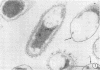
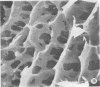
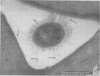
Abstract
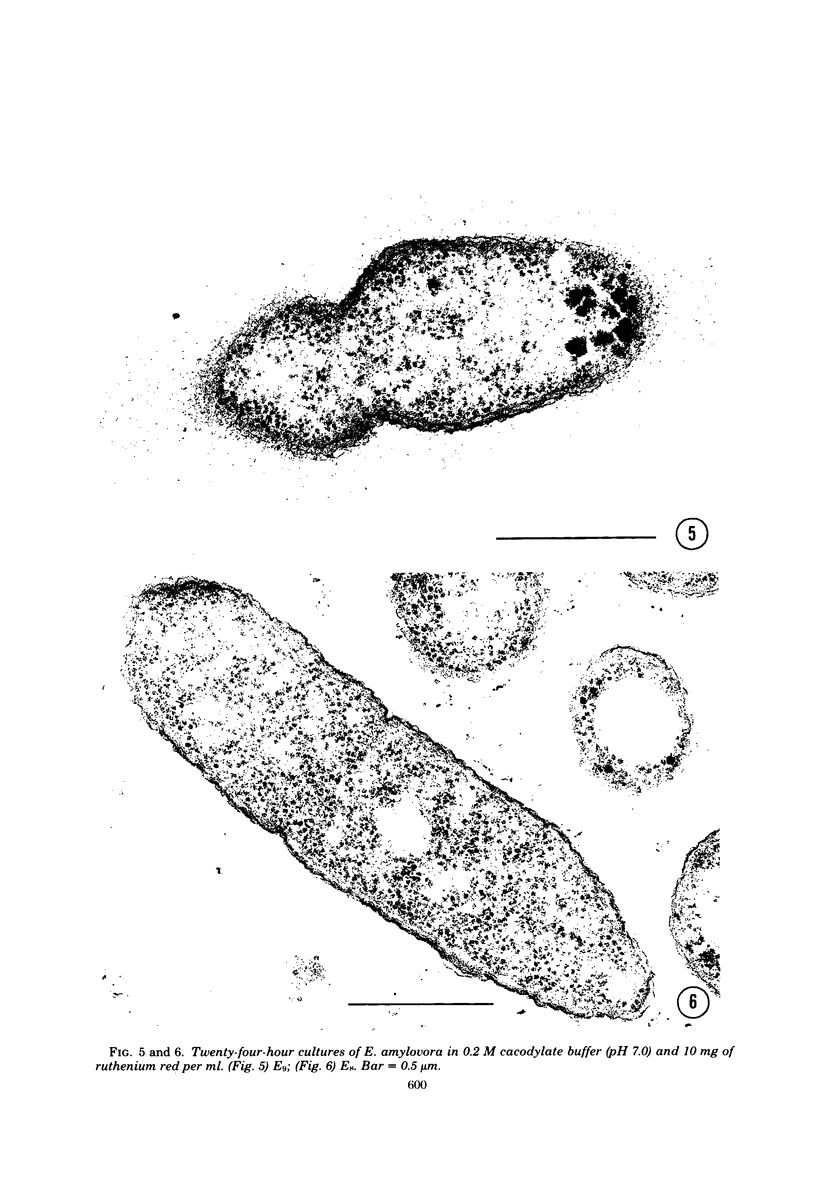
Virulent E9 and avirulent E8 strains of Erwinia amylovora were shown by means of light, transmission, and scanning microscopy to be, respectively, encapsulated and unencapsulated. Difficulty was encountered in stabilizing the fibrillar-appearing capsular extracellular polysaccharide. We suggest that the ephemeral nature of extracellular polysaccharide is due to the collapse of its extended structure upon dehydration. This occurs when bacteria are prepared for either transmission or scanning electron microscopy. The electron micrographs support our previous biochemical and immunological studies contending that the capsule is composed of tightly bound and loosely held components. The preparation of bacteria in freeze-dried colonies has permitted us to observe and explain the fluidity of the encapsulated strain. We suggest that this fluidity is a reflection of the loosely held extracellular polysaccharide or slime.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Ayers S. B., Goodman R. N. Extracellular Polysaccharide of Erwinia amylovora: a Correlation with Virulence. Appl Environ Microbiol. 1979 Oct;38(4):659–666. doi: 10.1128/aem.38.4.659-666.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagle G. D. Fine structure and distribution of extracellular polymer surrounding selected aerobic bacteria. Can J Microbiol. 1975 Mar;21(3):395–408. doi: 10.1139/m75-055. [DOI] [PubMed] [Google Scholar]
- Calvert H. E., Lalonde M., Bhuvaneswari T. V., Bauer W. D. Role of lectins in plant--microorganism interactions. IV. Ultrastructural localization of soybean lectin binding sites of Rhizobium japonicum. Can J Microbiol. 1978 Jul;24(7):785–793. doi: 10.1139/m78-132. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Ultrastructure of cell envelopes of bacteria of the bovine rumen. Appl Microbiol. 1975 Jun;29(6):841–849. doi: 10.1128/am.29.6.841-849.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Damgaard H. N., Cheng K. J. Cell envelope morphology of rumen bacteria. J Bacteriol. 1974 Jun;118(3):1132–1143. doi: 10.1128/jb.118.3.1132-1143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGUID J. P. The demonstration of bacterial capsules and slime. J Pathol Bacteriol. 1951 Oct;63(4):673–685. doi: 10.1002/path.1700630413. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Bacterial polysaccharide which binds Rhizobium trifolii to clover root hairs. J Bacteriol. 1979 Mar;137(3):1362–1373. doi: 10.1128/jb.137.3.1362-1373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B. A., Dugan P. R., Pfister R. M., Remsen C. C. Structure of exocellular polymers and their relationship to bacterial flocculation. J Bacteriol. 1969 Jun;98(3):1328–1334. doi: 10.1128/jb.98.3.1328-1334.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. N., Huang J. S., Huang P. Y. Host-Specific Phytotoxic Polysaccharide from Apple Tissue Infected by Erwinia amylovora. Science. 1974 Mar 15;183(4129):1081–1082. doi: 10.1126/science.183.4129.1081. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- Mackie E. B., Brown K. N., Lam J., Costerton J. W. Morphological stabilization of capsules of group B streptococci, types Ia, Ib, II, and III, with specific antibody. J Bacteriol. 1979 May;138(2):609–617. doi: 10.1128/jb.138.2.609-617.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Isaacson R. E., Pohlenz J. Mechanisms of association of enteropathogenic Escherichia coli with intestinal epithelium. Am J Clin Nutr. 1979 Jan;32(1):119–127. doi: 10.1093/ajcn/32.1.119. [DOI] [PubMed] [Google Scholar]
- Morris E. R., Rees D. A., Young G., Walkinshaw M. D., Darke A. Order-disorder transition for a bacterial polysaccharide in solution. A role for polysaccharide conformation in recognition between Xanthomonas pathogen and its plant host. J Mol Biol. 1977 Feb 15;110(1):1–16. doi: 10.1016/s0022-2836(77)80095-8. [DOI] [PubMed] [Google Scholar]
- Smith H. Microbial interference with host defence mechanisms. Monogr Allergy. 1975;9:13–38. [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker D. K., Drucker D. B. Scanning electron microscopy of intact colonies of microorganisms. J Bacteriol. 1970 Nov;104(2):902–909. doi: 10.1128/jb.104.2.902-909.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]