Abstract
IGF-I acts through endocrine and local, autocrine/paracrine routes. Disruption of both endocrine and local IGF-I action leads to neonatal lethality and impaired growth in various tissues including bone; however, the severity of growth and skeletal phenotype caused by disruption of endocrine IGF-I action is far less than with total IGF-I disruption. Based on these data and the fact that bone cells express IGF-I in high abundance, we and others predicted that locally produced IGF-I is also critical in regulating growth and bone accretion. To determine the role of local IGF-I, type 1α2 collagen-Cre mice were crossed with IGF-I loxP mice to generate Cre+ (conditional mutant) and Cre− (control) loxP homozygous mice. Surprisingly, approximately 40–50% of the conditional mutants died at birth, which is similar to total IGF-I disruption, but not observed in mice lacking circulating IGF-I. Expression of IGF-I in bone and muscle but not liver and brain was significantly decreased in the conditional mutant. Accordingly, circulating levels of serum IGF-I were also not affected. Disruption of local IGF-I dramatically reduced body weight 28–37%, femur areal bone mineral density 10–25%, and femur bone size 18–24% in growing mice. In addition, mineralization was reduced as early as during embryonic development. Consistently, histomorphometric analysis determined impaired osteoblast function as demonstrated by reduced mineral apposition rate (14–30%) and bone formation rate (35–57%). In conclusion, both local and endocrine IGF-I actions are involved in regulating growth of various tissues including bone, but they act via different mechanisms.
IGF-I was originally identified as a sulfation factor 50 yr ago in studies to understand how somatic growth was regulated by pituitary growth factor (1). Subsequent research on IGFs during the last 50 yr has provided convincing data that the IGFs are important regulators of pre- and postnatal growth. In addition, they are the most abundant growth factors stored in bone and produced by osteoblasts (2), and it has been well established that IGF-I plays an important role in regulating peak bone mineral density (BMD) and bone size (3, 4). It is well known, since the discovery of IGF-I, that it can act as an endocrine hormone in which it is released from the liver after stimulation by GH and acts on target tissues such as bone (5–8). It has also been suggested that IGF-I acts as a local growth factor through paracrine/autocrine actions (5, 6). To determine the relative contribution of systemic vs. local IGF-I, transgenic mouse models have been developed to disrupt IGF-I specifically in liver and other tissues. Specifically, using the Cre-loxP model, IGF-I production was disrupted in the liver, a tissue that contributes to about 75% of the circulating IGF-I. Surprisingly, when liver-derived IGF-I was disrupted and circulating IGF-I was reduced by about 70%, the effects on growth and skeletal parameters were minimal (9–11). However, when this mouse was crossed with a mouse lacking the acid-labile subunit (ALS), a protein that carries 70% of the circulating IGF-I, these mice demonstrated reduced growth and bone size, as well as a 90% reduction in circulating IGF-I (12), thus demonstrating the important role of circulating IGF-I in regulating growth. However, even with the 90% reduction in circulating IGF-I, the growth and skeletal phenotypes were much less severe than that observed in the total IGF-I knockout mouse. Therefore, based on these and other findings, we and others have suggested that, in addition to the endocrine actions of IGF-I, locally produced IGF-I acts in an autocrine/paracrine manner to regulate proliferation, differentiation, and apoptosis of osteoblast cells (10, 11, 13). In support of the proposed important role of local IGF-I in growth and development, it was recently demonstrated that local IGF-I signaling is required for optimal growth and bone volume (13) and overexpression of IGF-I in cells of osteoblastic lineage increased bone formation and resorption (14).
In terms of the importance of IGF-I in bone, it is known that cells of the osteoblastic lineage as well as chondroblasts and osteoclasts produce IGF-I (15, 16). However, the relative contribution of IGF-I produced by each cell type in regulating skeletal growth is not known. Based on the findings that disruption of 90% of circulating IGF-I is not as severe as the total IGF-I knockout mouse model and that local signaling through the IGF-I receptor is required for bone volume regulation, we hypothesized that disruption of IGF-I in type I collagen-expressing cells (primarily cells of the osteoblastic lineage) would result in reduced peak bone mass. Using the Cre-loxP model, we disrupted IGF-I in type 1α2 collagen (Col1α2)-expressing cells and demonstrate in this study that local production of IGF-I is critical for embryonic development, survival of newborn pups, optimal peak BMD, and bone size in mice.
Materials and Methods
Generation of Cre/loxP mice
Breeding pairs of transgenic mice in which Cre recombinase is driven by the procollagen, type IαII gene (Col1α2-Cre) were generated as previously described in an FVB/N background (17). Breeding pairs of transgenic mice in which exon 4 of the IGF-I gene is flanked by the loxP gene (IGF-Iflox/flox) were kindly provided by Dr. Derek LeRoith (Mt. Sinai School of Medicine, New York, NY) in a C57BL/6 background. Crosses to generate IGF-Iflox/flox/Col1α2-Cre mice (hereafter called Col1α2 conditional mutants or simply conditional mutants) were performed according to the following breeding scheme. IGF-Iflox/flox mice were bred to Col1α2-Cre-positive (+) mice to generate IGF-Iflox/−; Col1α2-Cre+ mice. These mice were then bred to IGF-Iflox/flox mice to generate conditional mutants. The conditional mutants were bred to IGF-Iflox/flox mice and their offspring were used for our experiments. The mice used for experiments were of a mixed genetic background; therefore, we used littermate controls to account for variation due to the mixed genetic background.
The experimental procedures performed in this study were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Studies Sub-committee at the Jerry L. Pettis Memorial Veterans Affairs Medical Center.
Genotyping of Cre/loxP mice
At 3 wk of age, DNA was extracted from ear or tail tissue using a Puregene DNA purification kit (Gentra Systems, Inc., Minneapolis, MN) according to the manufacturer’s protocol. PCR was performed to identify mice with Cre recombinase and/or loxP sites. Primers specific for the Cre recombinase gene (forward, 5′-TTAGCACCACGGCAGCAGGAGGTT-3′ and reverse, 5′-CAGGCCAGATCTCCTGTGCAGCAT-3′) were used under the following conditions: 94 C for 4 min; 33 cycles at 94 C for 1 min, 66 C for 1 min, 72 C for 10 min; 72 C from 10 min. loxP sites were amplified using primers previously described (18) under the following condition: 93 C for 2 min; 30 cycles at 93 C for 20 sec, 57 C for 1 min, 70 C for 1 min; 70 C for 10 min. The PCR products were run on a 2% agarose gel and the image taken with a ChemiImager 4400 (Alpha Innotech Corp., San Leandro, CA).
Bone densitometry by dual x-ray absorptiometry (DXA)
Bone mineral content (BMC) and areal BMD were measured by DXA, using the PIXImus instrument (Lunar Corp., Madison, WI). The precision for the BMC and BMD was ± 1% for repeat measurements of the same bones several times (19). Animals were anesthetized by a ketamine/xylaine (50/5 mg/kg body weight) injection before measurement.
Volumetric (v) BMD and geometric parameters
Volumetric BMD and geometric parameters at the middiaphysis of the femur and fourth and fifth lumbar vertebrae of bones isolated at 12 wk of age were determined by peripheral quantitative computed tomography (Norland Stratec XCT; Stratec Medzizintechnik GmbH, Madison, WI). Analysis of the scans was performed using the manufacturer-supplied software program (Stratec Medzizintechnik GmbH bone density software, version 5.40 C). Total BMD and geometric parameters were estimated with Loop analysis. The threshold was set at 230–630 mg/cm3. For femur analysis, nine scans per bone were measured and the data presented are the average of the fourth, fifth, and sixth scan (middiaphysis region). For the vertebrae analysis, six to nine scans at a set distance of 0.7 mm between each scan were measured, and the data presented are the average of the four to six scans that ran through the fourth and fifth vertebrae (scans between the two vertebrae or at the end of the vertebrae were eliminated). The coefficient of variation for total BMD, periosteal circumference, and endosteal circumference for repeat measurements of four mouse femurs (two to five measurements) were less than 3, less than 1, and less than 2%, respectively (19). The longitudinal lengths of the femurs and distance between the fourth and fifth lumbar vertebrae were measured with a caliper.
Immunohistochemistry analysis
Immunohistochemistry was used to assess Cre protein expression. Tissue sections were first heated in citrate buffer (pH 6.0) for 35 min at 95 C for antigen retrieval. Endogenous peroxidase activity and other nonspecific binding sites were blocked with 3% H2O2 and 20% normal goat serum, respectively. The sections were then incubated with an anti-Cre antibody (Novagen Laboratories, Madison, WI), at 1:2000 dilutions, at 37 C for 1 h. Subsequent detection procedures, including biotinylated antirabbit IgG antibody, streptavidin-HRP (Vector Labs, Burlingame, CA), and 3–3′ diaminobenzidine tetrahydrochloride-peroxide, were performed with an automated Ventana ES immunostainer (Ventana Medical Systems, Tucson, AZ) according to the manufacturer’s specification. All sections were examined using an Olympus BX-60 microscope (Olympus America, Melville, NY) and photomicrographs obtained with a Sony camera (Sony America, New York, NY).
Histological measurements
Growth rate of femurs between 9 and 14 d of age were determined as previously described (20, 21) using calcein (15 mg/kg body weight) as the double label.
Microcomputed tomography (µCT) analysis
Cortical and trabecular bone architecture were assessed using µCT (Inveon CT module; Siemens, Malver, PA). Specifically, femurs of control (n = 5) and conditional mutant (n = 5) females were scanned by x-ray (80 kVp volts; anode current at 250 µA) with an axial length of 1024 slices at 10 µm/slice and parallel length of 2048 slices. The voxel size was 10 µm. Reconstruction analysis was performed with COBRA software (Exxim, Pleasanton, CA). For analysis using Amira software (Mercury Computer Systems, Inc., Chelmsford, MA), 1-mm sections of the middiaphysis were used for cortical measurements and a fixed section of 3.2 mm at the distal end for trabecular measurements. Bone volume and surface area were determined and used to calculate trabecular volume, number, and thickness. The bones analyzed were adjusted for length such that the region of interest chosen for cortical and trabecular bone parameters was not different between the mutant and control bones.
Serum IGF-I RIA
IGF-I was measured by RIA using rabbit polyclonal antiserum and recombinant IGF-I as standard and tracer, respectively. IGF binding proteins (IGFBPs) were removed from serum before RIA by acid gel filtration protocol (22).
RNA extraction
RNA was extracted from the tissues using a lipid tissue minikit (QIAGEN Inc., Valencia, CA) according to the manufacturer’s protocol. For bone sample collection, muscle and tissue were removed, and bone marrow was flushed from the bone. RNA quality was determined using a 2100 Bioanalyzer (Agilent, Palo Alto, CA) and RNA was quantified using a NanoDrop spectrophotometer (Wilmington, DE).
Gene expression analysis
Quantitative real-time RT-PCR analysis was used to determine the expression levels of IGF-I; improved Cre (iCre); Col1α2; IGF-II; IGFBP-3, -4, -5; and peptidylprolyl isomerase A (endogenous control) as previously described (23). Primers used were validated as previously described (23). Δ Cycle threshold (CT) values were determined (CT value for gene of interest minus CT value for control gene) and comparisons of the CT values were used for relative quantification of gene expression (24).
Statistical analysis
Bone parameters analyzed by DXA analysis at 4, 8, and 12 wk of age were analyzed by repeated-measures ANOVA. All other data were analyzed by ANOVA and/or student’s t test where appropriate. Post hoc analysis was performed using Newman-Keuls analysis. Data were analyzed using Statistica 6 software (StatSoft, Tulsa, OK). Data are presented as mean ± se, and a significant difference was determined at P ≤ 0.05. For most of the growth and bone parameters evaluated after 2–4 wk of age, the expected gender difference with males being greater than females was observed; however, a gender-by-treatment interaction was not observed; therefore, male and female data were combined for the figures presented where appropriate.
Results
Reduced survival rate of conditional mutant mice
To obtain mice for our experiments, we bred Col1α2-Cre+, IGF-Iflox/− with Col1α2-Cre−, IGF-Iflox/flox mice to generate a ratio of 1:1:1:1 for the four potential genotypes generated by this breeding scheme. We used two lines of iCre mice (A23 and A26) (17). To our surprise, we did not obtain any live conditional mutant pups (Col1α2-Cre+, IGF-Iflox/flox) from our A23 line. However, we did observe the expected ratio of pups for the remaining three potential genotypes (Table 1). In contrast, we observed about half of the expected number of conditional mutants for line A26 (Table 1). In addition, when we obtained embryos between embryonic d (E) 12.5 and 18.5, we observed the expected ratio of 1:1:1:1 (data not shown), thus suggesting that 100% of the A23 and 50% of the A26 conditional mutants die at or shortly after birth. Based on these findings and the knowledge that 32–90% of total IGF-I knockout pups die at birth due to respiratory failure (25) and the findings that type I collagen is expressed during early lung development (26), we hypothesize that poor lung development caused by local disruption of IGF-I in the lung may attribute to increased mortality in the conditional mutants; however, further analysis is needed to identify the specific cause of death in these mice.
TABLE 1.
Reduced survival in mice lacking local production of IGF-I
| Line | Number of live pups |
loxP +/+ | loxP +/− | ||
|---|---|---|---|---|---|
| iCre+ | iCre− | iCre+ | iCre− | ||
| A23 | 120 | 0 | 30 | 48 | 42 |
| A26 | 149 | 22 | 44 | 48 | 35 |
Data are the number of pups for each genotype from two lines of iCre mice (A23 and A26). Lines A23 and A26 had a total of 19 and 20 litters, respectively. We anticipated a 1:1:1:1 ratio for the four potential genotypes based on the genotype of breeding pairs of mice used (iCre−/−, IGF-Iflox/flox × iCre+/−, IGF-Iflox/−).
Confirmation of specificity of iCre expression and reduced IGF-I expression in conditional mutant mice
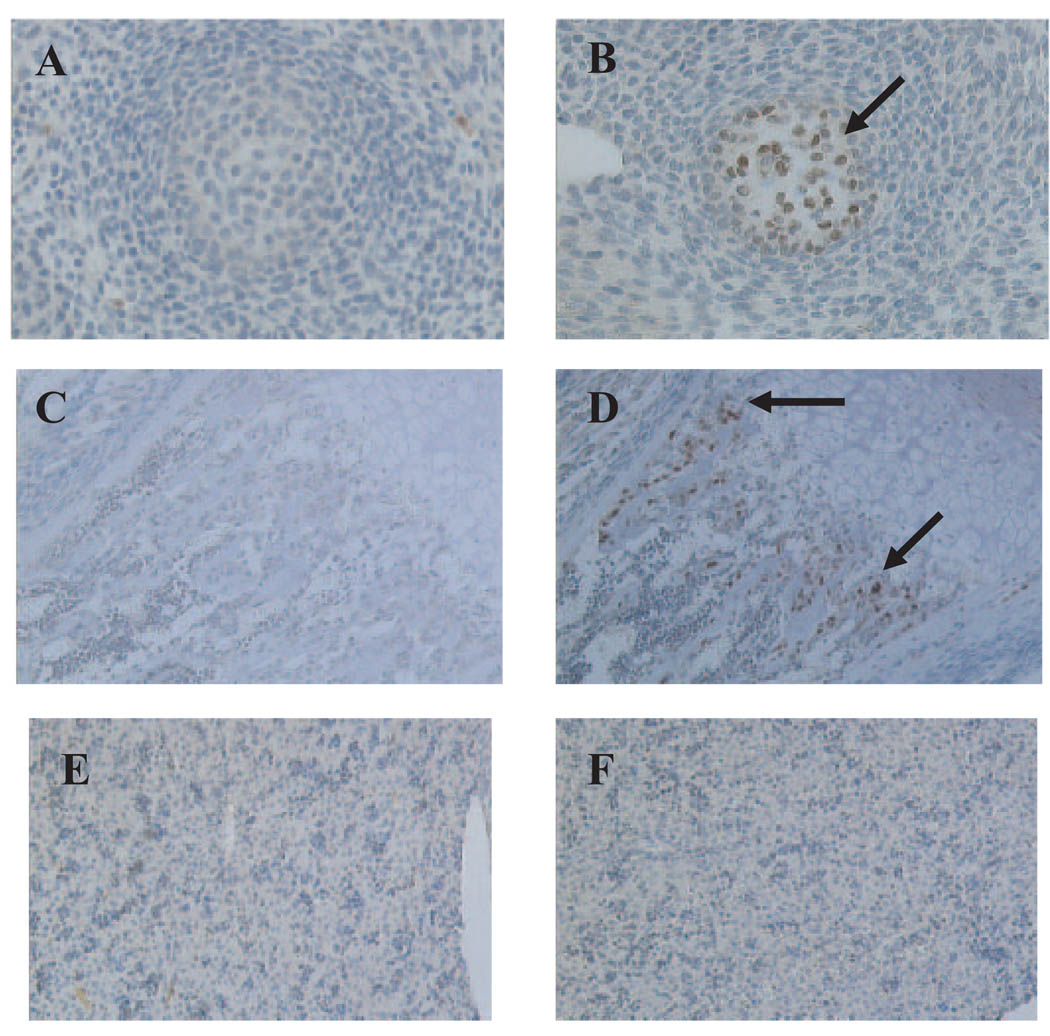
To confirm the expression of iCre recombinase in cells that express Col1α2, we obtained cross-sections of conditional mutant and control mice at E12.5 and newborn. We observed iCre expression in bone forming cells in the neck region of the conditional mutants as early as E12.5 (Fig. 1, A and B). In addition, iCre expression was observed in the bone-forming region of the tibia (primarily osteoblasts) in newborn conditional mutants but not control mice (Fig. 1, C and D). We did not observe any iCre expression in the livers of the conditional mutant and control mice (Fig. 1, E and F), thus demonstrating the specificity of iCre expression.
FIG. 1.
Cell type-specific iCre expression in embryos and newborn mice. Immunostaining was performed for iCre in cross-sections from the neck region of embryos at d 12.5 (A and B) and tibia (C and D) and liver (E and F) of newborn mice. Control (A, C, and E) and conditional mutant (B, D, and F) mice are shown. Arrows indicate iCre-specific immunostaining in bone-forming regions. iCre was not detectable in the livers of conditional mutants.
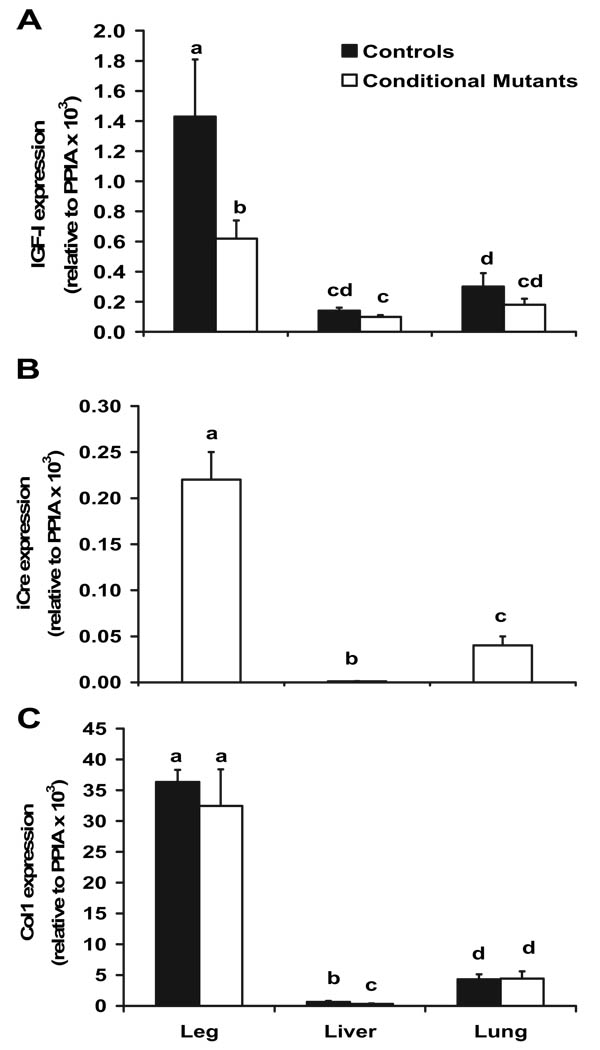
Because iCre expression is specific to cells that express Col1α2, we anticipated that IGF-I expression would be disrupted in these cells. Accordingly, we determined that IGF-I expression was reduced 50% in the legs of the embryos at E16.5 (Fig. 2A) but not the liver or lung tissues. These data are consistent with the greater iCre (Fig. 2B) and Col1α2 (Fig. 2C) expression in the legs, compared with the liver and lung tissue. We used the whole leg rather than bone from the embryos due to the difficulty in isolating poorly mineralized long bones from muscle at this embryonic stage. Although other tissues besides bone are present in the legs, the results in terms of mRNA expression are similar to our finding in long bones at 2 and 12 wk of age (data not shown). At 12 wk of age, we observed a positive correlation (R2 = 0.53; P < 0.0001) between iCre and Col1α2 expression in various tissues, thus resulting in the greatest amount of iCre and Col1α2 in bone (Table 2). Consistent with these data, mRNA expression of IGF-I was reduced the most in bone, compared with other tissues, that express some levels of Col1α2 at 12 wk of age (Fig. 3). In support of reduced local IGF-I, but no effect on endocrine IGF-I in our model, circulating levels of serum IGF-I were not different (283 ± 11 vs. 280 ± 11 ng/ml; P = 0.8810) between control and conditional mutant mice, respectively.
FIG. 2.
mRNA expression of IGF-I (A), iCre (B), and Col1 (C) in 16.5-d-old embryos. Data were analyzed for ΔCT values using ANOVA and are presented as mean ± sem relative to control gene expression [peptidylprolyl isomerase A (PPIA)]. Means with different letters (a, b, c, d) are significantly different at P ≤ 0.05. Leg, n = 5–7; liver, n = 4; lung, n = 4.
TABLE 2.
Col1α2 and iCre expression in different tissues of conditional mutant mice at 12 wk of age
| Gene | Bone | Liver | Kidney | Muscle | Brain | Heart |
|---|---|---|---|---|---|---|
| Col1 | 103 ± 11.4 | 0.06 ± 0.02a | 0.75 ± 0.12a | 6.1 ± 1.8a | 0.07 ± 0.01a | 6.9 ± 0.88a |
| iCre | 117 ± 27 | 0.14 ± 0.04a | 3.9 ± 1.0a | 35.6 ± 11.5a | 10.9 ± 3.5a | 28.6 ± 6.3a |
Data are presented as percent of long bone (mean ± sem), n = 5–7 mice/treatment.
Significant different from long bone at P ≤ 0.05.
FIG. 3.
Reduced local IGF-I expression. Data from 12-wk-old mice are expressed as fold change from controls and presented as mean ± sem, n = 6–17. *, Significant difference between conditional mutant and control mice (P < 0.05). Control values are set at 1-fold.
Reduced mineralization in conditional mutant mice
To determine whether reduced local production of IGF-I altered bone mineralization, we obtained embryos at E16.5 and stained for Alizarin Red and Alcian Blue. In addition, to a 14% reduction in body weight at this age (P < 0.05; data not shown), we observed reduced mineralization throughout the entire skeleton as demonstrated by the reduced intensity of the Alizarin Red stain (Fig. 4, A and B). Specifically, reduced mineralization was observed in the calvaria (Fig. 4, C and D), long bones (Fig. 4, E and F), and vertebrae (Fig. 4, G and H) of the conditional mutant mice, compared with controls.
FIG. 4.
Reduced mineralization in conditional mutant mice. Embryos at d 16.5 were stained for Alizarin Red and Alcian Blue. Control (B, D, F, and H) and conditional mutant (A, C, E, and G) mice.
Growth and bone parameters between 2 and 12 wk of age
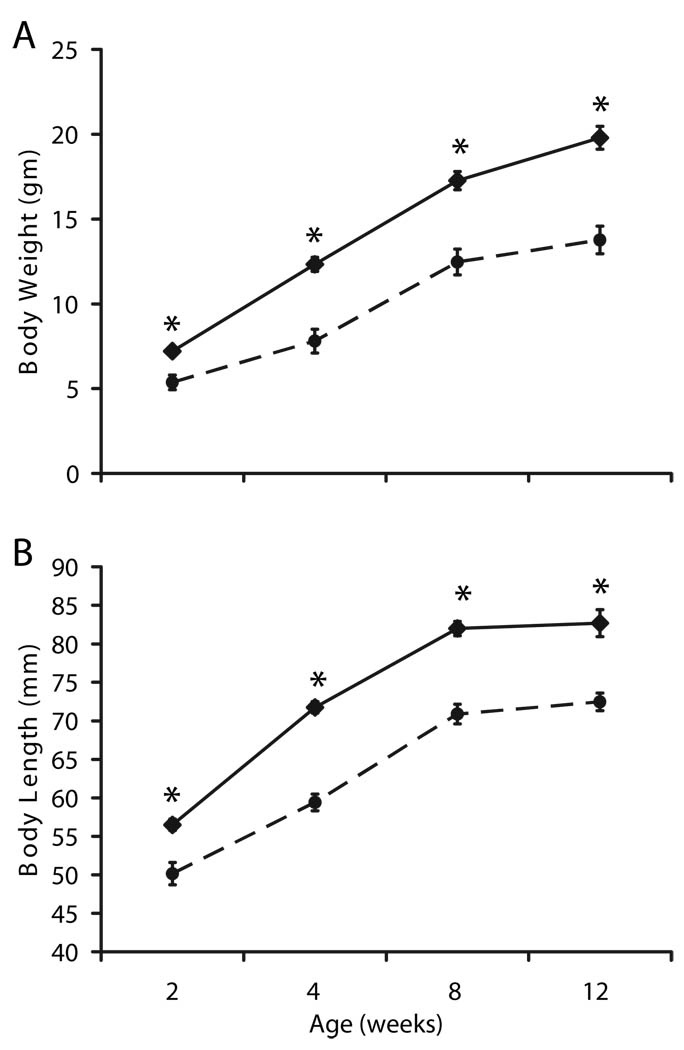
Lack of local IGF-I production in Col1α2-expressing cells reduced body weight by 26–37% as early as 2 wk of age, and this reduction was maintained through 12 wk of age (P < 0.0001; Fig. 5A). In addition, a reduction in body length by 12–14% was observed between 2 and 12 wk of age (P < 0.0001; Fig. 5B) in conditional mutant mice.
FIG. 5.
Reduced body weight (A) and length (B) in conditional mutants between 2 and 12 wk of age (n = 7–12 for 2 wk of age; n = 17–19 for 12 wk of age). All data are expressed as mean ± sem. At 2 wk of age, a significant effect of genotype was observed at P < 0.001. Solid line, Controls; dashed line, conditional mutants. *, Significant difference between conditional mutant and control mice (P < 0.0001).
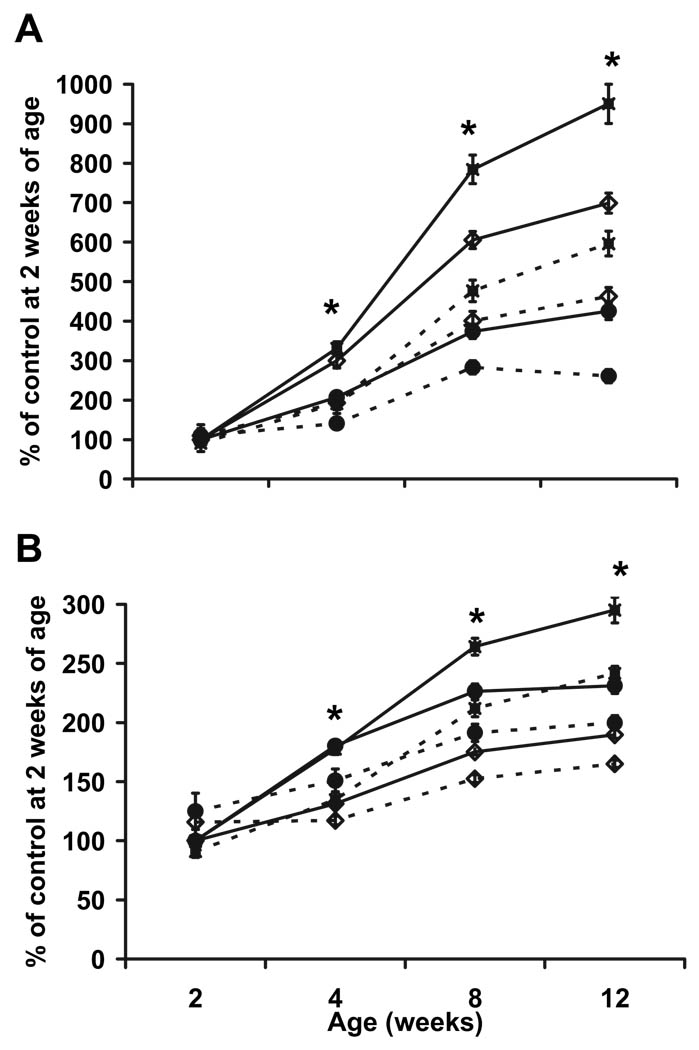
As determined by DXA analysis, total body BMC (Fig. 6A) and BMD (Fig. 6B) were reduced 35 and 10–13%, respectively, between 4 and 12 wk of age (P < 0.01) in conditional mutant mice. Specifically, femur BMC and BMD were reduced 37–41 and 18–25%, respectively, between 4 and 12 wk of age (P < 0.001) in conditional mutant mice. Similar to femur, tibia BMC and BMD were reduced between 4 and 12 wk of age (P < 0.001; data not shown). Vertebrae BMC and BMD were reduced 25–39 and 14–16%, respectively, between 4 and 12 wk of age (P < 0.05) in conditional mutants. At 2 wk of age, a significant difference in BMC and BMD was not observed for femur, vertebrae, and total body (P > 0.10). However, a reduction (P < 0.05) in tibia BMC and BMD (39 and 11%, respectively) was observed at 2 wk of age. The expected gender difference with males being greater than females was observed for several parameters after 4 wk of age. However, a gender-by-treatment interaction was not observed, so data were combined in the figures presented.
FIG. 6.
Reduced BMC (A) and BMD (B) in conditional mutant mice between 2 and 12 wk of age (n = 7–12 for 2 wk of age; n = 17–19 for 12 wk of age). All data are expressed as mean ± sem. Solid line with closed square, Control femur; dashed line with closed square, conditional mutant femur; solid line with closed circle, control vertebrae; dashed line with closed circle, conditional mutant vertebrae; solid line with open triangle, control total body; dashed line with open triangle, conditional mutant total body. *, Significant difference between conditional mutant and control mice (P < 0.05).
Volumetric BMD and geometric parameters
Because areal BMD measurements by DXA are subject to influence by bone size, we also measured vBMD of femur and vertebrae by peripheral quantitative computed tomography, which measures the amount of mineral per unit volume of bone. At 12 wk of age, a reduction in cortical vBMD (5%; P = 0.0015) and bone length (15%; P < 0.01) was observed in the femurs (data not shown). In the vertebrae of the mice at 12 wk of age, a 7, 5, and 8% reduction in total, trabecular, and cortical vBMD (P < 0.05), respectively, was observed. In addition, bone length was reduced by 17% (P < 0.0001) for the fourth and fifth lumbar vertebrae (data not shown).
Dynamic histomorphometric analysis
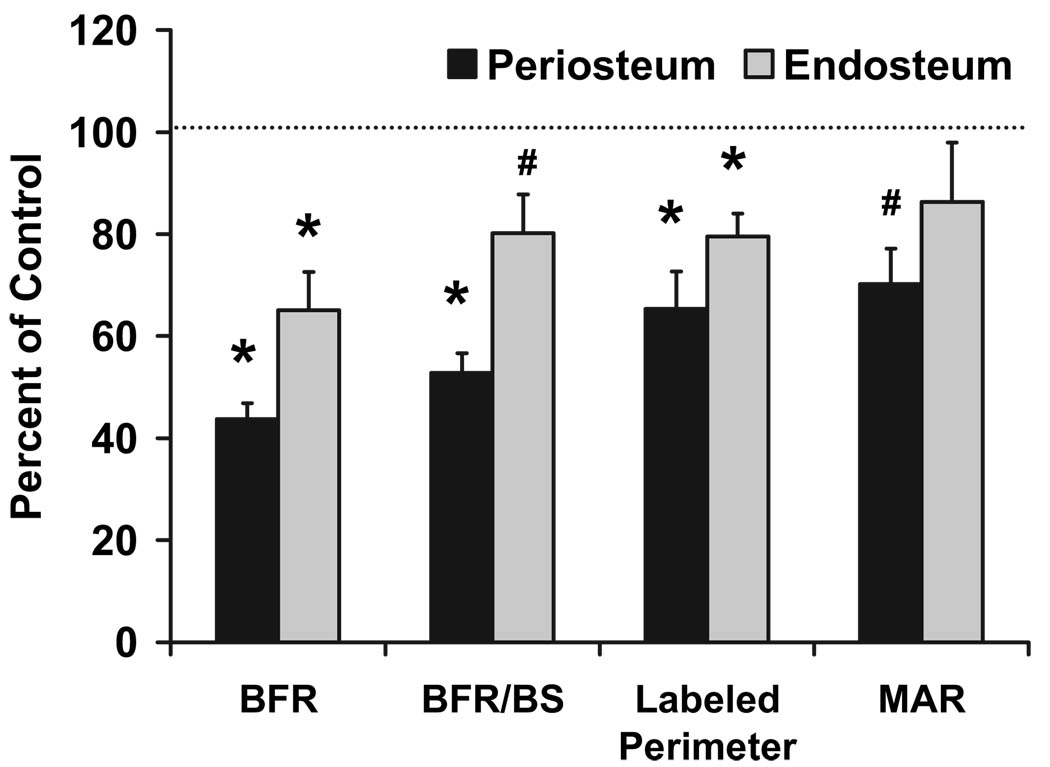
The mechanism behind the significantly reduced BMD was evaluated by performing dynamic histomorphometry. At the femoral middiaphysis, we observed a 32–34% reduction in total, bone, and medullary area (P < 0.05) in the conditional mutant mice (data not shown). Periosteal and endosteal perimeter were reduced by 18 and 21%, respectively (P < 0.05; data not shown). In the conditional mutant mice, the bone formation rate was reduced 57 and 38% in the periosteum and endosteum, respectively (P < 0.05; Fig. 7), demonstrating a reduction in total osteoblastic activity. To rule out the possibility that the reduced bone formation rate in the conditional mutants is not due to reduced bone size, we divided bone formation rate with bone surface in the two groups of mice to correct for bone size differences. As seen in Fig. 7, bone formation rate adjusted for bone surface was significantly reduced in the conditional mutant mice. In addition, there was tendency for a 38% reduction in mineral apposition rate in the periosteum (P = 0.0674; Fig. 7), suggesting impaired differentiation and/or function of osteoblasts as the major cause for reduced bone formation.
FIG. 7.
Dynamic histomorphometry demonstrates reduced bone formation and bone size in conditional mutant mice at 2 wk of age. BFR, Bone formation rate; BS, bone surface; MAR, mineral apposition rate. Controls, n = 6–8; conditional mutants, n = 8–12. *, P < 0.05 vs. control; #, P ≤ 0.07 vs. control. Data are expressed as a percent of controls and presented as mean ± sem.
µCT analysis
In the femurs of conditional mutant females, cortical and trabecular bone volume were reduced 27 and 57%, respectively (P < 0.01), and total volume was reduced 37 and 46%, respectively (P < 0.001; Fig. 8). Accordingly, bone volume/total volume (BV/TV) was slightly elevated in cortical bone (16%; P < 0.01) of conditional mutants but not significantly different from controls in trabecular bone (P = 0.15). We did not observe a significant difference (P > 0.25) in BV/TV (0.2135 ± 0.0141 vs. 0.1921 ± 0.0110 for control and conditional mutant, respectively), bone surface/volume (38.57 ± 2.43mm−1 vs. 34.64 ± 1.43mm−1 for control and conditional mutant, respectively), trabecular thickness (9.51 ± 0.55 mm vs. 10.54 ± 0.60 mm for control and conditional mutant, respectively), trabecular spacing (36.33 ± 4.38mm vs. 45.68 ± 5.76 mm for control and conditional mutant, respectively), and trabecular number (0.0232 ± 0.0032 mm−1 vs. 0.0187 ± 0.0021 mm−1 for control and conditional mutant, respectively).
FIG. 8.
Reduced bone and total volume in conditional mutants as determined by µCT analysis. A and C, Control. B and D, Conditional mutant. A and B, Cortical bone in cross-section of femur mid-shaft. C and D, Trabecular bone in cross-section of femur distal end. E, Controls (n = 5); conditional mutants (n = 5). *, Significant difference from control at P ≤ 0.01. Data are expressed as a percent of controls and presented as mean ± sem.
Reduced rate of gain in conditional mutant mice
During prepubertal growth between 2 and 4 wk of age, a period of rapid bone accretion, there was a 43–87% reduction in the rate of gain of total body, femur, and vertebrae BMC and BMD (P < 0.05; Fig. 9). However, from 4 to 8 wk of age, the rate of gain in total body and femur BMD was reduced about 35% (P < 0.05) but was not different for other parameters between conditional mutant and control mice.
FIG. 9.
Reduced rate of gain between 2 and 4 wk of age in conditional mutants. F, Femur; TB, total body; V, vertebrae. *, P < 0.05 vs. control; #, P ≤ 0.07 vs. control. All data are expressed as a percent of controls and presented as mean ± sem.
mRNA expression of IGF-I, IGF-II, and IGFBPs
To determine whether expression of other IGF system components were altered in the conditional mutants, we determined the expression of IGF-II and IGFBPs by real-time RT-PCR at various ages in the bones of control and conditional mutants (Fig. 9). Interestingly, expression of IGF-II increased 1.6- to 4.0-fold (P < 0.05) during embryonic and early postnatal development. Similarly, expression of IGFBP-3 increased 2-fold during these periods of growth (P < 0.05; Fig. 10). Expression of IGFBP-4 a key binding protein in bone, was reduced 2-fold during embryonic development (P < 0.01), but we did not observe a significant difference at later time points (P ≥ 0.5029; Fig. 10). Surprisingly, we did not detect a significant change in IGFBP-5 expression at any of the time points examined (P ≥ 0.3449).
FIG. 10.
Increased IGF-II and IGFBP-3 expression in the bones of conditional mutants during embryonic and early postnatal development. Gene expression was determined by real-time RT-PCR. Embryos, n = 4; 2 wk of age, n = 8; 12 wk of age, n = 7–8. *, Significant difference from controls at P ≤ 0.05. Data are expressed as fold change from controls and presented as mean ± sem. Control values are set at 1-fold.
Discussion
The Cre-loxP model is a well-established method to evaluate the role of a gene of interest in a specific tissue and/or cell type (27–29). In this model the efficiency of Cre recombinase and disruption of the gene of interest are critical. Our primary focus was on evaluating the role of local IGF-I in bone, and therefore, we used mice in which Cre recombinase was driven by the Col1α2 gene, which is predominantly expressed in osteoblasts. In addition, these mice were developed using an iCre sequence (30) and contain the entire Col1α2 gene, plus 60 kb of upstream regulatory sequences, thus providing us with an efficient Cre transgenic mouse model. The specificity of the iCre expression in Col1α2-expressing cells was previously shown (17). We confirmed the specificity of the iCre expression in tissues with high expression of Col1α2 (primarily bone) by immunostaining and real-time RT-PCR. In addition, we determined that the greatest disruption of IGF-I is observed in the bone tissue. Surprisingly, we did observe a small but significant reduction in IGF-I expression in other tissues (liver, kidney, and heart) besides bone, which could be explained by the expression of Col1α2 at high levels in specialized cells at certain stages of embryonic or postnatal development in nonbone tissues, which could have contributed to the iCre expression and consequently IGF-I gene disruption. Surprisingly, IGF-I expression was reduced 5-fold in the muscle despite much lower levels of type 1 collagen and iCre expression when compared with bone. It remains to be determined whether the reduced expression of IGF-I in muscle is in part due to decreased paracrine action of bone produced IGF-I. Importantly, our data on IGF-I expression in different tissues reveal that the Cre model chosen in this study exerts nearly a 70% reduction in bone produced IGF-I. These data, together with the finding that serum IGF-I is not altered at either 2 or 12 wk of age, are consistent with the idea that the observed skeletal effects are due to disruption of local but not circulating IGF-I. The issue of whether the small reduction in IGF-I in other tissues contributes to skeletal deficit by reduced production of systemic factors that influence bone accretion remains to be established.
In this study, we did not perform IGF-I protein measurements in the skeletal tissue extracts of conditional mutants and control mice because the relative contribution of IGF-I derived from osteoblasts vs. circulation to IGF-I stored in bone is not known. Because local production of IGF-I but not circulating IGF-I is diminished in the conditional mutant mice, the levels of IGF-I in bone extracts may not be a reliable measure of IGF-I protein production by osteoblasts if circulating IGF-I also contributes to the depot of IGF-I in bone. Future immunocytochemistry studies using reliable IGF-I antibody are needed to convincingly demonstrate that IGF-I protein expression is indeed reduced in other cell types besides bone of conditional mutant mice, compared with control mice.
One of the surprising findings in this study relates to the fact that 100% of the mice born to line A23 die at or before birth. This is in contrast to previous findings that 10–70% of total IGF-I knockout mice survive in different genetic backgrounds (25). Previous studies demonstrate that the total IGF-I knockout mice die due to inadequate lung development and respiratory failure (25, 31). It has also been demonstrated that type 1 collagen is detected in the diaphragm and lungs of developing mice at E12.5 and 14.5, respectively (26). In addition, IGF-I expression is detected as early as E12.5 in the lungs (32), and its expression is localized in the mesenchymal cells of the lungs and airway epithelium (33). It was previously demonstrated that IGF-I and -II knockout mice have similar hypercellularity and less alveolar separation of the developing lung (34, 35). Therefore, based on these previous findings and our identification of conditional mutants in both lines at the expected mendelian ratio during embryonic development, we hypothesize that these conditional mutants die due to inadequate lung development and/or respiratory failure. In terms of why there is increased mortality in the conditional mutants vs. total IGF-I knockout mice, one possibility is that in the case of total IGF-I knockout mice, all organs are correspondingly reduced in growth, and therefore, functional demand on the lungs is considerably less. However, in the case of the conditional mutants, the weak diaphragm and poorly developed lungs may not be able to support other organs that are normally developed, thus leading to increased lethality. Second, our mice are a mixed genetic background but primarily C57BL/6, which may increase the chances of mortality with reduced IGF-I (25). Further analysis is needed to identify the specific reason for increased mortality in these mice.
Our findings provide the first direct experimental evidence that locally produced IGF-I plays a critical role in embryonic and postnatal growth. Local IGF-I from Col1α2-producing cells is required for mineralization during embryonic development and postnatal growth. Interestingly, our findings that disruption of local IGF-I reduced the rate of gain in several growth and skeletal parameters during the prepubertal growth period are consistent with our previous findings that IGF-I is critical during the prepubertal growth period (4). Although it has previously been demonstrated that total disruption of IGF-I alters embryonic skeletal development (36), these are the first data demonstrating a direct role for local IGF-I in regulating bone accretion during all stages of development as demonstrated by reduced mineralization in newborn pups and reduced BMC and BMD between 2 and 12 wk of age.
The impairment in bone accretion during postnatal development in conditional mutant mice is due to decreased bone formation both at the periosteal and endosteal sites as revealed by our histomorphometric data (Fig. 7). This reduced bone formation can be due to decreased osteoblast cell number and/or activity because in vitro studies have shown that IGFs stimulate proliferation and differentiation and decrease apoptosis (37, 38). Accordingly, our histomorphometric studies revealed a significant decrease in labeled perimeter, an indirect measure of osteoblast number, in the bones from conditional IGF-I knockout mice, compared with corresponding control mice, thus demonstrating that lack of locally produced IGF-I leads to a decrease in osteoblast number at both the periosteum and endosteum. Furthermore, mineral apposition rate, which is a measure of osteoblast activity, was also decreased in the bones of conditional IGF-I knockout mice, compared with control mice. Thus, these data provide the first in vivo evidence that locally produced IGF-I is involved in regulating both osteoblast cell number and activity.
A previous study demonstrated a significant increase in BV/TV for trabecular bone with total IGF-I disruption (39). In our study, local disruption of IGF-I did not produce similar changes in the trabecular parameters that were observed in total IGF-I knockout mice (39, 40). In contrast, there was a small but statistically significant increase in BV/TV for cortical bone in the conditional IGF-I knockout mice, compared with corresponding control mice. Because IGF-I is known to be a positive regulator of bone formation, the observed increases in trabecular and cortical volume, respectively, in total IGF-I and local IGF-I knockout mice were surprising. Although the femurs of conditional mutant mice were about 15% shorter, compared with control mice, we adjusted the region of interest for their respective bone length such that the anatomical sampling site is equivalent for both sets of bones irrespective of their differences in length. Thus, the differences or their lack of in various parameters between the conditional mutants and controls cannot be explained on the basis of differences in the region of interest. Future studies are needed to determine whether the increases in trabecular and cortical bone volume in the total and conditional IGF-I knockout mice represent compensatory mechanisms to maintain bone strength that is compromised by large decreases in bone size and volumetric BMD in total IGF-I knockout mice and bone size in conditional IGF-I knockout mice and evaluate the mechanisms that contribute to the increases in trabecular and cortical bone volume in these mice.
It is well known that IGF-II is a critical growth factor for embryonic and early postnatal growth. Therefore, to determine whether reduced local production of IGF-I would alter IGF-II, we looked at its expression during different stages of development. Interestingly, expression of IGF-II was elevated during embryonic and early postnatal development in the long bones of conditional mutant mice. Although elevated IGF-II might suggest a potential compensatory mechanism, we did observe a significant reduction in BMD during early postnatal growth in the conditional mutant mice. These findings are consistent with recent reports that elevated IGF-II postnatally does not rescue the phenotype in total IGF-I knockout mice (41). It is known that IGF actions in bone are subject to modulation by IGFBPs (42, 43). We found that whereas the expression levels of stimulating IGFBP-5 were not altered, expression of inhibitory IGFBP-4 was reduced in long bones during embryonic development. Further analysis is needed to determine whether reduced IGFBP-4 expression is a direct effect of reduced local IGF-I or a result of the increased expression of IGF-II. Overall, these findings support the close regulation and interaction between the IGFs and their binding proteins in bone.
As previously discussed, it has been well established that IGF-I is critical for normal growth and development (4, 25, 31, 35, 40, 44). In addition, a certain level of circulating IGF-I is required for optimal bone development in mice; however, even in the liver-derived IGF-I/ALS knockout model, the phenotype is not as severe as the total IGF-I knockout mouse. Our data provide the first direct evidence that local production of IGF-I from Col1α2 cells is required for newborn survival, optimal postnatal growth, and peak bone mass and size in mice and support an important role for local IGF-I. Based on previous findings and our current data, we conclude that both local and endocrine sources of IGF-I play a role in regulating skeletal growth. The availability of mice with disruption of IGF-I gene in Col1α2-expressing cells should provide a model to evaluate the role of locally produced IGF-I in mediating the effects of systemic and local regulators of bone metabolism.
Acknowledgments
The authors acknowledge the contribution of Professor Derek LeRoith (Mt. Sinai School of Medicine, New York, NY) for providing us with IGF-1 loxP mice for these studies. All work was performed in facilities provided by the Department of Veterans Affairs. The authors thank Erica Winter, Catrina Raynor, Joe Rung-Aroon, Nancy Lowen, Carolyn Hargrave, Shamyne Hover, and Sheila Pourteymoor for technical assistance and Terry Dent for secretarial assistance.
This work was supported by Grant AR048139 from the National Institutes of Health (to S.M.).
Abbreviations
- BMC
Bone mineral content
- BMD
bone mineral density
- BV/TV
bone volume/total volume
- Col1α2
type 1α2 collagen
- Col1α2-Cre
Cre recombinase driven by procollagen, type IαII gene
- CT
cycle threshold
- µCT
microcomputed tomography
- DXA
dual x-ray absorptiometry
- E
embryonic d
- iCre
improved Cre
- IGFBP
IGF binding protein
- v
volumetric
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Salmon WD, Jr, Daughaday WH. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957;49:825–836. [PubMed] [Google Scholar]
- 2.Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- 3.Mohan S, Baylink DJ. Impaired skeletal growth in mice with haploin-sufficiency of IGF-I: genetic evidence that differences in IGF-I expression could contribute to peak bone mineral density differences. J Endocrinol. 2005;185:415–420. doi: 10.1677/joe.1.06141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohan S, Richman C, Guo R, Amaar Y, Donahue LR, Wergedal J, Baylink DJ. Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and -independent mechanisms. Endocrinology. 2003;144:929–936. doi: 10.1210/en.2002-220948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 6.Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- 7.Cohen P. Overview of the IGF-I system. Horm Res. 2006;65 Suppl 1:3–8. doi: 10.1159/000090640. [DOI] [PubMed] [Google Scholar]
- 8.Canalis E. Growth hormone, skeletal growth factors and osteoporosis. Endocr Pract. 1995;1:39–43. doi: 10.4158/EP.1.1.39. [DOI] [PubMed] [Google Scholar]
- 9.Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Tornell J, Isaksson OG, Jansson JO, Ohlsson C. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjogren K, Sheng M, Moverare S, Liu JL, Wallenius K, Tornell J, Isaksson O, Jansson JO, Mohan S, Ohlsson C. Effects of liver-derived insulin-like growth factor I on bone metabolism in mice. J Bone Miner Res. 2002;17:1977–1987. doi: 10.1359/jbmr.2002.17.11.1977. [DOI] [PubMed] [Google Scholar]
- 11.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 14.Jiang J, Lichtler AC, Gronowicz GA, Adams DJ, Clark SH, Rosen CJ, Kream BE. Transgenic mice with osteoblast-targeted insulin-like growth factor-I show increased bone remodeling. Bone. 2006;39:494–504. doi: 10.1016/j.bone.2006.02.068. [DOI] [PubMed] [Google Scholar]
- 15.Hayden JM, Mohan S, Baylink DJ. The insulin-like growth factor system and the coupling of formation to resorption. Bone. 1995;17:93S–98S. doi: 10.1016/8756-3282(95)00186-h. [DOI] [PubMed] [Google Scholar]
- 16.Ueland T. GH/IGF-I and bone resorption in vivo and in vitro. Eur J Endocrinol. 2005;152:327–332. doi: 10.1530/eje.1.01874. [DOI] [PubMed] [Google Scholar]
- 17.Florin L, Alter H, Grone HJ, Szabowski A, Schutz G, Angel P. Cre recombinase-mediated gene targeting of mesenchymal cells. Genesis. 2004;38:139–144. doi: 10.1002/gene.20004. [DOI] [PubMed] [Google Scholar]
- 18.Liu JL, Grinberg A, Westphal H, Sauer B, Accili D, Karas M, LeRoith D. Insulin-like growth factor-I affects perinatal lethality and postnatal development in a gene dosage-dependent manner: manipulation using the Cre/loxP system in transgenic mice. Mol Endocrinol. 1998;12:1452–1462. doi: 10.1210/mend.12.9.0162. [DOI] [PubMed] [Google Scholar]
- 19.Miyakoshi N, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that anabolic effects of PTH on bone require IGF-I in growing mice. Endocrinology. 2001;142:4349–4356. doi: 10.1210/endo.142.10.8436. [DOI] [PubMed] [Google Scholar]
- 20.Kasukawa Y, Baylink DJ, Guo R, Mohan S. Evidence that sensitivity to growth hormone (GH) is growth period and tissue type dependent: studies in GH-deficient lit/lit mice. Endocrinology. 2003;144:3950–3957. doi: 10.1210/en.2002-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salih DA, Mohan S, Kasukawa Y, Tripathi G, Lovett FA, Anderson NF, Carter EJ, Wergedal JE, Baylink DJ, Pell JM. Insulin-like growth factor-binding protein-5 induces a gender-related decrease in bone mineral density in transgenic mice. Endocrinology. 2005;146:931–940. doi: 10.1210/en.2004-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohan S, Baylink DJ. Development of a simple valid method for the complete removal of insulin-like growth factor (IGF)-binding proteins from IGFs in human serum and other biological fluids: comparison with acid-ethanol treatment and C18 Sep-Pak separation. J Clin Endocrinol Metab. 1995;80:637–647. doi: 10.1210/jcem.80.2.7531716. [DOI] [PubMed] [Google Scholar]
- 23.Govoni KE, Amaar YG, Kramer A, Winter E, Baylink DJ, Mohan S. Regulation of insulin-like growth factor binding protein-5, four and a half lim-2, and a disintegrin and metalloprotease-9 expression in osteoblasts. Growth Horm IGF Res. 2005;16:49–56. doi: 10.1016/j.ghir.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kesavan C, Mohan S, Oberholtzer S, Wergedal JE, Baylink DJ. Mechanical loading-induced gene expression and BMD changes are different in two inbred mouse strains. J Appl Physiol. 2005;99:1951–1957. doi: 10.1152/japplphysiol.00401.2005. [DOI] [PubMed] [Google Scholar]
- 25.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 26.Niederreither K, D’Souza R, Metsaranta M, Eberspaecher H, Toman PD, Vuorio E, De Crombrugghe B. Coordinate patterns of expression of type I and III collagens during mouse development. Matrix Biol. 1995;14:705–713. doi: 10.1016/s0945-053x(05)80013-7. [DOI] [PubMed] [Google Scholar]
- 27.Govoni KE, Lee SK, Chung YS, Behringer RR, Wergedal JE, Baylink DJ, Mohan S. Disruption of insulin-like growth factor (IGF)-I expression in type IIαI collagen expressing cells reduces bone length and width in mice. Physiol Genomics. 2007;30:354–362. doi: 10.1152/physiolgenomics.00022.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pluck A. Conditional mutagenesis in mice: the Cre/loxP recombination system. Int J Exp Pathol. 1996;77:269–278. [PMC free article] [PubMed] [Google Scholar]
- 29.Liu JL, Yakar S, LeRoith D. Mice deficient in liver production of insulin-like growth factor I display sexual dimorphism in growth hormone-stimulated postnatal growth. Endocrinology. 2000;141:4436–4441. doi: 10.1210/endo.141.12.7825. [DOI] [PubMed] [Google Scholar]
- 30.Shimshek DR, Kim J, Hubner MR, Spergel DJ, Buchholz F, Casanova E, Stewart AF, Seeburg PH, Sprengel R. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis. 2002;32:19–26. doi: 10.1002/gene.10023. [DOI] [PubMed] [Google Scholar]
- 31.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 32.Schuller AG, van Neck JW, Beukenholdt RW, Zwarthoff EC, Drop SL. IGF, type I IGF receptor and IGF-binding protein mRNA expression in the developing mouse lung. J Mol Endocrinol. 1995;14:349–355. doi: 10.1677/jme.0.0140349. [DOI] [PubMed] [Google Scholar]
- 33.Retsch-Bogart GZ, Moats-Staats BM, Howard K, D’Ercole AJ, Stiles AD. Cellular localization of messenger RNAs for insulin-like growth factors (IGFs), their receptors and binding proteins during fetal rat lung development. Am J Respir Cell Mol Biol. 1996;14:61–69. doi: 10.1165/ajrcmb.14.1.8534487. [DOI] [PubMed] [Google Scholar]
- 34.Silva D, Venihaki M, Guo WH, Lopez MF. Igf2 deficiency results in delayed lung development at the end of gestation. Endocrinology. 2006;147:5584–5591. doi: 10.1210/en.2006-0498. [DOI] [PubMed] [Google Scholar]
- 35.Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA. IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993;7:2609–2617. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Nishida S, Sakata T, Elalieh HZ, Chang W, Halloran BP, Doty SB, Bikle DD. Insulin-like growth factor-I is essential for embryonic bone development. Endocrinology. 2006;147:4753–4761. doi: 10.1210/en.2006-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kooijman R. Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytokine Growth Factor Rev. 2006;17:305–323. doi: 10.1016/j.cytogfr.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Linkhart TA, Mohan S, Baylink DJ. Growth factors for bone growth and repair: IGF, TGFβ and BMP. Bone. 1996;19:1S–12S. doi: 10.1016/s8756-3282(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Nishida S, Elalieh HZ, Long RK, Halloran BP, Bikle DD. Role of IGF-I signaling in regulating osteoclastogenesis. J Bone Miner Res. 2006;21:1350–1358. doi: 10.1359/jbmr.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bikle D, Majumdar S, Laib A, Powell-Braxton L, Rosen C, Beamer W, Nauman E, Leary C, Halloran B. The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res. 2001;16:2320–2329. doi: 10.1359/jbmr.2001.16.12.2320. [DOI] [PubMed] [Google Scholar]
- 41.Moerth C, Schneider MR, Renner-Mueller I, Blutke A, Elmlinger MW, Erben RG, Camacho-Hubner C, Hoeflich A, Wolf E. Postnatally elevated levels of insulin-like growth factor (IGF)-II fail to rescue the dwarfism of IGF-I-deficient mice except kidney weight. Endocrinology. 2007;148:441–451. doi: 10.1210/en.2006-0385. [DOI] [PubMed] [Google Scholar]
- 42.Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- 43.Mohan S, Baylink DJ. IGF system components and their role in bone metabolism. In: Rosenfeld RG, Roberst C, editors. IGFs in health and diseases. Totawa, NJ: Humana Press; 1999. pp. 457–496. [Google Scholar]
- 44.Wang J, Zhou J, Cheng CM, Kopchick JJ, Bondy CA. Evidence supporting dual, IGF-I-independent and IGF-I-dependent, roles for GH in promoting longitudinal bone growth. J Endocrinol. 2004;180:247–255. doi: 10.1677/joe.0.1800247. [DOI] [PubMed] [Google Scholar]