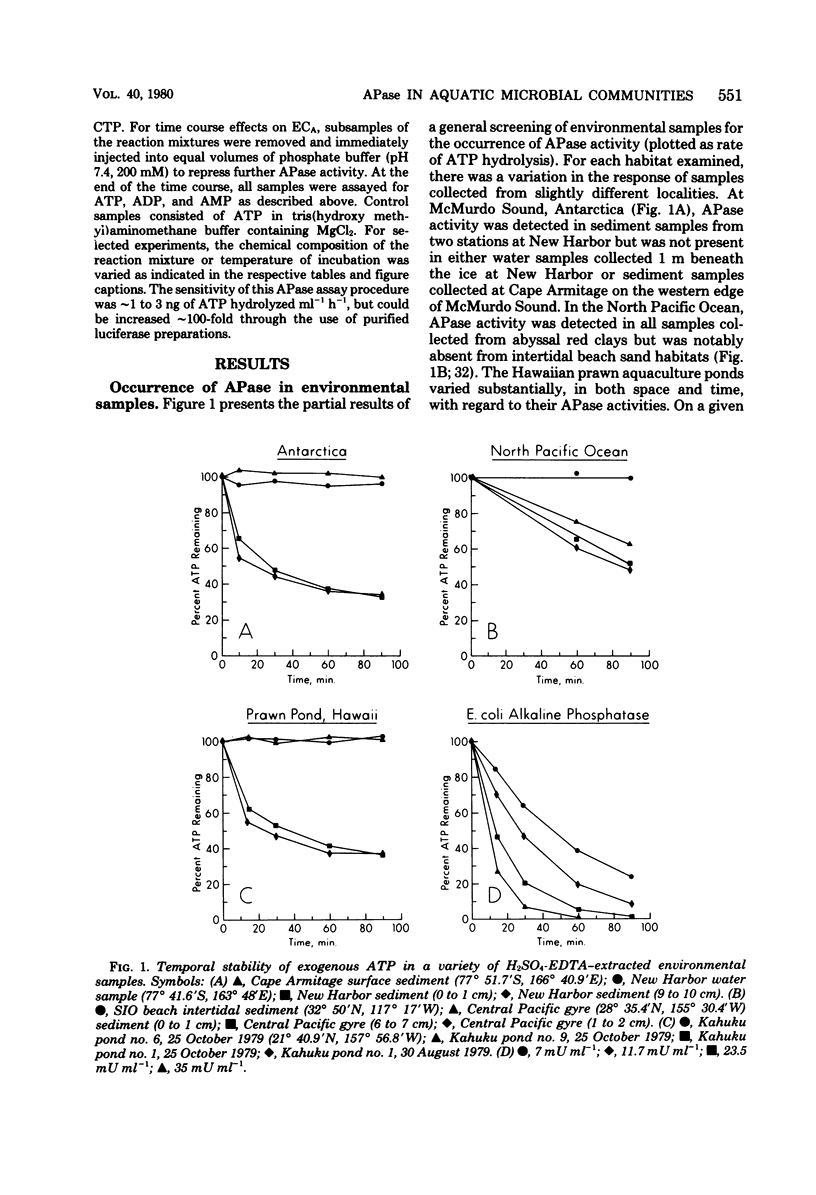
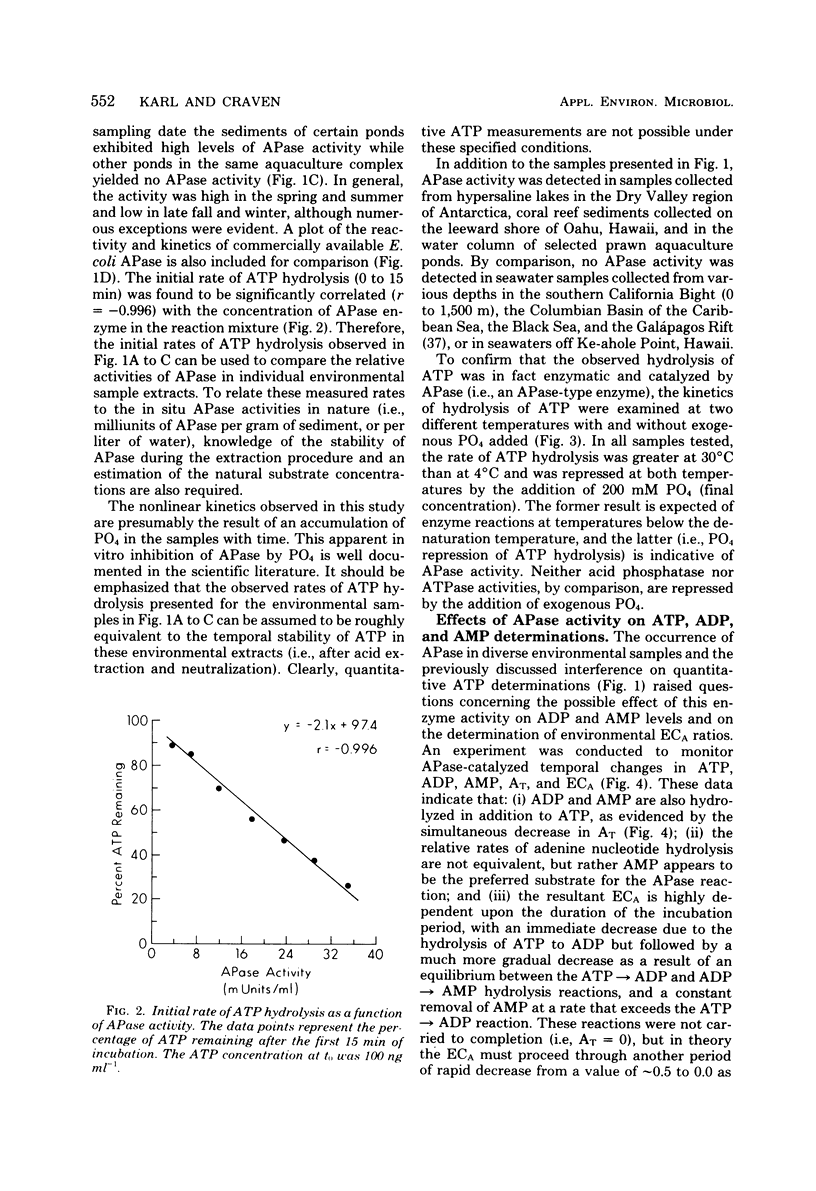
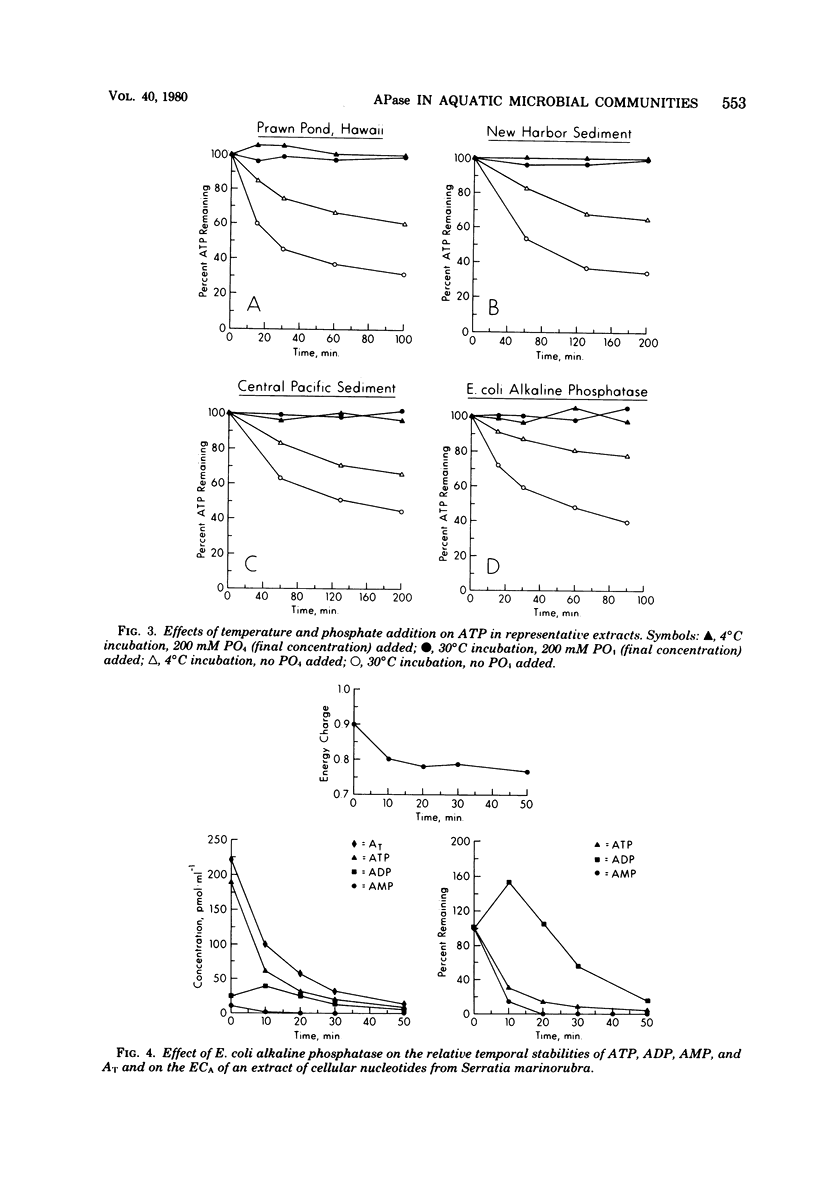
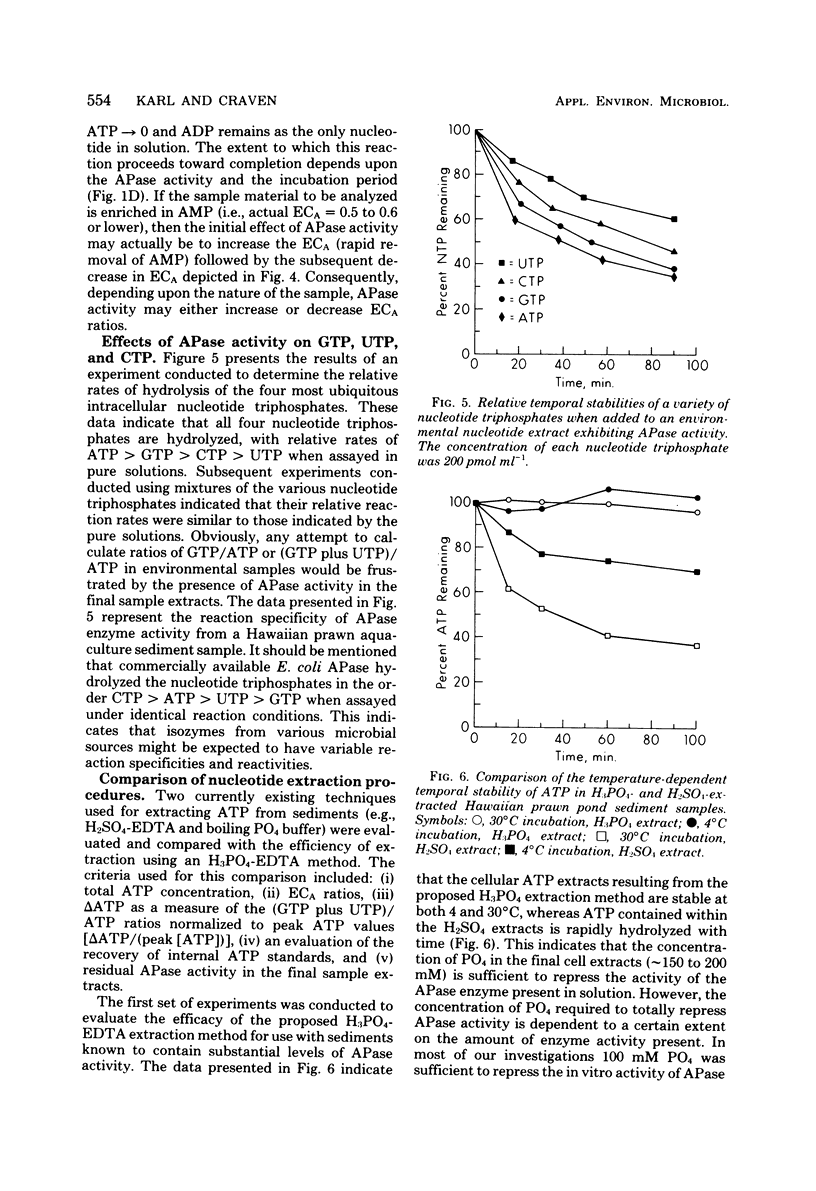
Alkaline phosphatase (APase) activity was detected in aquatic microbial assemblages from the subtropics to Antarctica. The occurrence of APase in environmental nucleotide extracts was shown to significantly affect the measured concentrations of cellular nucleotides (adenosine triphosphate, adenosine diphosphate, adenosine monophosphate, guanosine triphosphate, uridine triphosphate, and cytidine triphosphate), adenylate energy charge, and guanosine triphosphate/adenosine triphosphate ratios, when conventional methods of nucleotide extraction were employed. Under the reaction conditions specified in this report, the initial rate of hydrolysis of adenosine triphosphate was directly proportional to the activity of APase in the sample extracts and consequently can be used as a sensitive measure of APase activity. A method was devised for obtaining reliable nucleotide measurements in naturally occurring microbial populations containing elevated levels of APase activity. The metabolic significance of APase activity in microbial cells is discussed, and it is concluded that the occurrence and regulation of APase in nature is dependent upon microscale inorganic phosphate limitation of the autochthonous microbial communities.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman T. Phosphatase release of inorganic phosphorus in Lake Kinneret. Nature. 1969 Dec 20;224(5225):1231–1232. doi: 10.1038/2241231b0. [DOI] [PubMed] [Google Scholar]
- Bobbie R. J., Morrison S. J., White D. C. Effects of substrate biodegradability on the mass and activity of the associated estuarine microbiota. Appl Environ Microbiol. 1978 Jan;35(1):179–184. doi: 10.1128/aem.35.1.179-184.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone D. H. Relationship between phosphates and alkaline phosphatase of Anabaena flos-aquae in continuous culture. Arch Mikrobiol. 1971;80(2):147–153. doi: 10.1007/BF00411879. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Alkaline phosphatase activity of rumen bacteria. Appl Environ Microbiol. 1977 Nov;34(5):586–590. doi: 10.1128/aem.34.5.586-590.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin A. R., Jr, Macgregor A. N. Soil adenosine triphosphate: extraction, recovery and half-life. Bull Environ Contam Toxicol. 1972 May;7(5):296–300. doi: 10.1007/BF01684528. [DOI] [PubMed] [Google Scholar]
- Davison J. A., Fynn G. H. The assay of ATP by the luciferin-luciferase method. Interference by a bacterial phosphatase enzyme stable to perchlorate treatment. Anal Biochem. 1974 Apr;58(2):632–637. doi: 10.1016/0003-2697(74)90233-4. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Cheng K. J. The constitutive nature of alkaline phosphatase in rumen bacteria. Can J Microbiol. 1980 Feb;26(2):268–272. doi: 10.1139/m80-043. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Lam K. Use of adenosine 5'-triphosphate as an indicator of the microbiota biomass in rumen contents. Appl Environ Microbiol. 1977 Mar;33(3):528–537. doi: 10.1128/aem.33.3.528-537.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Grusky G. E., Aaronson S. Cytochemical changes in aging Ochromonas; evidence for an alkaline phosphatase. J Protozool. 1969 Nov;16(4):686–689. doi: 10.1111/j.1550-7408.1969.tb02327.x. [DOI] [PubMed] [Google Scholar]
- HORIUCHI T., HORIUCHI S., MIZUNO D. A possible negative feedback phenomenon controlling formation of alkaline phosphomonoesterase in Escherichia coli. Nature. 1959 May 30;183(4674):1529–1530. doi: 10.1038/1831529b0. [DOI] [PubMed] [Google Scholar]
- Hill H. D., Summer G. K., Waters M. D. An automated fluorometric assay for alkaline phosphatase using 3-O-methylfluorescein phosphate. Anal Biochem. 1968 Jul;24(1):9–17. doi: 10.1016/0003-2697(68)90054-7. [DOI] [PubMed] [Google Scholar]
- KUO M. H., BLUMENTHAL H. J. Absence of phosphatase repression by inorganic phosphate in some micro-organisms. Nature. 1961 Apr 1;190:29–31. doi: 10.1038/190029a0. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., Nyc J. F., Brown D. M. A repressible alkaline phosphatase in Neurospora crassa. II. Isolation and chemical properties. J Biol Chem. 1968 Jun 10;243(11):3076–3082. [PubMed] [Google Scholar]
- Karl D. M. A rapid sensitive method for the measurement of guanine ribonucleotides in bacterial and environmental extracts. Anal Biochem. 1978 Sep;89(2):581–595. doi: 10.1016/0003-2697(78)90387-1. [DOI] [PubMed] [Google Scholar]
- Karl D. M. Measurement of microbial activity and growth in the ocean by rates of stable ribonucleic Acid synthesis. Appl Environ Microbiol. 1979 Nov;38(5):850–860. doi: 10.1128/aem.38.5.850-860.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl D. M. Occurrence and ecological significance of GTP in the ocean and in microbial cells. Appl Environ Microbiol. 1978 Aug;36(2):349–355. doi: 10.1128/aem.36.2.349-355.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin A., Thore A. Comparison of methods for extraction of bacterial adenine nucleotides determined by firefly assay. Appl Microbiol. 1975 Nov;30(5):713–721. doi: 10.1128/am.30.5.713-721.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIRSON A., KUHL A. Uber den Phosphathaushalt von Hydrodictyon. I. Arch Mikrobiol. 1958;30(2):211–225. [PubMed] [Google Scholar]
- Patni N. J., Dhawale S. W., Aaronson S. Extracellular phosphatases of Chlamydomonas reinhardi and their regulation. J Bacteriol. 1977 Apr;130(1):205–211. doi: 10.1128/jb.130.1.205-211.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayler G. S., Puziss M., Silver M. Alkaline phosphatase assay for freshwater sediments: application to perturbed sediment systems. Appl Environ Microbiol. 1979 Nov;38(5):922–927. doi: 10.1128/aem.38.5.922-927.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Wiebe W. J., Bancroft K. Use of the adenylate energy charge ratio to measure growth state of natural microbial communities. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2112–2115. doi: 10.1073/pnas.72.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A. S. Physiological factors in the regulation of alkaline phosphatase synthesis in Escherichia coli. J Bacteriol. 1972 May;110(2):616–623. doi: 10.1128/jb.110.2.616-623.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


