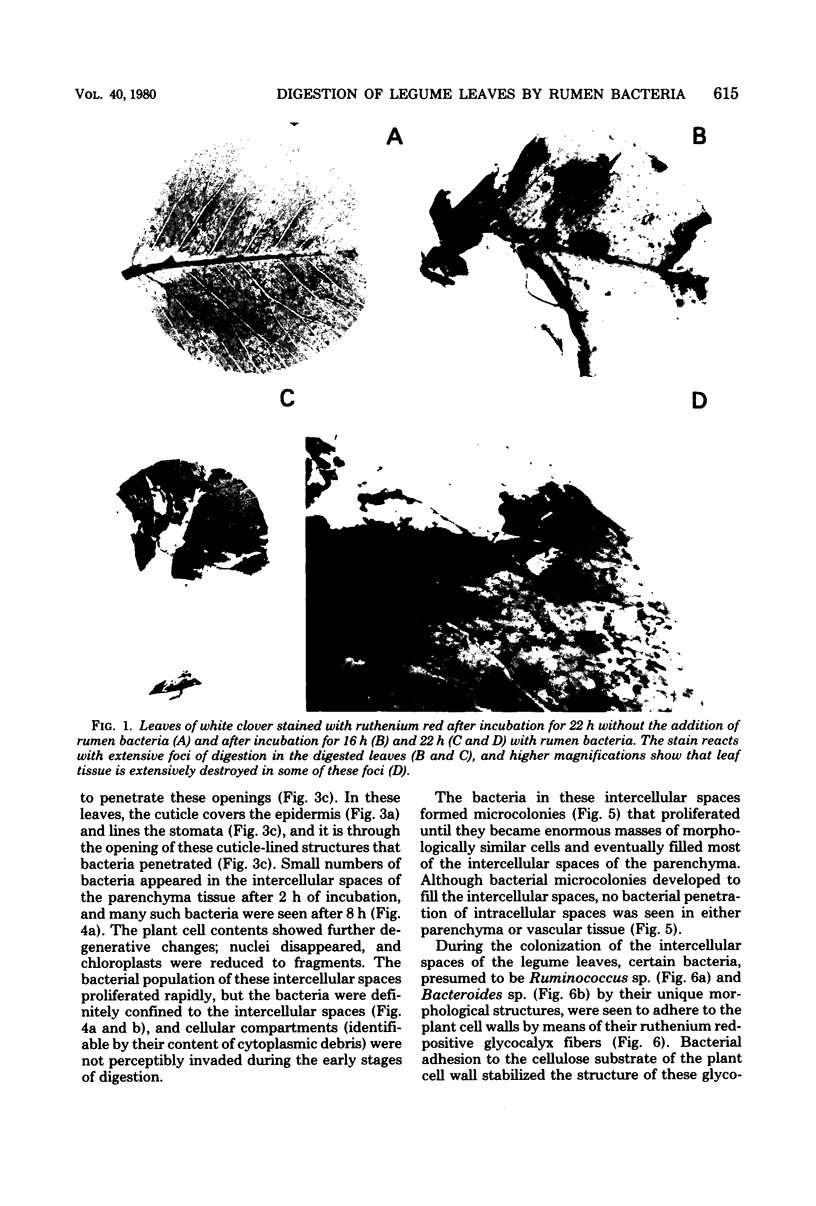
Abstract
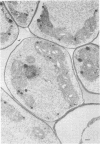
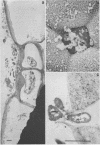
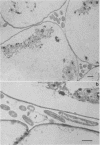
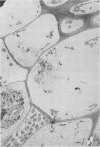
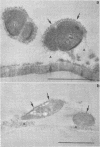
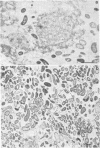
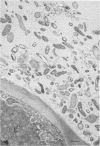
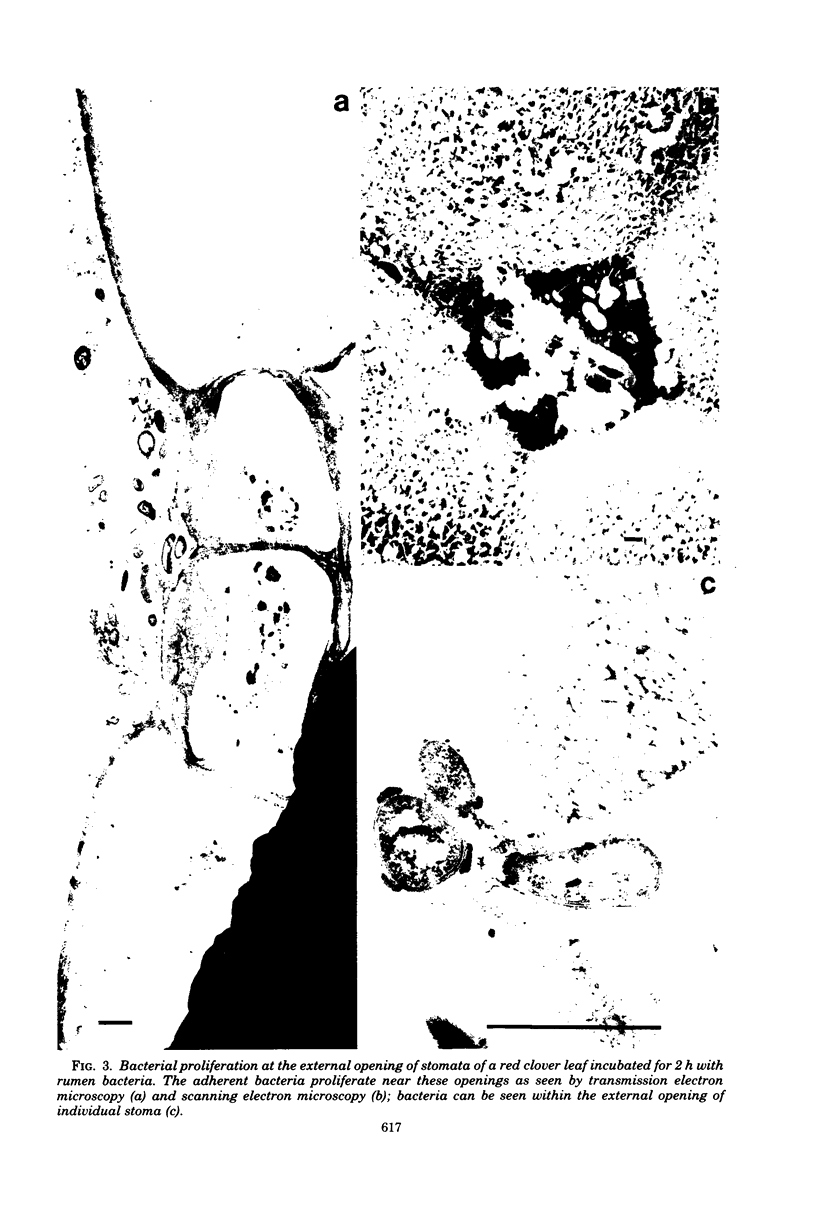
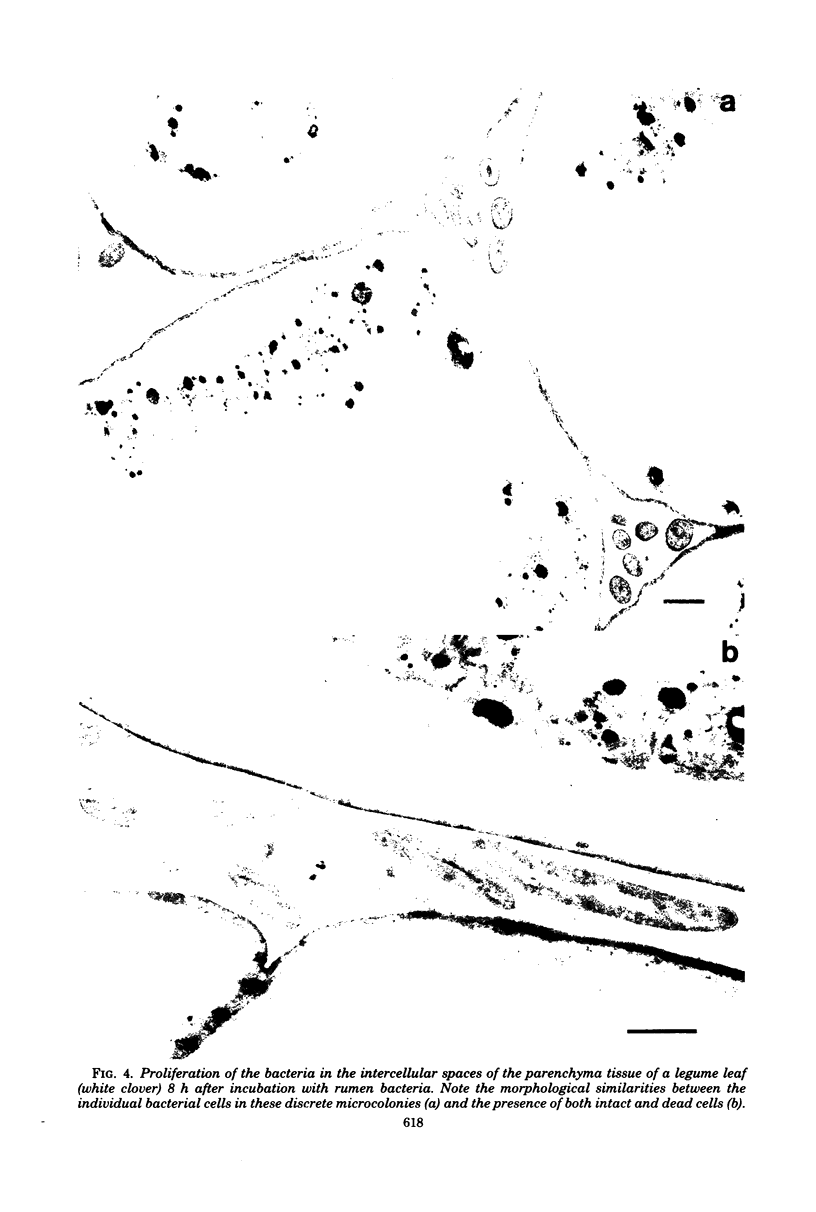
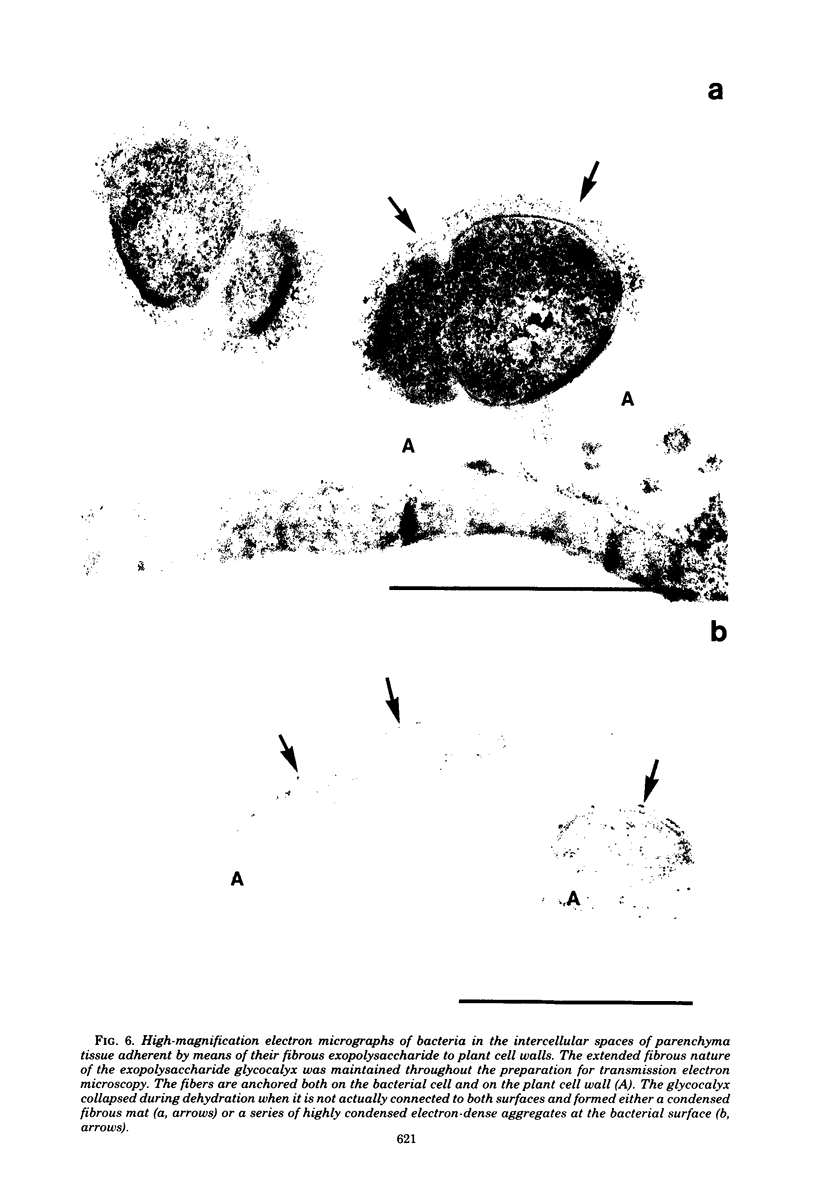
When fresh whole leaves of six different species of forage legumes were suspended in an artificial rumen medium and inoculated with rumen bacteria, bacterial adhesion and proliferation were noted at the stomata, and penetration of the stomate by these bacteria was documented by electron microscopy. The invading bacteria adhered to surfaces within the intercellular space of the leaf and produced very extensive exopolysaccharide-enclosed microcolonies. After some of the legume leaf cell walls were disorganized and ruptured by bacterial digestion, these cells (notably, parenchyma and epidermal cells) were invaded by bacteria, with subsequent formation of intracellular microcolonies. However, other cells were neither ruptured nor colonized (notably, stomata guard cells and vascular tissue). At all stages of the digestion of intact legume leaves, the rumen bacteria grew in microcolonies composed of cells of single or mixed morphological types, and a particular ecological niche was often completely and consistently occupied by a very large microcolony of cells of single or mixed morphological types.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Amos H. E. Rumen bacterial degradation of forage cell walls investigated by electron microscopy. Appl Microbiol. 1975 May;29(5):692–701. doi: 10.1128/am.29.5.692-701.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E., Burdick D., Michaels G. E. Rumen bacterial interrelationships with plant tissue during degradation revealed by transmission electron microscopy. Appl Microbiol. 1974 Jun;27(6):1149–1156. doi: 10.1128/am.27.6.1149-1156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Akin D. E., Costerton J. W. Rumen bacteria: interaction with particulate dietary components and response to dietary variation. Fed Proc. 1977 Feb;36(2):193–197. [PubMed] [Google Scholar]
- Cheng K. J., Dinsdale D., Stewart C. S. Maceration of Clover and Grass Leaves by Lachnospira multiparus. Appl Environ Microbiol. 1979 Oct;38(4):723–729. doi: 10.1128/aem.38.4.723-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., McCowan R. P., Costerton J. W. Adherent epithelial bacteria in ruminants and their roles in digestive tract function. Am J Clin Nutr. 1979 Jan;32(1):139–148. doi: 10.1093/ajcn/32.1.139. [DOI] [PubMed] [Google Scholar]
- Dinsdale D., Morris E. J., Bacon J. S. Electron microscopy of the microbial populations present and their modes of attack on various cellulosic substrates undergoing digestion in the sheep rumen. Appl Environ Microbiol. 1978 Jul;36(1):160–168. doi: 10.1128/aem.36.1.160-168.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Lam K. Use of adenosine 5'-triphosphate as an indicator of the microbiota biomass in rumen contents. Appl Environ Microbiol. 1977 Mar;33(3):528–537. doi: 10.1128/aem.33.3.528-537.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMLIN L. J., HUNGATE R. E. Culture and physiology of a starch-digesting bacterium (Bacteroides amylophilus n. sp.) from the bovine rumen. J Bacteriol. 1956 Oct;72(4):548–554. doi: 10.1128/jb.72.4.548-554.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Adhesion of Bacteroides succinogenes in pure culture and in the presence of Ruminococcus flavefaciens to cell walls in leaves of perennial ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jun;35(6):1166–1173. doi: 10.1128/aem.35.6.1166-1173.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Ruminococcus flavefaciens Cell Coat and Adhesion to Cotton Cellulose and to Cell Walls in Leaves of Perennial Ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jan;35(1):156–165. doi: 10.1128/aem.35.1.156-165.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- Malick L. E., Wilson R. B. Modified thiocarbohydrazide procedure for scanning electron microscopy: routine use for normal, pathological, or experimental tissues. Stain Technol. 1975 Jul;50(4):265–269. doi: 10.3109/10520297509117069. [DOI] [PubMed] [Google Scholar]
- Patterson H., Irvin R., Costerton J. W., Cheng K. J. Ultrastructure and adhesion properties of Ruminococcus albus. J Bacteriol. 1975 Apr;122(1):278–287. doi: 10.1128/jb.122.1.278-287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]