Abstract
AphB is a LysR-type activator that initiates the expression of the virulence cascade in Vibrio cholerae by cooperating with the quorum-sensing-regulated activator AphA at the tcpPH promoter on the Vibrio pathogenicity island (VPI). To identify the ancestral chromosomal genes in V. cholerae regulated by AphB, we carried out a microarray analysis and show here that AphB influences the expression of a number of genes that are not associated with the VPI. One gene strongly activated by AphB is cadC, which encodes the ToxR-like transcriptional activator responsible for activating the expression of lysine decarboxylase, which plays an important role in survival at low pH. Other genes activated by AphB encode a Na+/H+ antiporter, a carbonic anhydrase, a member of the ClC family of chloride channels, and a member of the Gpr1/Fun34/YaaH family. AphB influences each of these genes directly by recognizing a conserved binding site within their promoters, as determined by gel mobility shift assays. Transcriptional lacZ fusions indicate that AphB activates the expression of these genes under aerobic conditions in response to low pH and also under anaerobic conditions at neutral pH. Further experiments show that the regulation of cadC by AphB in response to low pH and anaerobiosis is mirrored in the heterologous organism Escherichia coli, is independent of the global regulators Fnr and ArcAB, and depends upon the region of the promoter that contains the AphB binding site. These results raise the possibility that the activity of AphB is influenced by the pH and oxygen tension of the environment.
The ability of pathogenic bacteria to respond to environmental cues allows them to rapidly adapt to niches both within and outside the host. The human pathogen Vibrio cholerae colonizes the epithelium of the small intestine and is also found attached to the surface of plankton in aquatic environments. The expression of its two primary virulence factors, toxin-coregulated pilus (TCP) and cholera toxin (CT), is influenced by a variety of environmental stimuli, such as pH, temperature, oxygen tension, bile, unsaturated fatty acids, c-di-GMP, and quorum sensing (6, 36, 38). These stimuli have been found to influence gene expression at a number of different levels within the virulence cascade (38; see also below). The complexity of this regulation stems from the need to integrate horizontally acquired virulence genes into the existing regulatory circuits of the organism to optimize its survival both inside and outside the host.
ToxT is an AraC-type regulator encoded on the Vibrio pathogenicity island (VPI) that directly activates the transcription of the tcp, ctx, and accessory colonization factor genes (5). The ability of ToxT to activate transcription is influenced by a number of factors, such as bile (13, 52), unsaturated fatty acids (6, 33), and bicarbonate (1). Expression of toxT is dependent upon cooperation between two homologous pairs of transmembrane regulatory proteins encoded by the toxRS operon on chromosome I (17, 29) and tcpPH encoded on the VPI (3, 14). Although the specific signals regulating the activities of ToxRS and TcpPH are not yet clear, pH and temperature have been found to influence the levels of TcpP via intramembrane proteolysis (37). A large part of the responsiveness of the virulence cascade to environmental signals is due to transcriptional silencing by the DNA-binding protein H-NS (44). H-NS is an abundant nucleoid-associated protein that directly represses the expression of the toxT, tcpA, and ctx promoters (44, 57, 63). In addition, c-di-GMP, an important second messenger in bacterial cell signaling, impacts the virulence cascade by an as-yet-undefined mechanism through the VieSAB signal transduction system (59).
The induction of the virulence cascade by activation of the tcpPH promoter is dependent upon two transcription factors separately encoded within the ancestral genome, AphA and AphB (21, 55), and it is influenced by environmental stimuli such as pH, temperature, and quorum sensing (3, 42, 64). AphA is a winged-helix transcription factor (8) that facilitates the binding of the LysR-type regulator AphB to the promoter (23, 24, 27). AphA also has additional roles in V. cholerae. It functions as a repressor of an operon involved in the production of acetoin (28). The metabolic function of acetoin production is to counteract lethal acidification when cells are grown in the presence of excess glucose by redirecting pyruvic acid into neutral rather than acidic end products. AphA also plays a role in enhancing biofilm formation (62). Quorum sensing influences the expression of the virulence cascade and acetoin production in response to cell density by controlling the expression of aphA (25). This is due to the ability of HapR, the central quorum-sensing regulator, to bind to the aphA promoter at high cell density and interfere with its activation by Lrp and the biofilm regulator VpsR (32).
The results presented here shed light on the physiological role of AphB in V. cholerae. Microarray analysis led to the identification of a number of genes directly activated by AphB that have roles in pH homeostasis. One of these, cadC, encodes a member of the ToxR-like family of transmembrane transcriptional regulators (40) that directly activates the expression of the cadBA operon, which encodes a lysine/cadaverine antiporter (CadB) and lysine decarboxylase (CadA). Lysine decarboxylases have been shown to play an important role in adaptive acid tolerance responses in Salmonella enterica serovar Typhimurium and in V. cholerae (39, 47) where exposure to moderately acidic environments for a short period provides protection against subsequent challenge at a normally lethal pH. The lysine decarboxylase activity of CadA converts lysine to cadaverine and in the process consumes a proton and produces carbon dioxide. Cadaverine is transported out of the cell by CadB in exchange for lysine (56).
Other genes directly activated by AphB include nhaB, encoding an Na+/H+ antiporter, a gene encoding carbonic anhydrase, a gene encoding a member of the ClC family of chloride channels, and a gene encoding member of the poorly characterized Gpr1/Fun34/YaaH family. AphB bound directly to the promoters of each of these genes by using a recognition sequence similar to the LysR-type motif identified at the tcpPH promoter, and it stimulated their expression, as well as that of tcpPH, at pH 5.5 to a greater degree than at pH 7.0. These results indicate that AphB plays a more significant role in activating the expression of its target genes at low pH. AphB also stimulated the expression of these genes in response to anaerobiosis at neutral pH. These findings raise the possibility that AphB responds independently to both the pH and the oxygen tension of the environment.
MATERIALS AND METHODS
Bacterial strains and media.
The strains and plasmids used in this study are described in Table 1 . Strains were maintained at −70°C in Luria-Bertani (LB) medium (41) containing 30% (vol/vol) glycerol. AKI medium was previously described (18). Antibiotics were used at the following concentrations in LB medium: ampicillin, 100 μg/ml; kanamycin, 45 μg/ml; polymyxin B, 50 units/ml; and streptomycin, 1 mg/ml. 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was used in LB agar at 40 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype and relevant characteristics (endpoint position) | Reference or source |
|---|---|---|
| Strains | ||
| V. cholerae | ||
| C6706 str2 | El Tor Inaba Smr | Laboratory collection |
| O395 | Classical Ogawa Smr | Laboratory collection |
| GK138 | KSK725ΔaphB | 21 |
| GK142 | KSK262 aphB-lacZ | 21 |
| GK954 | C6706 ΔaphB | This work |
| GK976 | KSK1105 ΔaphB | This work |
| GK1222 | KSK262 Δclc-lacZ | This work |
| GK1224 | GK1222 ΔaphB | This work |
| GK1232 | KSK262 ΔnhaB-lacZ | This work |
| GK1234 | GK1232 ΔaphB | This work |
| GK1240 | C6706 ΔnhaB | This work |
| GK1242 | C6706 Δclc | This work |
| KSK262 | C6706 str2 ΔlacZ3 | 21 |
| KSK725 | C6706 str2 ΔlacZ3 tcpP-lacZ | 21 |
| KSK1105 | C6706 ΔtcpPH promoter | 28 |
| KSK2896 | KSK262 cacC+cadC-lacZ | This work |
| KSK2900 | KSK2896 ΔaphB | This work |
| KSK2917 | KSK262 cacC+cadC-lacZ (−63) | This work |
| WL344 | KSK262 ΔVC0770-lacZ | This work |
| WL346 | WL344 ΔaphB | This work |
| WL361 | C6706 ΔVC0770 | This work |
| WL540 | C6706 ΔcadC | This work |
| WL547 | KSK262 ΔcadC-lacZ | This work |
| WL558 | KSK262 Δcah-lacZ | This work |
| WL562 | WL558 ΔaphB | This work |
| WL564 | C6706 Δcah | This work |
| WL737 | KSK262 cacC+cadC-lacZ (−102) | This work |
| WL762 | KSK262 cacC+cadC-lacZ (−91) | This work |
| WL786 | KSK262 cacC+cadC-lacZ (−78) | This work |
| WL972 | WL547 Δfnr ΔarcA | This work |
| E. coli | ||
| KSK2925 | MC1061 cadC-lacZ pMF3 | This work |
| KSK2926 | KSK2925 pGKK263 | This work |
| Plasmids | ||
| pGKK263 | aphB in pMF3 | This work |
| pGKK360 | ΔnhaB in pKAS154 | This work |
| pGKK361 | Δclc in pKAS154 | This work |
| pGKK362 | ΔnhaB-lacZ in pKAS154 | This work |
| pGKK364 | Δclc-lacZ in pKAS154 | This work |
| pGKK368 | Δfnr in pKAS154 | This work |
| pKAS359 | cadC-lacZ in pRS415 | This work |
| pWEL92 | ΔVC0770 in pKAS154 | This work |
| pWEL93 | ΔVC0770-lacZ in pKAS154 | This work |
| pWEL134 | ΔcadC in pKAS154 | This work |
| pWEL135 | ΔcadC-lacZ in pKAS154 | This work |
| pWEL136 | Δcah in pKAS154 | This work |
| pWEL138 | Δcah-lacZ in pKAS154 | This work |
| pWEL151 | ΔarcA in pKAS154 | This work |
| pWEL153 | cadC-lacZ in pKAS180 | This work |
Construction of mutants with in-frame deletions and lacZ fusions.
The in-frame deletion mutations were constructed by amplifying DNA fragments upstream and downstream of each gene from C6706 str2 chromosomal DNA by using primers as follows (Table 2 ): for cadC, CadC3/CadC1 and CadC5/CadC4; for VC0770, GprB/GprN1 and GprN2/GprE; for nhaB, NhaB7/NhaB5 and NhaB6/NhaB8; for cah, CA6/CA5 and CA7/CA8; for clc, Clc8/Clc6 and Clc5/Clc7; fnr, FnrN2/FnrB2 and FnrN1/FnrE; and for arcA, FexA1/FexA2 and FexA3/FexA4. The fragments were inserted into pKAS154 (25) joined by a NotI restriction site, and the resulting plasmids are listed in Table 1. To generate each lacZ fusion, the appropriate in-frame deletion plasmid was digested with NotI, and a promoterless lacZ gene from pVC200 (48) was ligated in the correct orientation. The deletion constructs and fusions were transferred onto the chromosome of V. cholerae by allelic exchange (54). To construct the merodiploid cadC+ cadC-lacZ chromosomal fusion strain, the EcoRI-BglII fragment containing the cadC promoter was digested out of pWEL135 and ligated into pKAS180 (26), generating pWEL153. The resulting fusion was then introduced in place of the lacZ gene in KSK262, generating KSK2896. The ΔaphB mutant was described previously (21).
TABLE 2.
Oligonucleotides used in this study
| Primer | Oligonucleotide sequence (5′-3′) |
|---|---|
| CA3 | TCGCTAATACCCACGTTGTC |
| CA5 | GATCGGCGGCCGCCGTTGTCTTTTTCATAGTCCC |
| CA6 | GATCGAGATCTTCAATATGTGCCATGTGTGC |
| CA7 | GATCGGCGGCCGCATTGCAAGCCGACTAAATACAAGG |
| CA8 | GATCGGAATTCATCGATTTCGACGTTGATGG |
| CA9 | ATTCGGCCGTGATGAGTTTC |
| CA10 | TATATTCCGAACATATTCATTTTG |
| CadC1 | GATCGGCGGCCGCGATTCCAATCATAGAATAGC |
| CadC3 | GATCGGAATTCAGAGCCAGCAACAAGTCACG |
| CadC4 | GATCGAGATCTTCAACCTGATAAACACCACC |
| CadC5 | GATCGGCGGCCGCACGTTTACTGTAACGCTCGTC |
| CadC6 | ATGACGTTGTCTGATGGTTAAAC |
| CadC7 | ATAGCATCGGTAAGAGGC |
| CadC8 | AAGTTTGCAATTTTTAGCCTTATC |
| CadC19 | GATCGGGATCCGATTCCAATCATAGAATAGC |
| CadC28 | GATCGGAATTCTACCACTAGGAATGACGTTG |
| CadC43 | GATCGGAATTCATGACGTTGTCTGATGGTTAAAC |
| CadC47 | GATCGGAATTCATGGTTAAACAACCTAAGTTTGC |
| Clc5 | GATCGGCGGCCGCATCTTGTTGACATACTAATGACACC |
| Clc6 | GATCGGCGGCCGCAGCCCCAACTCATAAAAGTC |
| Clc7 | GATCGTCTAGACGGAACTCGATTGATCATGCTG |
| Clc8 | GATCGGAATTCTAAATGCAGTAATAAGGCTG |
| Clc9 | TCCATCAAATCAATCATATATGG |
| Clc10 | TAAACGTCTCTCTTGTTGAC |
| Clc11 | AATATTTCAACTTTATCGCTATAG |
| CO2-6 | GATCGGAATTCAAGCCATGCAAATGGCGGCC |
| CO2-35 | GATCGGTCGACCGGGATGTGAGATTTATA |
| FexA1 | AGCTGGAATTCACCCTCACTTTCACCCAATCA |
| FexA2 | AGCTGGCGGCCGCACGGGGTTTGCATTAGCGTTAC |
| FexA3 | AGCTGGCGGCCGCGAAGATTAACCTCTTCTTTTA |
| FexA4 | AGCTGAGATCTGCGAGAAGTGATTGCGAAGAT |
| FnrB2 | TGCACAGATCTAACGGACTCTAGCATGTCGTG |
| FnrE | TGCACGAATTCTTAGGCCTTTACATCGGACGC |
| FnrN1 | TGCACGCGGCCGCGCTTTTCAGAAATCATAACAACC |
| FnrN2 | TGCACGCGGCCGCTAACTTCAATGATGTAGCTC |
| Gpr1 | GAACTTACCTTCAGAGTGTG |
| Gpr14 | ACTGTTTCAACAATCTAAGTTTCC |
| Gpr15 | GGCGATTTTTTACTCGCATC |
| GprB | GATCGGGATCCGACACAAGGTGATGAACCTTC |
| GprE | GATCGGAATTCTTGGCATGAGCTTTAAGCTG |
| GprN1 | GATCGGCGGCCGCTAGCTTAGTTGACATGTGAAC |
| GprN2 | GATCGGCGGCCGCAGCTGCATAAGTCAAACATTTGG |
| NhaB5 | GATCGGCGGCCGCCATCGGCATGATGATTACTC |
| NhaB6 | GATCGGCGGCCGCAAGTGGCCACTAAATTTCAATTG |
| NhaB7 | GATCGAGATCTGGACACAGCTCACACTATCC |
| NhaB8 | GATCGGAATTCCATACCACCTTGCTACAGTC |
| NhaB9 | ACACTATCCTATTTCTAATTAGC |
| NhaB10 | CTAAGTGGTGTAAGCATAAC |
| NhaB11 | AAACTTACTCGAAAGAGGTA |
Construction of the cadC-lacZ fusion in E. coli.
A cadC promoter-lacZ fusion was constructed by amplifying a 500-bp product from C6706 str2 by using primers CadC3 and CadC19. The resulting fragment was ligated into the lacZ operon fusion pRS415 (53) to generate pKAS359. This cadC-lacZ fusion was recombined onto λ RS45 and integrated onto the chromosome of E. coli MC1061. A low-copy-number plasmid expressing aphB from its native promoter was constructed by amplifying a region from O395 using primers CO2-6 and CO2-35. The resulting fragment was digested with EcoRI and SalI and ligated into the mini-F vector pMF3 (34) to generate pGKK263.
Construction of cadC-lacZ promoter deletion mutants.
The cadC promoter deletion-lacZ fusion mutants were constructed by amplifying regions of the cadC promoter from C6706 str2 chromosomal DNA by using primers with different upstream endpoints (CadC28 [position −102], CadC43 [position −91], CadC47 [position −78], and CadC8 [position −63]) together with the downstream primer CadC1. Each fragment was ligated to a promoterless lacZ gene from pVC200 (48) in the correct orientation in pKAS180 (26), generating pWEL191 (position −102), pWEL199 (position −91), pWEL200 (position −78), and pKAS358 (position −63). The resulting fusions were introduced into the chromosome of V. cholerae by allelic exchange.
Isolation of RNA.
Cultures of KSK1105 and GK976 were inoculated from single colonies into 10 ml of AKI medium lacking bicarbonate and incubated statically at 37°C for 3.5 h. RNA was extracted using TRIzol (Invitrogen). The RNA was treated with DNase I (Ambion) and purified using RNeasy columns (Qiagen) according to the manufacturer's instructions.
Labeling of cDNA probes and hybridization to microarray.
Total RNA (10 μg) was reverse transcribed in the presence of amino allyl dUTP by using Superscript II (Invitrogen) and random hexamers for priming. cDNA was then coupled to monofunctional Alexa Fluor 555 or 647 dye (Invitrogen). Hybridization took place underneath a coverslip in 25% formamide at 42°C overnight. Arrays consisted of 50-mer and 70-mer oligonucleotides representing 3,946 probes from 3,929 unique genes (a few large genes have more than one probe) as well as 10 positive and 15 negative controls. Bioinformatics and oligonucleotide synthesis were performed by Illumina. Arrays were printed on Corning Ultra GAPS slides by using a GMS 417 arrayer with 50% dimethyl sulfoxide (DMSO) as the printing buffer. Postprocessing was carried out according to the manufacturer's instructions.
Microarray data analysis and statistics.
Slides were scanned in a GMS 418 scanner, and images were quantified using Scanalyze software (Michael Eisen, Stanford University). Array images and intensity data were further analyzed using GeneTraffic (Iobion Informatics). Three independent RNA preparations from each strain were used for hybridization. Statistical analyses were performed using Cyber T (University of California at Irvine), with a Bayesian confidence value of 10 and a sliding window size of 121.
Gel mobility shift experiments.
The DNA fragments for the gel mobility shift assays were amplified by PCR from C6706 str2. For each promoter region, the larger fragment has a more distal upstream endpoint such that it contains both the left and right arms of the putative region of dyad symmetry and the smaller fragment has a more proximal upstream endpoint such that it contains only the right arm, as follows: for cadC, CadC6/CadC7 (141 bp) and CadC7/CadC8 (113 bp); for VC0770, Gpr1/Gpr15 (142 bp) and Gpr1/Gpr14 (115 bp); for nhaB, NhaB9/NhaB10 (144 bp) and NhaB10/NhaB11 (116 bp); for cah, CA3/CA9 (152 bp) and CA3/CA10 (124 bp); and for clc, Clc9/Clc10 (141 bp) and Clc11/Clc10 (113 bp). The fragments were gel purified and end labeled with digoxigenin as described previously (23). Binding reactions for AphB were carried out as previously described (23). The samples were applied to a 5% polyacrylamide gel and subjected to electrophoresis. The DNA was transferred to nylon membranes by electroblotting, probed with anti-digoxigenin-alkaline phosphatase (AP) antibody (Amersham Pharmacia), and visualized using chemiluminescence.
Acid tolerance assays.
Strains were grown aerobically in LB medium to an optical density at 600 nm (OD600) of 0.5. For acid shock assays, 1-ml aliquots of the cells were pelleted and then resuspended in LB challenge medium adjusted to pH 4.0 with HCl (inorganic) or LB challenge medium containing 8.7 mM acetic acid, 2.5 mM butyric acid, and 3.7 mM propionic acid adjusted to pH 4.5 with HCl (organic) (39) for various lengths of time. For acid adaptation assays, 1-ml aliquots of the cells were pelleted and then resuspended in LB medium adjusted to pH 5.7 with HCl (inorganic) or in LB medium containing 6.5 mM acetic acid, 1.8 mM butyric acid, and 2.7 mM propionic acid adjusted to pH 5.7 with HCl (organic) (39) and incubated for 1 h at 37°C with shaking before pelleting again and resuspending in challenge medium.
β-Galactosidase assays.
β-Galactosidase assays were carried out as previously described (41). Aerobic cultures were grown in a 3-ml volume on a rotator. Static cultures were grown in a 10-ml volume without rotation. Anaerobic cultures were grown in filled 15-ml conical tubes with sealed screw caps on a rotator.
RESULTS
Identification of genes activated by AphB in V. cholerae by using microarray analyses.
Whole-genome microarray analysis was carried out in El Tor biotype strain C6706 to identify the genes in V. cholerae regulated by AphB. It is known that induction of the virulence cascade in the El Tor biotype requires specialized conditions, referred to as AKI conditions, in which the strains are incubated in a peptone-based medium containing bicarbonate statically for 3.5 h and then shifted to shaking conditions for 4 h (18). Since AphB strongly activates the expression of tcpPH in this biotype when the strains are incubated statically in AKI medium lacking bicarbonate for 3.5 h (21), we chose this condition for microarray analysis to identify other genes regulated by AphB. To avoid identifying the subset of genes also regulated by tcpPH, RNAs from cultures of KSK1105 (C6706 ΔtcpPH promoter) and GK976 (C6706 ΔtcpPH promoter, ΔaphB) were isolated and compared. Table 3 lists genes with Bayesian P statistic values of 0.01 or lower and which showed a >2.5-fold change in expression. By these criteria, 18 genes were found to be activated by AphB and 3 genes were repressed.
TABLE 3.
Genes regulated by AphB under AKI conditions, as determined by microarray analysis
| Gene (locus tag) | Bayes P | Folda | Gene product (gene name)b |
|---|---|---|---|
| VC0278 | 9.2E−07 | −6.18 | Transcriptional activator (cadC) |
| VC0280 | 1.7E−10 | −6.05 | Cadaverine/lysine antiporter (cadB) |
| VC0281 | 1.2E−06 | −4.95 | Lysine decarboxylase (cadA) |
| VC0364 | 3.1E−05 | 3.50 | Bactoferritin-associated ferredoxin (bfd) |
| VC0770 | 6.3E−06 | −8.34 | Gpr1/Fun34/YaaH family protein |
| VC1777 | 2.9E−04 | −2.58 | Conserved hypothetical protein |
| VC1780 | 7.5E−04 | −2.62 | Conserved hypothetical protein |
| VC1781 | 9.1E−05 | −2.81 | Conserved hypothetical protein |
| VC1901 | 4.6E−05 | −2.57 | Na+/H+ antiporter (nhaB) |
| VC2100 | 3.2E−04 | −2.60 | Conserved hypothetical protein |
| VC2102 | 2.1E−04 | −2.64 | Conserved hypothetical protein |
| VC2772 | 1.1E−06 | −3.89 | ParB family protein |
| VCA0013 | 3.7E−04 | −2.60 | Maltodextrin phosphorylase (malP) |
| VCA0186 | 1.7E−15 | 7.56 | Hypothetical protein |
| VCA0188 | 9.0E−05 | 2.93 | Hypothetical protein |
| VCA0274 | 8.7E−07 | −6.41 | Carbonic anhydrase (cah) |
| VCA0526 | 4.6E−06 | −5.86 | ClC-type chloride channel |
| VCA0834 | 4.3E−06 | −3.41 | Hypothetical protein |
| VCA0944 | 1.9E−04 | −3.58 | Maltose ABC transporter, permease protein (malF) |
| VCA1027 | 8.1−04 | −2.89 | Maltose operon periplasmic protein, putative |
| VCA1099 | 9.6−04 | −2.72 | ABC transporter, permease protein |
Positive numbers show repression and negative numbers show activation.
Boldface type indicates genes directly activated by AphB, as determined by gel mobility shift analysis.
With regard to the genes activated by AphB, VC0770, which showed the strongest activation (8-fold), encodes a protein that is a member of the poorly characterized Gpr1/Fun34/YaaH family. Although these proteins are predicted to be transmembrane proteins, no functions for these family members in bacteria have yet been defined. The cadC gene (VC0278) was upregulated 6-fold, and this was consistent with the finding that AphB in V. vulnificus also activates the expression of cadC and plays a role in acid tolerance (49). VCA0274, encoding a carbonic anhydrase, was also upregulated 6-fold by AphB. Carbonic anhydrase catalyzes the interconversion of carbon dioxide and bicarbonate and has been found to play an important role in acid survival of Helicobacter pylori due to its ability to buffer the periplasm (35). VCA0526, which was upregulated almost 6-fold by AphB, encodes a protein that is a member of the ClC family of chloride channels (10). The ClC family comprises a group of integral membrane proteins whose major action is to translocate chloride (Cl−) ions across cell membranes. This protein has previously been shown to confer mild acid pH resistance to V. cholerae (9). VC1901, upregulated 2.5-fold by AphB, encodes NhaB, a member of a group of Na+/H+ antiporters with roles in pH homeostasis and NaCl tolerance (16).
Other genes activated by AphB include VC2772, encoding a ParB family protein required for efficient plasmid and chromosome partitioning in many bacterial species (50), several genes involved in maltose uptake and utilization (VCA0013, VCA0944, and VCA1027), an ABC transporter gene (VCA1099), and six genes annotated as hypotheticals (VC1777, VC1780, VC1781, VC2100, VC2102, and VCA0834). AphB repressed a gene encoding bacteroferritin-associated ferredoxin (VC0364) and two genes annotated as hypotheticals (VCA0186 and VC0188).
AphB activates a number of its target genes by binding directly to their promoter regions.
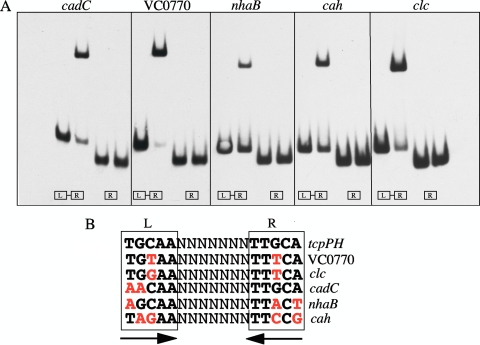
To determine which genes in Table 3 were directly activated by AphB, DNA fragments containing each of the upstream regions of the genes or operons were PCR amplified and used in gel mobility shift assays under the conditions optimized for binding at the tcpPH promoter (23). As shown in Fig. 1 A, purified AphB bound to DNA fragments containing the upstream regions of only five genes from Table 3: VC0278 (cadC), VC0770, VC1901 (nhaB), VCA0274 (cah), and VCA0526 (clc). Within each of these fragments is a LysR-type recognition sequence fairly well conserved relative to that from the tcpPH promoter (Fig. 1B). This sequence at the VC0770 and clc promoters has only one mismatch, in the same position, in the left and right dyad arms, at cadC it has two mismatches in the left dyad arm, and at nhaB and cah it has three and four mismatches, respectively. For each promoter region, binding was lost when the fragments contained only the right dyad arm of the motif (Fig. 1A). These results strongly indicate that the recognition sequence identified in each promoter is critical for AphB binding.
FIG. 1.
(A) Binding of purified AphB to various promoter fragments. For each promoter, the first fragment contains both the left (L) and right (R) dyad arms of the LysR motif shown below in panel B whereas the second fragment has only the right dyad arm. The first lane in each set has no protein added, the second lane has 160 nM (100 ng) AphB. The sequence of the AphB binding sites at the various promoters is shown in panel B compared to the site identified at the tcpPH promoter (23). Mismatches are shown in red.
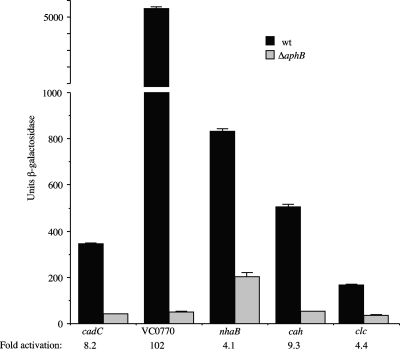
To verify that AphB activates the expression of the five genes listed above, we made transcriptional fusions of these promoter regions with lacZ and compared their expression levels under conditions similar to those used for the original microarray (AKI medium static for 3.5 h). As shown in Fig. 2, the five promoter fusions show various degrees of activation by AphB under these conditions. The VC0770 promoter was clearly the strongest, showing 102-fold activation by AphB. The cah promoter was upregulated 9.3-fold, the cadC promoter was upregulated 8.2-fold, and the nhaB and clc promoters were each upregulated 4-fold. With the exception of VC0770, for which the degree of activation was significantly higher, the degrees of activation of all of the lacZ fusions by AphB were similar to the data obtained from the microarray.
FIG. 2.
Influence of a ΔaphB mutation on the expression of various promoter-lacZ fusions in V. cholerae. Strains were grown in AKI medium lacking bicarbonate statically for 3.5 h. The strains are as follows, from left to right: KSK2896 (cadC-lacZ), KSK2900 (ΔaphB), WL344 (VC0770-lacZ), WL346 (ΔaphB), GK1232 (nhaB-lacZ), GK1234 (ΔaphB), WL558 (cah-lacZ), WL562 (ΔaphB), GK1222 (clc-lacZ), and GK1224 (ΔaphB). wt, wild type.
Transcriptional regulation of AphB-activated genes is independently influenced by pH and oxygen in both V. cholerae and E. coli.
The decrease in the pH of the static cultures used to identify the various AphB-regulated genes from 7.0 to approximately 5.7 after 3.5 h of incubation raised the possibility that the expression of a number of AphB-regulated genes could be upregulated in response to low pH, consistent with their known or presumed roles in pH regulation. To determine whether the expression of the AphB-regulated genes was influenced by low pH under aerobic conditions, the V. cholerae lacZ fusion strains were compared after aerobic growth in LB medium held constant at pH 7.0 (buffered with 100 mM Trizma; final pH at the end of growth, 7.0) and at pH 5.5 (buffered with 100 mM MES [morpholineethanesulfonic acid]; final pH at the end of growth, 5.6). All of the fusion strains (which had gene deletions and lacZ replacements except for the cadC strain, which is a merodiploid, since the deletion strain grew very slowly at pH 5.5) were stimulated when the pH was lowered from 7.0 to 5.5 (Table 4). The expression level of clc increased nearly 7-fold at pH 5.5, the expression levels of cah and tcpPH increased 4-fold, and the expression levels of nhaB and cadC increased close to 3-fold. In each of these cases, the pH regulation was lost or significantly reduced when the ΔaphB mutation was introduced into the fusion strains (Table 4). In the case of the VC0770 mutant (Table 5), expression of the fusion was so high in LB medium at pH 7.0 that it was stimulated only 1.7-fold when the pH was lowered to 5.5. However, growth of the strain in minimal medium at pH 7.0 significantly reduced the expression of the fusion relative to its growth in LB medium, and under these conditions, lowering the pH from 7.0 to 6.0 resulted in a 5-fold increase in expression, which was completely prevented in the presence of the ΔaphB mutation (Table 5). These results indicate that the expression of all the genes directly activated by AphB in Table 3 increases at a lower pH during aerobic growth and is consistent with their known or possible roles in pH homeostasis.
TABLE 4.
Activation of AphB regulated promoter-lacZ fusions by low pH
| Strain | β-Galactosidase activitya |
Fold change | |
|---|---|---|---|
| pH 7.0b | pH 5.5c | ||
| GK1222 (Δclc-lacZ) | 32 ± 1 | 217 ± 7 | 6.8 |
| GK1224 (ΔaphB) | 22 ± 1 | 49 ± 1 | 2.2 |
| WL558 (Δcah-lacZ) | 71 ± 7 | 315 ± 3 | 4.4 |
| WL562 (ΔaphB) | 56 ± 11 | 78 ± 1 | 1.3 |
| KSK725 (ΔtcpPH-lacZ) | 377 ± 56 | 1,631 ± 47 | 4.3 |
| GK138 (ΔaphB) | 122 ± 1 | 226 ± 5 | 1.8 |
| GK1232 (ΔnhaB-lacZ) | 238 ± 5 | 687 ± 10 | 2.9 |
| GK1234 (ΔaphB) | 225 ± 42 | 242 ± 2 | 1.0 |
| KSK2896 (ΔlacZ::cadC-lacZ) | 48 ± 1 | 129 ± 1 | 2.7 |
| KSK2900 (ΔaphB) | 42 ± 1 | 40 ± 1 | 0.95 |
Units per OD600 of culture.
LB medium buffered with 100 mM Trizma.
LB medium buffered with 100 mM MES.
TABLE 5.
Activation of a VC0770 promoter-lacZ fusion by low pH
| Strain | β-Galactosidase activitya |
Fold change | |
|---|---|---|---|
| LB medium, pH 7.0b | LB medium, pH 5.5c | ||
| WL344 (ΔVC0770-lacZ) | 2,591 ± 65 | 4,403 ± 42 | 1.7 |
| WL346 (ΔaphB) | 79 ± 1 | 51 ± 1 | 0.64 |
| M9 medium, pH 7.0 | M9 medium, pH 6.0 | ||
|---|---|---|---|
| WL344 (ΔVC0770-lacZ) | 210 ± 12 | 1,149 ± 32 | 5.5 |
| WL346 (ΔaphB) | 45 ± 2 | 41 ± 5 | 0.91 |
Units per OD600 of culture.
Buffered with 100 mM Trizma.
Buffered with 100 mM MES.
To address whether the expression of the AphB-regulated genes was stimulated by a lack of oxygen at neutral pH, the fusion strains were grown aerobically and anaerobically in LB medium held constant at pH 7.0 (buffered with 100 mM Trizma; the final pH at the end of aerobic growth was 7.0 and that at the end of anaerobic growth was 6.7 to 6.8). The levels of expression of all of the fusions were stimulated to various degrees by anaerobic growth (Table 6). The expression levels of cadC and cah increased 12-fold, that of tcpPH increased 7-fold, that of clc increased 4-fold, and those of nhaB and VC0770 increased 2-fold; in each case, this regulation was completely dependent on the presence of AphB. In addition, the expression of these genes was not increased further under anaerobic conditions when the pH was lowered (data not shown). These results indicate that the expression of all the genes directly activated by AphB is also stimulated by anaerobiosis at neutral pH.
TABLE 6.
Activation of AphB-regulated promoter-lacZ fusions by anaerobiosis
| Strain | β-Galactosidase activitya |
Fold change | |
|---|---|---|---|
| Aerobicb | Anaerobicb | ||
| KSK2896 (ΔlacZ::cadC-lacZ) | 48 ± 1 | 575 ± 23 | 12.0 |
| KSK2900 (ΔaphB) | 42 ± 1 | 68 ± 5 | 1.6 |
| WL558 (Δcah-lacZ) | 71 ± 7 | 849 ± 50 | 11.9 |
| WL562 (ΔaphB) | 56 ± 11 | 86 ± 8 | 1.5 |
| KSK725 (ΔtcpPH-lacZ) | 377 ± 56 | 2,731 ± 25 | 7.2 |
| GK138 (ΔaphB) | 122 ± 1 | 230 ± 23 | 1.9 |
| GK1222 (Δclc-lacZ) | 32 ± 1 | 126 ± 4 | 3.9 |
| GK1224 (ΔaphB) | 22 ± 1 | 35 ± 5 | 1.6 |
| GK1232 (ΔnhaB-lacZ) | 238 ± 5 | 555 ± 69 | 2.3 |
| GK1234 (ΔaphB) | 225 ± 42 | 177 ± 5 | 0.79 |
| WL344 (ΔVC0770-lacZ) | 2,591 ± 65 | 5,665 ± 142 | 2.2 |
| WL346 (ΔaphB) | 79 ± 1 | 89 ± 12 | 1.1 |
Units per OD600 of culture.
LB medium (pH 7.0) buffered with 100 mM Trizma.
The results listed above indicate that the expression of AphB-activated genes is influenced independently by low pH and by low oxygen levels. To address whether this regulation is due to the influence of these signals on the expression of aphB itself, an aphB-lacZ fusion was analyzed under the same conditions. With respect to pH 5.5, the expression of aphB did not significantly change, and with respect to anaerobiosis, the expression of aphB increased slightly more than 2-fold. Since it is unlikely that the influence of oxygen on the various AphB-activated genes can be completely accounted for by the 2-fold increase in aphB expression under these conditions, these results raise the possibility that the activity of AphB may be responding to both low pH and low oxygen levels. Alternatively, AphB could possibly function together with another regulatory protein that is influenced by these stimuli.
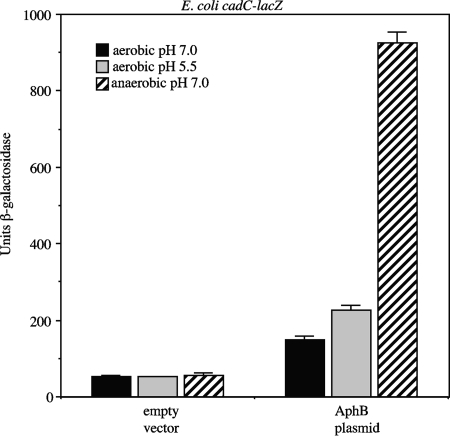
To further investigate this, we focused on cadC, since it is the most well characterized of the various genes activated by AphB (40, 49). We first examined whether AphB was capable of activating the expression of a cadC-lacZ fusion in the heterologous organism E. coli in response to low pH and oxygen. Introduction of a single-copy plasmid expressing aphB into an E. coli cadC-lacZ strain increased the expression of the fusion 2-fold when the cells were grown aerobically at pH 7.0, 4-fold when they were grown aerobically at pH 5.5, and 17-fold when they were grown anaerobically at pH 7.0 (Fig. 3). These results indicate that the expression of cadC is activated by AphB in response to low pH and low oxygen in the absence of other V. cholerae proteins and suggest that the mechanisms involved in this regulation are not exclusive to Vibrio.
FIG. 3.
Influence of AphB on the expression of a cadC-lacZ fusion in E. coli. Strains were grown aerobically in LB medium buffered to pH 7.0 with 100 mM Trizma, aerobically in LB medium buffered to pH 5.5 with 100 mM MES, or anaerobically in LB medium buffered to pH 7.0 with 100 mM Trizma. The first three bars represent the values obtained with empty vector pMF3, and the second three bars represent the values obtained with the AphB plasmid (pGKK263).
Neither Fnr nor ArcAB plays a role in the regulation of cadC expression in response to reduced oxygen levels.
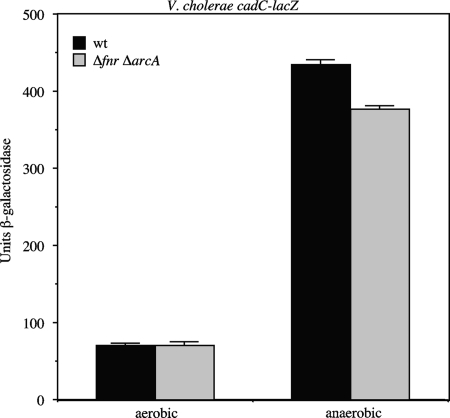
Anaerobic induction of gene expression in enteric bacteria is controlled by two global transcription factors, Fnr (fumarate and nitrate reductase regulation) and ArcAB (aerobic respiratory control) (2, 51). Fnr is a one-component transcriptional regulator that has been established as a major activator of genes involved in anaerobic metabolism in E. coli, and ArcAB is a two-component regulator involved in activating and repressing transcription under both anaerobic and aerobic conditions. The finding that the expression of the V. cholerae cadC promoter was strongly activated under anaerobic conditions in E. coli as well as in V. cholerae raised the possibility that either Fnr or ArcAB might play a role at the cadC promoter together with AphB in both organisms. To address this, mutations in fnr and arcA were introduced into a V. cholerae cadC-lacZ fusion strain and anaerobic induction of the promoter was assessed. As shown in Fig. 4, a strain lacking both fnr and arcA still showed significant induction of cadC expression under anaerobic conditions. These results indicate that neither Fnr nor ArcAB is responsible for the anaerobic induction of cadC, but the results leave open the possibility that other regulators in common to both V. cholerae and E. coli may still play a role in this process.
FIG. 4.
Influence of Fnr and ArcAB on the expression of a cadC-lacZ fusion in V. cholerae. Strains (WL547 [ΔcadC-lacZ] and WL972 [Δfnr ΔarcA]) were grown in LB medium either aerobically or anaerobically.
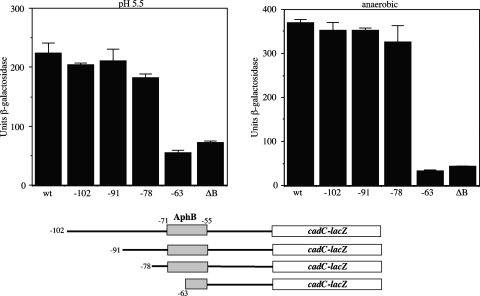
A region of the cadC promoter between −78 and −63 is required for AphB-dependent activation in response to pH and oxygen.
As shown in Fig. 1, a LysR-type binding site is present in the cadC promoter that is required for AphB binding, which spans a region from positions −71 to −55 relative to the start of transcription (40). To confirm that this region of the promoter is important for transcriptional activation of cadC, a deletion series was constructed in the cadC-lacZ merodiploid V. cholerae fusion strain. As shown in Fig. 5, fusion strains with endpoints at positions −102, −91, and −78 were capable of being activated by AphB in response to both low pH and low oxygen. However, a fusion with an endpoint at position −63, which contains only the right dyad arm of the LysR motif and is incapable of binding AphB (Fig. 1), was unable to be activated in response to these signals, similar to that of a ΔaphB (null) mutant. These results indicate that the region of the cadC promoter important for activation in response to pH and oxygen coincides with the location of the AphB binding site. However, it remains possible that regions of the promoter downstream of the AphB binding site are also involved in the response to these signals.
FIG. 5.
Activation of cadC-lacZ promoter deletions in V. cholerae. (Left graph) Strains were grown aerobically in LB medium buffered to pH 5.5 with 100 mM MES. (Right graph) Strains were grown anaerobically in LB medium buffered to pH 7.0 with 100 mM Trizma. The strains are as follows, from left to right: KSK2896 (cadC-lacZ), WL737 (−102), WL762 (−91), WL786 (−78), KSK2917 (−63), and KSK2900 (ΔaphB [ΔB]).
Role of cadC and the other AphB-regulated genes in acid tolerance.
CadA is an acid-inducible lysine decarboxylase that has previously been shown to play an important role in the acid tolerance response of V. cholerae (39). In addition, the protein encoded by VCA0526 (clc) has been shown to confer mild resistance to inorganic acid (9). The finding here that cadCBA, clc, and a number of other genes are directly regulated by AphB in response to both low pH and low oxygen prompted us to assess the role of these genes in the survival of V. cholerae under acid stress. This response encompasses resistance to both inorganic acid (low pH) and organic acid (low pH plus short chain fatty acids).
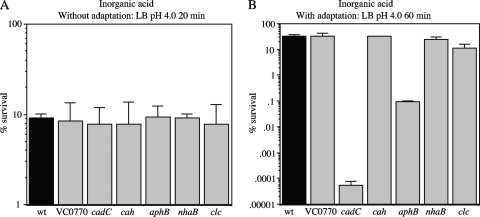
Acid shock assays were initially carried out similarly to those described previously (9). Cells were grown aerobically in LB medium to an OD600 of 0.5. To examine exposure to inorganic acid, 1-ml aliquots of the cells were resuspended in LB challenge medium adjusted to pH 4.4 with HCl, and after 60 min, no significant reduction in viability was observed with any of the strains (data not shown). Lowering the pH to 4.0 with HCl and decreasing the exposure time to 20 min resulted in approximately 10% survival for all of the strains (Fig. 6 A). To examine exposure to organic acid, 1-ml aliquots of cells grown as described above were resuspended in LB challenge medium containing 8.7 mM acetic acid, 2.5 mM butyric acid, and 3.7 mM propionic acid at pH 4.5 (39). After 20 min of incubation, all of the strains showed approximately 10% survival with no significant differences between them (Fig. 7 A). From these results, it appears that after logarithmic growth in LB medium, none of the genes activated by AphB plays a significant role in survival to inorganic or organic acid shock.
FIG. 6.
Inorganic acid tolerance assays. (A) Strains were exposed to LB challenge medium at pH 4.0 for 20 min without adaptation. (B) Strains were adapted for 60 min to LB medium at pH 5.7 and then exposed to LB challenge medium at pH 4.0 for 60 min. The strains are as follows, from left to right: C6706 (wt), WL361 (ΔVC0770), WL540 (ΔcadC), WL564 (Δcah), GK954 (ΔaphB), GK1240 (ΔnhaB), and GK1242 (Δclc).
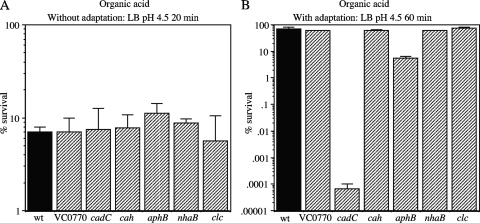
FIG. 7.
Organic acid tolerance assays. (A) Strains were exposed to LB challenge medium containing organic acids at pH 4.5 for 20 min without adaptation. (B) Strains were adapted for 60 min to LB medium containing organic acids at pH 5.7 and then exposed to LB challenge medium containing organic acids at pH 4.5 for 60 min. The strains are as follows, from left to right: C6706 (wt), WL361 (ΔVC0770), WL540 (ΔcadC), WL564 (Δcah), GK954 (ΔaphB), GK1240 (ΔnhaB), and GK1242 (Δclc).
It has previously been shown that if cells are adapted to conditions that are mildly acidic prior to their exposure to a lethal low pH, the percentage of survival is much higher than that of cells exposed to the lethally low-pH environment directly (11). In V. cholerae, cadA has been shown to play an important role in this adaptive response to both inorganic and organic acid challenges (39). To address the role of the other AphB-regulated genes in this response, the various mutant strains were grown in a manner similar to that described above, but instead of being placed directly in acid challenge, they were first incubated in LB medium at pH 5.7 for 1 h with aeration and then subsequently challenged with LB medium at pH 4.0. Without adaptation, exposure to LB medium at pH 4.0 for 20 min resulted in only 10% viability (Fig. 6A), whereas with adaptation, all the strains showed close to 100% viability when subjected to this condition (data not shown). However, after 60-min exposure to this condition, the levels of survival of the wild-type strain and ΔVC0770, Δcah, ΔnhaB, and Δclc mutants were reduced to between 15 to 30% (Fig. 6B). In contrast, the ΔaphB mutant showed only 0.096% survival (a 333-fold decrease), and the ΔcadC mutant showed essentially no survival (Fig. 6B). Without adaptation, none of the strains survived the 60-min incubation (less than 0.0001% survival). These results indicate that both AphB and CadC significantly contribute to the inorganic acid adaptation response.
A similar result was observed for the organic acid adaptation response. Adaptation of the cells to LB medium containing organic acids at pH 5.7 for 1 h resulted in complete survival after challenge with LB medium at pH 4.5 containing organic acids as described above for 20 min (data not shown), but often 60 min resulted in approximately 70% survival for the wild-type strain and ΔVC0770, Δcah, ΔnhaB, and Δclc mutants (Fig. 7B). Under these conditions, the ΔaphB mutant showed 5.6% survival (a 12.5-fold decrease) and the ΔcadC mutant showed essentially no survival. Thus, both AphB and CadC significantly contribute to the acid tolerance response to both inorganic and organic acids.
DISCUSSION
The virulence cascade in V. cholerae is influenced by a wide variety of environmental and physiological signals. However, since this regulation is complex, involving multiple promoters, activators, and repressors responding to the different stimuli simultaneously, it has been challenging to elucidate the mechanisms by which each signal impacts the expression of the cascade. The identification here of additional AphB-activated promoters in V. cholerae begins to shed light on the specific signals that influence the virulence cascade through AphB.
Microarray analysis led to the identification at least five other promoters besides tcpPH in V. cholerae for which AphB binds directly to a LysR-type motif to activate gene expression. For each of these promoters, cadC, VC0770, clc, nhaB, cah, and tcpPH, AphB-dependent activation was greater at pH 5.5 than at pH 7.0 under aerobic conditions. In addition, the expression of these promoters was greater under reduced-oxygen conditions than under aerobic conditions at neutral pH. These results indicate that the six different promoters in V. cholerae directly activated by AphB appear to be responsive to two different environmental signals: pH when grown aerobically and anaerobiosis at neutral pH.
With respect to the cadC promoter, AphB was capable of activating its expression in response to low pH and anaerobiosis in E. coli as well as in V. cholerae. The response to low pH in E. coli was similar to that in V. cholerae (approximately 2-fold cadC induction), and the response to anaerobiosis in E. coli was slightly greater than that in V. cholerae (17-fold versus 12-fold). Although it is not yet known if other proteins in addition to AphB play a role in this regulation, we found that neither of the two global regulators responsible for anaerobic induction of gene expression in enteric bacteria, Fnr or ArcAB, influenced the anaerobic induction of cadC expression in V. cholerae. In addition, loss of cadC induction in response to both low pH and anaerobiosis occurred in a region of the promoter between positions −78 and −63 which contains the AphB binding site. Since the expression of aphB was not influenced by pH and did not vary much more than 2-fold with respect to oxygen levels, these results raise the possibility that the activity of AphB may be influenced by both of these signals.
A large number of genes, including genes encoding several amino acid decarboxylases, have been shown to be influenced by both pH and oxygen levels (15, 45). The E. coli cadBA operon is induced in response to low pH, low oxygen concentrations, and lysine (7, 43, 60). In E. coli, the expression of cadC is constitutive and its activity is influenced by low pH and lysine (43, 58). CadC binds to two sites upstream of the cadBA promoter which are essential for transcriptional activation (30). Binding of H-NS upstream of these CadC binding sites is necessary for repression of cadBA under noninducing conditions (30). Decreasing the oxygen tension at low pH enhances the expression of cadBA (60). This appears to be due to a reduction in H-NS-mediated repression of the promoter, which occurs under aerobic conditions (30). In both E. coli and V. cholerae, cadBA is not fully induced under anaerobiosis unless the pH is also low (39, 60), presumably because CadC is not active. Although it is not known if H-NS plays a repressive role at the cadBA promoter in V. cholerae, we have found that H-NS does not play a role in regulating the expression of cadC in this organism with respect to oxygen limitation (data not shown). Since anaerobiosis may signal an impending encounter with low pH, the induction of cadC expression by anaerobiosis at neutral pH in V. cholerae may serve to ensure that sufficient levels of CadC are present when the pH begins to drop.
It has previously been shown in V. vulnificus that AphB activates the expression of cadC (49). Unlike the situation in V. cholerae, it appears that AphB-dependent activation of cadC is not influenced by pH (49). In addition, it has been shown in V. vulnificus that AphB plays a global role in the cell by regulating a large number of genes (>400) (20). Many of these genes are involved in the transport and metabolism of amino acids, carbohydrates, inorganic ions, nucleotides, and secondary metabolites. In addition, AphB influences processes such as motility, secretion, membrane and envelope biogenesis, and DNA replication, recombination, and repair (20). This raises the possibility that the pH dependence of AphB mediated regulation may be specific to V. cholerae.
Colonization of the human digestive tract by enteric bacteria requires transient survival through the stomach at a very low pH as well as resistance to short chain fatty acids produced by intestinal commensal bacteria. Inducible amino acid decarboxylases play an important role in the maintenance of pH homeostasis upon exposure to acid stress. In E. coli, glutamate and arginine decarboxylases are part of an extreme acid resistance response that allows survival at pH values as low as 2 (4, 31). Salmonella enterica serovar Typhimurium and V. cholerae lack an extreme acid resistance response but possess lysine decarboxylases that play a role in an adaptive acid tolerance response (39, 47). E. coli also has a lysine decarboxylase that contributes to survival at pH 5.5 in the presence of short chain fatty acids (12). Consistent with previous results (39), we found that cadC plays an important role in the responses to both inorganic acid (low pH) and organic acid (low pH plus short chain fatty acids). In addition, AphB, by virtue of its role in activating the expression of cadC, also plays a role in these responses but a slightly smaller one, most likely due to some basal level expression of cadC in its absence.
Besides cadC, none of the other genes directly regulated by AphB played a role in acid survival under the conditions examined here. E. coli has been shown to use two members of the ClC family with overlapping functions, EriC and MriT, in the extreme acid resistance response (19). Although the V. cholerae El Tor genome does not appear to encode another ClC family protein, it is possible that in strain C6706 another protein compensates for the absence of VCA0526. It was previously reported that in strain N16961, VCA0526 plays a role in the response to inorganic acid stress but not organic acid stress (9). We did not observe this phenotype in C6706, raising the possibility that it may have a different susceptibility to acid relative to N16961. Helicobacter pylori has a periplasmic α-type carbonic anhydrase that contributes to its survival at low pH (35). The El Tor genome has three genes annotated as carbonic anhydrase genes, VCA0274 (cah), VC0058, and VC0586. Since it was possible that they had partially redundant functions, we constructed a triple mutant lacking all three genes, yet it still did not display sensitivity to either inorganic or organic acid (data not shown). Thus, it appears that carbonic anhydrases do not play a role in acid survival of V. cholerae.
Na+/H+ antiporters are membrane proteins that play important roles in pH and Na+ homeostasis (46). In E. coli, nhaA is the main antiporter important for survival at high Na+ concentrations and high pH and it is activated by a member of the LysR family, NhaR. A second antiporter gene, nhaB, is expressed constitutively and becomes essential only when nhaA is absent. Unlike E. coli, V. cholerae contains a third antiporter, nhaD, and triple mutants lacking nhaA, nhaB, and nhaD are not significantly impaired at high Na+ concentrations and high pH, apparently because it possesses an electron transport-linked Na+ pump (NQR) that contributes to high Na+ resistance (16). The finding that the expression of nhaB in V. cholerae is not constitutive but is induced by AphB at low pH and low oxygen tensions suggests that this regulation provides a benefit to the cell under these conditions.
None of the genes identified here as being directly regulated by AphB influenced the expression of the virulence cascade in vitro under AKI conditions or influenced colonization in the infant mouse cholera model. Although cadA was initially identified as a gene induced during infection, the finding that it is not essential for colonization (39) is consistent with the results observed here for cadC. Since growing V. cholerae under acidic conditions prior to infection has been shown to significantly increase the ability of the organism to colonize (39), this result suggests that adaptation to acid conditions does have potential importance in the pathogenesis of cholera.
It was recently reported that bicarbonate induces virulence gene expression in V. cholerae by enhancing the activity of ToxT (1). In addition, the presence of the carbonic anhydrase inhibitor ethoxyzolamide significantly reduced virulence gene expression, suggesting that the activity of these enzymes plays a role in virulence induction. However, we found that the triple carbonic anhydrase deletion mutant (ΔVCA0274 ΔVC0058 ΔVC0586) did not appear to influence the expression of the virulence cascade under AKI conditions or influence colonization in infant mice. Thus, a role for carbonic anhydrase or any of the other AphB-regulated genes besides tcpPH in virulence cannot be established.
A major difference between the classical and El Tor biotypes of V. cholerae is that the induction of the virulence cascade requires different in vitro growth conditions. The classical biotype requires aerobic growth in LB medium, pH 6.5, at 30°C, whereas the El Tor biotype requires AKI conditions under which the cells are first incubated statically for 3.5 h before they are shifted to vigorous aeration (18). This difference in virulence gene expression between the two biotypes is due to a single nucleotide change in the AphB binding site within the tcpPH promoter, such that its affinity for AphB is reduced in the El Tor biotype (22, 24). In light of the observation here that low oxygen levels enhance the ability of AphB to activate the expression of its regulated genes, this may explain why the AKI method has been successful in inducing the expression of the virulence cascade in the El Tor biotype. The initial phase in which the cells grow statically for 3.5 h lowers the oxygen levels and increases the expression of tcpPH sufficiently to induce the expression of the cascade. Once induced, high-level expression of TCP and CT is obtained by vigorous aeration.
The results presented here show that AphB, in addition to initiating the virulence cascade, directly activates the expression of at least five other genes in V. cholerae. Although AphB may regulate additional genes in V. cholerae under environmental conditions different from those used here, we have thus far been unable to confirm that any other genes are regulated directly. However, it has recently been shown that AphB binds to the toxR promoter and contributes to its activation under stationary-phase conditions (61). The results here also show that the five genes directly regulated by AphB are activated independently in response to low pH and anaerobiosis, two signals encountered by V. cholerae during the early stages of the infection process. How this occurs is not yet known. Activation of promoters by LysR-type transcriptional regulators typically requires the binding of a small-molecule coinducer to the protein, which causes a conformational change in the structure of the protein-DNA complex that influences transcription. It is not yet known if AphB requires a coinducer to activate transcription. AphB could respond directly to these signals or, more likely, indirectly to them as a consequence of their influence on cellular physiology. It is tempting to speculate that the activity of AphB may in some way respond to both of these signals, and possible mechanisms involved in this are currently being investigated.
Acknowledgments
We thank Ronald Taylor and Carol Ringelberg for setup and assistance with the microarray analysis and Deborah Hogan and George O'Toole for helpful discussions. We are also grateful to Karen Thompson for assistance with the manuscript and Ronald Taylor and Raquel Martinez for critical reading of the manuscript.
This work was supported by grant NIH AI41558 to K.S.
Footnotes
Published ahead of print on 18 June 2010.
REFERENCES
- 1.Abuaita, B. H., and J. H. Withey. 2009. Bicarbonate induces Vibrio cholerae virulence gene expression by enhancing ToxT activity. Infect. Immun. 77:4111-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer, C. E., S. Elsen, and T. H. Bird. 1999. Mechanisms for redox control of gene expression. Annu. Rev. Microbiol. 53:495-523. [DOI] [PubMed] [Google Scholar]
- 3.Carroll, P. A., K. T. Tashima, M. B. Rogers, V. J. DiRita, and S. B. Calderwood. 1997. Phase variation in tcpH modulates expression of the ToxR regulon in Vibrio cholerae. Mol. Microbiol. 25:1099-1111. [DOI] [PubMed] [Google Scholar]
- 4.Castanie-Cornet, M.-P., T. A. Penfound, D. Smith, J. F. Elliott, and J. W. Foster. 1999. Control of acid resistance in Escherichia coli. J. Bacteriol. 181:3525-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Champion, G. A., M. N. Neely, M. A. Brennan, and V. J. DiRita. 1997. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol. Microbiol. 23:323-331. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee, A., P. K. Dutta, and R. Chowdhury. 2007. Effect of fatty acids and cholesterol present in bile on expression of virulence factors and motility of Vibrio cholerae. Infect. Immun. 75:1946-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dell, C. L., M. N. Neely, and E. R. Olson. 1994. Altered pH and lysine signalling mutants of cadC, a gene encoding a membrane-bound transcriptional activator of the Escherichia coli cadBA operon. Mol. Microbiol. 14:7-16. [DOI] [PubMed] [Google Scholar]
- 8.De Silva, R. S., G. Kovacikova, W. Lin, R. K. Taylor, K. Skorupski, and F. J. Kull. 2005. Crystal structure of the virulence gene activator AphA from Vibrio cholerae reveals it is a novel member of the winged helix transcription factor superfamily. J. Biol. Chem. 280:13779-13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding, Y., and M. K. Waldor. 2003. Deletion of a Vibrio cholerae ClC channel results in acid sensitivity and enhanced intestinal colonization. Infect. Immun. 71:4197-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutzler, R. 2006. The ClC family of chloride channels and transporters. Curr. Opin. Struct. Biol. 16:439-446. [DOI] [PubMed] [Google Scholar]
- 11.Foster, J. W., and H. K. Hall. 1991. Inducible pH homeostasis and the acid tolerance response of Salmonella typhimurium. J. Bacteriol. 173:5129-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilfoyle, D. E., and I. N. Hirshfield. 1996. The survival benefit of short-chain organic acids and the inducible arginine and lysine decarboxylase genes for Escherichia coli. Lett. Appl. Microbiol. 22:393-396. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, S., and R. Chowdhury. 1997. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect. Immun. 65:1131-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Häse, C. C., and J. J. Mekalanos. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 95:730-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes, E. T., J. C. Wilks, P. Sanfilippo, E. Yohannes, D. P. Tate, B. D. Jones, M. D. Radmacher, S. S. BonDurant, and J. L. Slonczewski. 2006. Oxygen limitation modulates pH regulation of catabolism and hydrogenases, multidrug transporters, and envelope composition in Escherichia coli K-12. BMC Microbiol. 6:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herz, K., S. Vimont, E. Padan, and P. Berche. 2003. Roles of NhaA, NhaB, and NhaD Na+/H+ antiporters in survival of Vibrio cholerae in a saline environment. J. Bacteriol. 185:1236-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins, D. E., and V. J. DiRita. 1994. Transcriptional control of toxT, a regulatory gene in the ToxR regulon of Vibrio cholerae. Mol. Microbiol. 14:17-29. [DOI] [PubMed] [Google Scholar]
- 18.Iwanaga, M., K. Yamamoto, N. Higa, Y. Ichinose, N. Nakasone, and M. Tanabe. 1986. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol. Immunol. 30:1075-1083. [DOI] [PubMed] [Google Scholar]
- 19.Iyer, R., T. M. Iverson, A. Accardi, and C. Miller. 2002. A biological role for prokaryotic ClC chloride channels. Nature 419:715-718. [DOI] [PubMed] [Google Scholar]
- 20.Jeong, H. G., and S. H. Choi. 2008. Evidence that AphB, essential for the virulence of Vibrio vulnificus, is a global regulator. J. Bacteriol. 190:3768-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacikova, G., and K. Skorupski. 1999. A Vibrio cholerae LysR homolog, AphB, cooperates with AphA at the tcpPH promoter to activate expression of the ToxR virulence cascade. J. Bacteriol. 181:4250-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovacikova, G., and K. Skorupski. 2000. Differential activation of the tcpPH promoter by AphB determines biotype specificity of virulence gene expression in Vibrio cholerae. J. Bacteriol. 182:3228-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovacikova, G., and K. Skorupski. 2001. Overlapping binding sites for the virulence gene regulators AphA, AphB and cAMP-CRP at the Vibrio cholerae tcpPH promoter. Mol. Microbiol. 41:393-407. [DOI] [PubMed] [Google Scholar]
- 24.Kovacikova, G., and K. Skorupski. 2002. Binding site requirements of the virulence gene regulator AphB: differential affinities for the Vibrio cholerae classical and El Tor tcpPH promoters. Mol. Microbiol. 44:533-547. [DOI] [PubMed] [Google Scholar]
- 25.Kovacikova, G., and K. Skorupski. 2002. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol. Microbiol. 46:1135-1147. [DOI] [PubMed] [Google Scholar]
- 26.Kovacikova, G., and K. Skorupski. 2002. The alternative sigma factor σE plays an important role in intestinal survival and virulence in Vibrio cholerae. Infect. Immun. 70:5355-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacikova, G., W. Lin, and K. Skorupski. 2004. Vibrio cholerae AphA uses a novel mechanism for virulence gene activation that involves interaction with the LysR-type regulator AphB at the tcpPH promoter. Mol. Microbiol. 53:129-142. [DOI] [PubMed] [Google Scholar]
- 28.Kovacikova, G., W. Lin, and K. Skorupski. 2005. Dual regulation of genes involved in acetoin biosynthesis and motility/biofilm formation by the virulence activator AphA and the acetate-responsive LysR-type regulator AlsR in Vibrio cholerae. Mol. Microbiol. 57:420-433. [DOI] [PubMed] [Google Scholar]
- 29.Krukonis, E. S., R. R. Yu, and V. J. DiRita. 2000. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol. Microbiol. 38:67-84. [DOI] [PubMed] [Google Scholar]
- 30.Kuper, C., and K. Jung. 2005. CadC-mediated activation of the cadBA promoter in Escherichia coli. J. Mol. Microbiol. Biotechnol. 10:26-39. [DOI] [PubMed] [Google Scholar]
- 31.Lin, J., I. S. Lee, J. Frey, J. L. Slonczewski, and J. W. Foster. 1995. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J. Bacteriol. 177:4097-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin, W., G. Kovacikova, and K. Skorupski. 2007. The quorum sensing regulator HapR downregulates the expression of the virulence gene transcription factor AphA in Vibrio cholerae by antagonizing Lrp- and VpsR-mediated activation. Mol. Microbiol. 64:953-967. [DOI] [PubMed] [Google Scholar]
- 33.Lowden, M. J., K. Skorupski, M. Pellegrini, M. G. Chiorazzo, R. K. Taylor, and J. F. Kull. 2010. Structure of Vibrio cholerae ToxT reveals a mechanism for fatty acid regulation of virulence genes. Proc. Natl. Acad. Sci. U. S. A. 107:2860-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manis, J. J., and B. C. Kline. 1977. Restriction endonuclease mapping and mutagenesis of the F sex factor replication region. Mol. Gen. Genet. 152:175-182. [DOI] [PubMed] [Google Scholar]
- 35.Marcus, E. A., A. P. Moshfegh, G. Sachs, and D. R. Scott. 2005. The periplasmic α-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation. J. Bacteriol. 187:729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marrero, K., A. Sanchez, A. Rodriguez-Ulloa, L. J. Gonzalez, L. Castellanos-Serra, D. Paz-Lago, J. Campos, B. L. Rodriguez, E. Suzarte, T. Ledon, G. Padron, and R. Fando. 2009. Anaerobic growth promotes synthesis of colonization factors encoded at the Vibrio pathogenicity island in Vibrio cholerae El Tor. Res. Microbiol. 160:48-56. [DOI] [PubMed] [Google Scholar]
- 37.Matson, J. S., and V. J. DiRita. 2005. Degradation of the membrane-localized virulence activator TcpP by the YaeL protease in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 102:16403-16408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matson, J. S., J. H. Withey, and V. J. DiRita. 2007. Regulatory networks controlling Vibrio cholerae virulence gene expression. Infect. Immun. 75:5542-5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merrell, D. S., and A. Camilli. 1999. The cadA gene of Vibrio cholerae is induced during infection and plays a role in acid tolerance. Mol. Microbiol. 34:836-849. [DOI] [PubMed] [Google Scholar]
- 40.Merrell, D. S., and A. Camilli. 2000. Regulation of Vibrio cholerae genes required for acid tolerance by a member of the “ToxR-like” family of transcriptional regulators. J. Bacteriol. 182:5342-5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 42.Miller, M. B., K. Skorupski, D. H. Lenz, R. K. Taylor, and B. L. Bassler. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303-314. [DOI] [PubMed] [Google Scholar]
- 43.Neely, M. N., and E. R. Olson. 1996. Kinetics of expression of the Escherichia coli cad operon as a function of pH and lysine. J. Bacteriol. 178:5522-5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nye, M. B., J. D. Pfau, K. Skorupski, and R. K. Taylor. 2000. Vibrio cholerae H-NS silences virulence gene expression at multiple steps in the ToxR regulatory cascade. J. Bacteriol. 182:4295-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olson, E. R. 1993. Influence of pH on bacterial gene expression. Mol. Microbiol. 8:5-14. [DOI] [PubMed] [Google Scholar]
- 46.Padan, E., M. Venturi, Y. Gerchman, and N. Dover. 2001. Na+/H+ antiporters. Biochim. Biophys. Acta 1505:144-157. [DOI] [PubMed] [Google Scholar]
- 47.Park, Y. K., B. Bearson, S. H. Bang, I. S. Bang, and J. W. Foster. 1996. Internal pH crisis, lysine decarboxylase and the acid tolerance response of Salmonella typhimurium. Mol. Microbiol. 20:605-611. [DOI] [PubMed] [Google Scholar]
- 48.Parsot, C., and J. J. Mekalanos. 1990. Expression of ToxR, the transcriptional activator of the virulence factors in Vibrio cholerae, is modulated by the heat shock response. Proc. Natl. Acad. Sci. U. S. A. 87:9898-9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhee, J. E., H. G. Jeong, J. H. Lee, and S. H. Choi. 2006. AphB influences acid tolerance of Vibrio vulnificus by activating expression of the positive regulator CadC. J. Bacteriol. 188:6490-6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saint-Dic, D., B. P. Frushour, J. H. Kehrl, and L. S. Kahng. 2006. A parA homolog selectively influences positioning of the large chromosome origin in Vibrio cholerae. J. Bacteriol. 188:5626-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salmon, K., S.-P. Hung, K. Mekjian, P. Baldi, G. W. Hatfield, and R. P. Gunsalus. 2003. Global gene expression profiling in Escherichia coli K12: the effects of oxygen availability and FNR. J. Biol. Chem. 278:29837-29855. [DOI] [PubMed] [Google Scholar]
- 52.Schuhmacher, D. A., and K. E. Klose. 1999. Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae. J. Bacteriol. 181:1508-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 54.Skorupski, K., and R. K. Taylor. 1996. Positive selection vectors for allelic exchange. Gene 169:47-52. [DOI] [PubMed] [Google Scholar]
- 55.Skorupski, K., and R. K. Taylor. 1999. A new level in the Vibrio cholerae ToxR virulence cascade: AphA is required for transcriptional activation of the tcpPH operon. Mol. Microbiol. 31:763-771. [DOI] [PubMed] [Google Scholar]
- 56.Soksawatmaekhin, W., A. Kuraishi, K. Sakata, K. Kashiwagi, and K. Igarashi. 2004. Excretion and uptake of cadaverine by CadB and its physiological functions in Escherichia coli. Mol. Microbiol. 51:1401-1412. [DOI] [PubMed] [Google Scholar]
- 57.Stonehouse, E., G. Kovacikova, R. K. Taylor, and K. Skorupski. 2008. Integration host factor positively regulates virulence gene expression in Vibrio cholerae. J. Bacteriol. 190:4736-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tetsch, L., C. Koller, I. Haneburger, and K. Jung. 2008. The membrane-integrated transcriptional activator CadC of Escherichia coli senses lysine indirectly via the interaction with the lysine permease LysP. Mol. Microbiol. 67:570-583. [DOI] [PubMed] [Google Scholar]
- 59.Tischler, A. D., and A. Camilli. 2005. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect. Immun. 73:5873-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watson, N., D. S. Dunyak, E. L. Rosey, J. L. Slonczewski, and E. R. Olson. 1992. Identification of elements involved in the transcriptional regulation of the Escherichia coli cad operon by external pH. J. Bacteriol. 174:530-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu, X., A. M. Stern, Z. Liu, B. Kan, and J. Zhu. 2010. Virulence regulator AphB enhances toxR transcription in Vibrio cholerae. BMC Microbiol. 10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang, M., E. M. Frey, Z. Liu, R. Bishar, and J. Zhu. 2010. The virulence transcriptional activator AphA enhances biofilm formation by Vibrio cholerae by activating the expression of the biofilm regulator VpsT. Infect. Immun. 78:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu, R. R., and V. J. DiRita. 2002. Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation. Mol. Microbiol. 43:119-134. [DOI] [PubMed] [Google Scholar]
- 64.Zhu, J., M. B. Miller, R. E. Vance, M. Dziejman, B. L. Bassler, and J. J. Mekalanos. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 99:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]