Abstract
The interplay between vector and pathogen is essential for vector-borne disease transmission. Dissecting the molecular basis of refractoriness of some vectors may pave the way to novel disease control mechanisms. A pathogen often needs to overcome several physical barriers, such as the peritrophic matrix, midgut epithelium and salivary glands. Additionally, the arthropod vector elicites immune responses that can severely limit transmission success. One important step in the transmission of most vector-borne diseases is the entry of the disease agent into the salivary glands of its arthropod vector. The salivary glands of blood-feeding arthropods produce a complex mixture of molecules that facilitate blood feeding by inhibition of the host haemostasis, inflammation and immune reactions. Pathogen entry into salivary glands is a receptor-mediated process, which requires molecules on the surface of the pathogen and salivary gland. In most cases, the nature of these molecules remains unknown. Recent advances in our understanding of malaria parasite entry into mosquito salivary glands strongly suggests that specific carbohydrate molecules on the salivary gland surface function as docking receptors for malaria parasites.
Keywords: Malaria, Plasmodium, Vector-borne diseases, Parasite-vector interactions
1. Introduction
Vector-borne diseases continue to be a major public health threat throughout the world. Usually transmitted by arthropods, their causative agents include helminths and protozoa, as well as microbial pathogens and viruses. Few vaccines are available and disease control and prevention in the majority of cases relies on vector control and, to a lesser extent, on drug treatment of the infected human population. Since the 1970s, vector-borne diseases have been on the rise. The underlying causes are complex and include political, sociological and biological factors, such as emergence of drug-resistant strains of pathogens, as well as insecticide-resistant vector populations. Other approaches to disease control are envisioned. These include the use of transmission-blocking vaccines against the pathogen (most recently reviewed by Coutinho-Abreu and Ramalho-Ortigao, 2010) or vector (Trager, 1939), and the use of refractory vectors for population replacement. The development of these strategies requires an intimate knowledge of vector biology and pathogen-vector interactions in order to identify potential molecular targets. In this article, we summarize our current understanding of salivary gland invasion, arguably the most important step for transmission in the majority of vector-borne diseases, using malaria as the best-studied example.
2. Salivation is the most common transmission strategy employed by vector-borne disease agents
Pathogens always enter their vector species with the blood meal. However, their transmission to the vertebrate host is more varied. While this transfer usually occurs during blood feeding on the vertebrate host, different exit strategies are used. Table 1 summarizes the different mechanisms of horizontal transmission, including salivation, active and passive escape through the cuticle, posterior exit with arthropod feces (stercoration), regurgitation, and in rare cases, ingestion of the vector. Host entry by salivation is the most commonly utilized and efficient transmission method, e.g. all known arthropod-borne viruses are transmitted this way. The pathogen is directly injected into the host during probing or blood feeding. This method cannot be employed by pathogens that remain in the gut or the hemocoel of the vector as it requires entry into the salivary glands. Therefore, invasion of salivary glands can be considered as one of the most important events required for vector-borne disease transmission.
Table 1.
Vector-borne pathogens/parasites and their mechanisms of transmission.
| Infectious agent Parasites | Class | Vector | Transmission | Disease |
|---|---|---|---|---|
| Parasites | ||||
| Babesia microti | Apicomplexa | Tick | salivation | Babesiosis |
| Brugia malayi | Nematoda | Mosquito | active escape | Filariasis |
| Drancunculus medinensis | Nematoda | Copepod | ingestion | Dracunculiasis |
| Leishmania spp. | Euglenozoa | Sandfly | regurgitation | Leishmaniasis |
| Loa loa | Nematoda | Deer fly | active escape | Filariasis |
| Mansonella ozzardi | Nematoda | Blackfly | active escape | Mansonellosis |
| Mansonella perstans | Nematoda | Biting midge | active escape | Mansonellosis |
| Onchocerca volvulus | Nematoda | Blackfly | active escape | River blindness |
| Plasmodium spp. | Apicomplexa | Mosquito | salivation | Malaria |
| Trypanosoma brucei | Euglenozoa | Tsetse fly | salivation | Sleeping sickness |
| Trypanosoma cruzi | Euglenozoa | Triatome bug | stercorarian | Chagas disease |
| Wuchereria bancrofti | Nematoda | Mosquito | active escape | Filariasis |
| Viruses | ||||
| CCHF virusc | Bunyaviridae | Ixodid tick | salivation | Crimean Congo hemorrhagic fever |
| Chikungunya virusc | Togaviridae | Mosquito | salivation | Chikungunya fever |
| DEN virus | Flavirviridae | Mosquito | salivation | Dengue |
| JE virusb | Flavirviridae | Mosquito | salivation | Japanese Encephalitis |
| LCE virusb | Bunyaviridae | Mosquito | salivation | LaCrosse Encephalitis |
| Ross River virus | Togaviridae | Mosquito | salivation | Ross River fever |
| RSSE virusc, CEE virusc | Flavirviridae | Ixodid tick | salivation | Tick-Borne Encephalitis |
| RVF virusa | Bunyaviridae | Mosquito | salivation | Rift Valley Fever |
| SLE virus | Flavirviridae | Mosquito | salivation | Saint Louis Encephalitis |
| VEE virusb | Togaviridae | Mosquito | salivation | Venezuelan Equine Encephalomyelitis |
| WNE virusb | Flavirviridae | Mosquito | salivation | West Nile Encephalitis |
| Yellow fever virusc | Flavirviridae | Mosquito | salivation | Yellow Fever |
| Bacteria | ||||
| Anaplasma phagocytophilum | Alphaproteobacteria | Hard Tick | salivation | Human Granulocytic Anaplasmosis |
| Bartonella bacilliformis | Alphaproteobacteria | Sand fly | salivation | Carrion’s disease |
| Bartonella quintana | Alphaproteobacteria | Louse | stercorarian | Trench fever |
| Borrelia burgdorferi | Spirochaeta | Tick | salivation | Lyme Disease |
| Borrelia hermsii | Spirochaeta | Soft Tick | salivation | Tick-borne relapsing fever |
| Borrelia recurrentis | Spirochaeta | Louse | passive escape | Louse-borne relapsing fever |
| Ehrlichia chaffeensis | Alphaproteobacteria | Hard Tick | salivation | Human Monocytic Ehrlichiosis |
| Francisella tularensisa | Gammaproteobacteria | Hard Tick, Deer fly | salivation | Tularemia |
| Orientia tsutsugamushi | Alphaproteobacteria | Mite | salivation | Scrub typhus |
| Rickettsia prowazekiib | Alphaproteobacteria | Louse | stercorarian | Epidemic typhus |
| Rickettsia rickettsiic | Alphaproteobacteria | Hard Tick | salivation | Rocky Mountain spotted fever |
| Rickettsia typhic | Alphaproteobacteria | Flea | salivation | Murine typhus |
| Yersinia pestisa | Gammaproteobacteria | Flea | regurgitation | Plague |
National Institute of Allergy and Infectious Diseases Biodefense Category Aa, Bb, Cc priority pathogens (http://www.niaid.nih.gov/topics/biodefenserelated/biodefense/research/pages/cata.aspx)
The molecular make-up of salivary glands of a variety of hematophagous arthropods, including of several mosquito species (Valenzuela et al., 2002, 2003; Calvo et al., 2004, 2007, 2010; Ribeiro et al., 2004b; Arca et al., 2005, 2007), sandflies (Ribeiro et al., 2000), blackflies (Andersen et al., 2009), triatome bugs (Ribeiro et al., 2004a), fleas (Andersen et al., 2007), and hard and soft tick species (Ribeiro et al., 1991; Santos et al., 2004; Francischetti et al., 2005; Mans et al., 2008), have been analyzed in detail. As a consequence, we have detailed understanding of the saliva components, which contribute to blood feeding in different ways, including regulation of blood haemostasis by vasodilators, inhibitors of blood clotting and platelet aggregation. In contrast, the salivary gland surface molecules that are required for pathogen entry are mostly unknown. Additionally, the cell biological events occurring during this invasion process have only been described for a handful of vector-borne disease pathogens. The following sections discuss the currently best-understood system of salivary gland invasion: the cross-talk of the malaria parasite, Plasmodium spp. with its mosquito vector, Anopheles spp..
3. The cell biology of malaria parasite invasion of mosquito epithelia
The Plasmodium parasite undergoes a complex life cycle encompassing heterophasic generational changes, and obligatorily fulfills its sexual life cycle in the mosquito. Male and female gametocytes, taken up with the blood meal, undergo gametogenesis within the lumen of the mosquito midgut. Fertilization takes place within approximately 2 h after a blood meal and the resulting zygote undergoes meiosis and develops into the motile ookinete. Approximately 1 day after the infectious blood meal, the ookinete traverses the peritrophic matrix and subsequently the midgut epithelium itself. The ookinete then rounds up and forms the oocyst, the stage in which sporogony occurs. Approximately 2 weeks after the blood meal sporozoites are released into the hemocoel. They then reach the salivary glands and again traverse an epithelium, in this case to penetrate into the salivary gland lumen, where they mix with the saliva and are injected into the next vertebrate host (recently reviewed by Baton and Ranford-Cartwright, 2005b).
3.1. Midgut invasion
The cellular processes that occur during ookinete invasion and traversal of the midgut epithelium (Fig. 1A) have been under intense investigation and various aspects have recently been reviewed extensively (Baton and Ranford-Cartwright, 2005a, b; Kumar and Barillas-Mury, 2005; Vinetz, 2005; Vlachou et al., 2006; Whitten et al., 2006). The ookinete presumably migrates actively in the blood bolus before encountering its first barrier, the peritrophic matrix (Sieber et al., 1991). Some parasite species require the secretion of chitinase to cross this barrier in order to reach the microvilli of the midgut epithelium (Huber et al., 1991; Tsai et al., 2001). Midgut cell entry is thought to be mediated by a yet to be identified specific receptor-ligand interaction and occurs into the apical-lateral membrane where three epithelial cells converge (Baton and Ranford-Cartwright, 2004). Ookinete invasion is an active process that requires gliding motility, a type of movement typical for all invasive apicomplexan parasites (Keeley and Soldati, 2004). The invasion induces tyrosine nitration in the invaded midgut cells, which involves nitric oxide synthase (NOS) up-regulation followed by increased peroxidase activity (Kumar and Barillas-Mury, 2005). Such a defense reaction generating toxic chemicals is potentially harmful to the host, often leading to apoptosis. Indeed, ookinete invasion of midgut epithelia induces apoptosis of the invaded cells, which are expelled from the epithelium by actin-based restitution mechanisms (Time-bomb model; Han et al., 2000; Gupta et al., 2005). A single ookinete often serially invades several cells, which all become apoptotic and are excluded from the epithelium. The parasite exits the midgut epithelium at its basal side and is at that stage covered by lamellipodia that form a “hood” around the parasite (Vlachou et al., 2006). The accumulation of actin around the parasite at the time of egress has been also noted (Vernick et al., 1995; Whitten et al., 2006). The interaction of the extracellular ookinete with the basal lamina is believed to induce transformation to the oocyst stage (Weathersby, 1952). The passage of an individual ookinete is thought to take no more than 30 min. However, ookinete invasion is asynchronous and continuous up to 36 h after a blood meal. Eventually, the remaining parasites present in the midgut lumen are excreted with the digested blood meal.
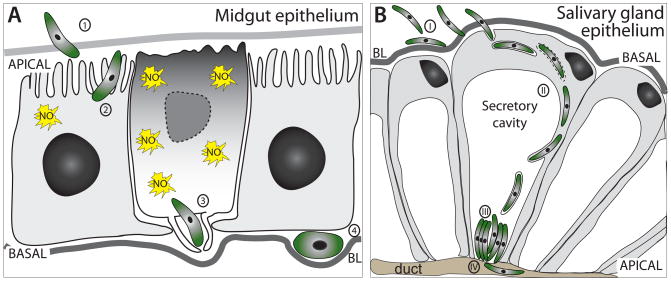
Fig. 1.
Comparison of malaria parasite entry into midgut and salivary gland epithelia. A) Ookinete invasion of midgut epithelia. The ookinete crosses the peritrophic matrix (1) and actively enters the epithelial cell 24–28 h after a bloodmeal, often sequentially invading several midgut cells (2). The ookinete egresses the epithelial cell at the basal site, which is accompanied by lamellipodia formation by the invaded cell as well as its neighbors (3). In the extracellular space of the basal labyrinth the ookinete starts to round up beneath the basal lamina (BL) and transforms into the oocyst (4). The invaded midgut cells undergo many severe physiological changes, among those nitric oxide (NO) production. Ultimately, the cells undergo apoptosis and are expelled from the epithelium. B) Salivary gland infection by sporozoites can be divided into several stages: (I) Sporozoite attachment, likely due to receptor-ligand interactions; (II) sporozoite invasion of epithelial cells with the formation of a transient vacuole; and (III) maturation phase, where sporozoites rest in the extracellular space of the secretory cavity, while (IV) few enter the salivary duct.
3.2. Reaching the salivary gland
Once oocysts are established, mitotic divisions occur and ultimately sporozoites are formed. Upon egress from mature oocysts, sporozoites travel via the hemolymph to the salivary glands, where they invade salivary gland secretory cells. Sporozoites can be found throughout the mosquito hemocoel (Garnham, 1966; Golenda et al., 1990) and are passively carried through the hemolymph to the salivary glands (Akaki and Dvorak, 2005; Hillyer et al., 2007). Less than 20% of the sporozoites entering the hemocoel ultimately reside within the salivary gland and the remaining 80% of parasites are eliminated from the hemolymph (Hillyer et al., 2007) and within the salivary gland (Pinto et al., 2008) by an unknown mechanism.
3.3. Salivary gland invasion
Morphological studies of the passage of Plasmodium sporozoites through the salivary gland of their mosquito vectors have been performed by electron microscopy using avian and rodent malaria models (Sterling et al., 1973; Meis et al., 1992; Pimenta et al., 1994; Ando et al., 1999). Similar to midgut invasion, the sporozoite takes an intracellular route through the salivary gland epithelium to reach its final destination in the mosquito, the salivary gland duct. However, the cell-biological events that occur during sporozoite invasion of salivary gland epithelial cells (summarized in Fig. 1B) differ substantially from those occurring during midgut invasion. The sporozoite approaches the salivary gland epithelium from the basal side and first has to recognize, attach to and subsequently penetrate the basal lamina in order to reach the epithelial cell. Invasion itself, as in midgut epithelia, is thought to be mediated via receptor-ligand interactions and involves parasite gliding motility (Sultan et al., 1997) and the possible formation of moving junctions (Pimenta et al., 1994). During cell invasion, the sporozoite is initially surrounded by a second membrane (Pimenta et al., 1994), which has been interpreted as a transient parasitophorous vacuole (PV) (Rodriguez and Hernandez-Hernandez Fde, 2004). The origin of this membrane is unclear. The sporozoite subsequently escapes the vacuole by an unknown mechanism, and exits the host cell into the secretory cavity. The majority of sporozoites remain in these cavities and only a few enter the salivary duct (Frischknecht et al., 2004). The invasion process has little visible effect on the invaded host cell. No apoptosis or actin rearrangements of parasite-invaded salivary gland epithelial cells have been described. In contrast to midgut invasion, NOS is either not transcriptionally altered or is down-regulated in Plasmodium berghei-infected salivary glands (Rosinski-Chupin et al., 2007; Dimopoulos et al., 1998), and the enzyme is not detectable by immunofluorescence analysis (Pinto et al., 2008).
4. Parasite molecules required for Plasmodium spp. salivary gland invasion
The molecular mechanisms responsible for the interaction between the malaria parasites and molecules on the surface of mosquito salivary glands are complex and not well understood. Apicomplexan parasites are named after their apical complex, an anterior structure formed by three organelles: the rhoptries, dense granules and micronemes. While molecules released from the rhoptries participate in PV formation (Lingelbach and Joiner, 1998), molecules from dense granules complete the establishment of parasites in their host cell, and the content of micronemes is required for host cell invasion. Micronemes are vesicles containing molecules that after secretion are maintained on the surface of the parasite. These molecules participate in adhesion to target cells, gliding motility and the invasion process (Donahue et al., 2000).
To date, no data formally show rhoptry, dense granule or microneme secretions to be required for sporozoite invasion of the mosquito salivary gland. However, several micronemal proteins have been shown to be important in salivary gland invasion: the circumsporozoite protein (CSP), the apical membrane antigen/erythrocyte binding-like protein (MAEBL), the thrombospondin-related anonymous protein (TRAP), and the up-regulated-in-oocysts sporozoites protein 3 (UOS3) (Mikolajczak et al., 2008), also called S6/TREP (Combe et al., 2009; Steinbuechel and Matuschewski, 2009). CSP is a parasite surface molecule essential for sporozoite development within oocysts and invasion of salivary glands (Menard et al., 1997, Sidjanski, 1997). An 18 amino acid N-terminal peptide fragment of CSP that includes Region I has been shown to specifically bind to salivary glands (Sidjanski et al., 1997; Myung et al., 2004). Together with CSP, the malaria sporozoite utilizes a second protein, MAEBL, to facilitate attachment to salivary glands (Kariu et al., 2002). MAEBL displays adhesive features involved in attachment of target organs. Mutant parasites developed normally within the mosquito vector but were unable to invade salivary glands or hepatocytes. While MAEBL expression is necessary for salivary gland invasion, conflicting data exist on its role in sporozoite motility (Kariu et al., 2002), (F. Frischknecht, K. Michel, A.K. Mueller, unpublished data). Midgut and salivary gland sporozoites express two dominant alternatively spliced MAEBL mRNAs, encoding a transmembrane and a secreted MAEBL isoform (Singh et al., 2004). Using knockout and allelic replacement experiments, Saenz et al., (2008) recently showed that the MAEBL transmembrane isoform is essential for salivary gland invasion by human malaria parasites.
The third micronemal protein, TRAP, also displays complex interactions with salivary glands (Sultan et al., 1997; Kappe et al., 1999). TRAP is a member of a family comprising at least six type I transmembrane invasins, which also include circumsporozoite and TRAP-related protein (CTRP; Dessens et al., 1999), merozoite TRAP (MTRAP, Baum et al., 2006), Plasmodium thrombospondin-related apical merozoite protein (PTRAMP; Thompson et al., 2004), TRAP-related protein (TREP, originally named UOS3/S6), and TRAP-like protein (TLP). In contrast to CSP and MAEBL, TRAP is not required for initial attachment to salivary glands but for the gliding movement of sporozoites, by functioning as a bridge between the outer membrane surface and the intracellular actin-myosin motor complex through binding to the actin-bridging molecule aldolase. TRAP is also needed for the invasion process of salivary glands and hepatocytes (Sultan et al., 1997; Kappe et al., 1999; Matuschewski et al., 2002a). These two functions can be genetically isolated and are mediated by distinct domains of the protein (Kappe et al., 1999; Matuschewski et al., 2002a). The extracellular portion of TRAP needs to be shed from the parasite surface for gliding motility and host cell invasion to occur. This process is potentially mediated by the rhomboid protease ROM4, which in cell-based assays cleaves TRAP within its transmembrane domain and causes the release of its extracellular portion (Baker et al., 2006). The function of the cytoplasmic domain of TRAP during sporozoite invasion of mosquito salivary glands and hepatocytes can be partially complemented by the cytoplasmic domain of at least one other member of the type I transmembrane invasins, TLP (Heiss et al., 2008). However, knockout-TLP P. berghei parasites produce normal numbers of salivary gland sporozoites, indicating that TLP might not be involved or only plays a non-redundant role for sporozoite invasion of salivary glands (Heiss et al., 2008). In contrast, TLP is required for cell/tissue traversal in the vertebrate host, as knockout parasites exhibit defects in hepatocyte cell traversal in vitro and in mouse infectivity in vivo (Moreira et al., 2008).
Whole genome approaches have been used to identify parasite genes required for infection of specific tissues (Matuschewski et al., 2002b; Kaiser et al., 2004; Mikolajczak et al., 2008; Tarun et al., 2008). Two of these studies identified a putative transmembrane protein, UOS3/S6, a member of the TRAP protein family, also called TREP (Combe et al., 2009). Unlike TRAP, UOS3/S6 lacks apparent adhesion motifs such as the Von Willebrand factor A-domain and only contains a degenerate thrombospondin type I motif in its extracelluar region. UOS3/S6 knockout sporozoites display reduced infectivity to salivary glands (Steinbuechel and Matuschewski, 2009). Using advanced microscopy techniques, UOS3/S6 has been shown to mediate attachment and detachment to substrate surfaces, and to interact with TRAP and TLP during gliding motility (Hegge et al., 2010).
In addition to the proteins described above, the sporozoite surface proteins Cysteine Repeat Modular Proteins (CRMPs) 1 and 2 are also required for salivary invasion (Thompson et al., 2007). Knockout of these two proteins does not seem to affect motility and sporozoites remain infectious to the vertebrate host, however no salivary gland sporozoites were observed.
5. Salivary gland surface molecules required for parasite binding
As described in Section 4, sporozoite attachment to salivary glands is facilitated by several different proteins on the sporozoite surface. The sporozoite encounters the salivary gland from the basal side. It initially attaches to the basal lamina of this epithelium and subsequently binds to the basolateral membrane of the epithelial cells. Initial attachment and presumably invasion are facilitated by the interaction of sporozoite surface molecules with molecules present on the salivary gland surface; so what do we know about its molecular composition?
Mosquito salivary glands bind a variety of lectins, indicative of sugar molecules on their surface, most likely present in the basal lamina (Molyneux et al., 1990; Mohamed and Ingram, 1993; Barreau et al., 1995). Indeed, salivary glands prepared from adult female Anopheles stephensi mosquitoes contain chondroitin sulfate and heparan sulfate, members of the glycosaminoglycan family of heteropolysaccharides (Sinnis et al., 2007). These sugar moiteties are likely to participate in parasite entry as lectin binding to Aedes aegypti salivary glands blocks Plasmodium gallinaceum sporozoite invasion (Barreau et al., 1995). The parasite surface molecule CSP binds to mammalian heparan sulfate (Sinnis et al., 1994; Ying et al., 1997; Rathore et al., 2001), which is required for liver cell invasion (Frevert et al., 1993; Pinzon-Ortiz et al., 2001). The demonstration that CSP binds to mosquito heparan sulfates (Sinnis et al., 2007) suggests that this may be the salivary gland molecule to which CSP binds.
In addition to lectins, polyclonal antibodies raised against salivary gland extracts can also block sporozoite salivary gland invasion (Barreau et al., 1995; Brennan et al., 2000). These antibodies recognize epitopes in the basal lamina, suggesting that this specialized extracelluar matrix may have receptors that mediate sporozoite attachment (Barreau et al., 1995). Subsequently, using purified monoclonal antibodies that specifically bound to median and distal lateral salivary gland lobes, the regions preferentially invaded by sporozoites (Sterling et al., 1973), a new Salivary Gland Surface (SGS) protein family was identified (Korochkina et al., 2006). SGS1 is a large protein of more than 220 kDa that is present in the basal lamina of female salivary glands. It contains heparin-binding and tyrosin O-sulfation motifs, suggesting that this protein is highly glycosylated in vivo. How SGS1 contributes to parasite invasion is unclear. Based on its heparin-binding domain, it is possible that SGS proteins bind a soluble heparin-like glycosaminoglycan present in the haemolymph that in turn could bind to CSP (Korochkina et al., 2006).
Two additional proteins that are only expressed in the distal lobes of female salivary glands have been shown to serve as sporozoite receptors (Brennan et al., 2000; Korochkina et al., 2006). One of these proteins, called Saglin, is a secreted protein that contains several putative glycosylation sites (Okulate et al., 2007) and its expression is induced by blood feeding (Korochkina et al., 2006). Furthermore, Saglin can bind to the A domain of TRAP in vitro (Ghosh et al., 2009), the same domain that had previously been shown to be required for parasite invasion (Matuschewski et al., 2002a). It is likely that the binding of TRAP to Saglin is also required in vivo, as this interaction can be inhibited in vivo by a synthetic peptide, called SM1, which binds to Saglin and abolishes the parasite’s ability to invade salivary glands (Ghosh et al., 2001).
Taken together, these studies indicate that malaria sporozoites utilize at least two receptor-ligand interactions to bind to the mosquito salivary glands. Given that at least five other sporozoite surface proteins with unknown binding partners are involved in the invasion process, the interaction between parasite and gland surface is likely to be more complex.
6. Epithelial defense mechanisms limit parasite success during salivary gland invasion
Once the sporozoite has attached to and migrated through the basal lamina, it actively invades the salivary gland epithelial cells. Under laboratory conditions, invasion of often hundreds to thousands of malaria sporozoites into a single salivary gland has seemingly little effect on the invaded cells (Pimenta et al., 1994; Pinto et al., 2008). In contrast, ookinete invasion of the mosquito midgut epithelium causes major cytoskeleton restructuring that ultimately leads to apoptosis and expulsion of the invaded cells from the epithelium (Han and Barillas-Mury, 2002; Baton and Ranford-Cartwright, 2004; Vlachou et al., 2004). In parallel, midgut and haemolymph-derived factors kill nearly 80% of the invading ookinete population (Blandin et al., 2004). These observations are reflected in the transcriptional changes observed in these two epithelia during parasite infection. While up to 7% of the mosquito midgut transcriptome was altered (Abraham et al., 2004; Vlachou et al., 2005; Xu et al., 2005; Dong et al., 2006), less than 1% of salivary gland transcripts changed (Rosinski-Chupin et al., 2007). Among these were 37 immune-related genes, indicating that salivary glands mount an acute immune response against invading sporozoites. Interestingly, four genes showed similar regulation in both epithelia: a fatty acid synthase was downregulated by parasite invasion in both tissues, while a GTP-binding nuclear protein, a lysosomal thioreductase precursor and SRPN6, (which belongs to a mosquito-specific expansion group (Michel et al., 2005) that is ortholgous to Drosophila Spn28D, (Scherfer et al., 2008)) were up-regulated in both tissues. SRPN6 knockdown in mosquito midguts and salivary glands leads to an increased number of parasites in the respective tissue (Abraham et al., 2005; Pinto et al., 2008), indicative of a common epithelial immune response to malaria parasite invasion.
Additionally, transcriptomes of naïve salivary glands of blood-feeding arthropods encode for anti-microbial peptides, lysozyme and pathogen pattern recognition polypeptides (Arca et al., 2005; Calvo et al., 2010). Their potential effect on pathogens and parasites within the salivary gland has not been characterized.
7. Future perspectives
Our understanding of pathogen and parasite interactions with salivary glands of their arthropod vectors remains incomplete. Over the last 10 years substantial progress has been made in the identification of the molecular interactions between Plasmodium spp. sporozoites and Anopheles spp. salivary glands. Two receptor-ligand interactions have been characterized and several other sporozoite surface molecules are now known to be required for invasion. Additionally, an epithelial immune response common in midguts and salivary glands against invading ookinetes and sporozoites, respectively, has been identified. However, even in this arguably best understood parasite-vector system, several basic questions are unanswered. Firstly, no detailed morphological description of the sporozoite invasion process of mosquito salivary glands exists for any of the human malaria parasite species. Second, proteomic studies of sporozoite surface or micronemes have not been performed and the molecular make-up of the salivary gland basal lamina or the surface of the basolateral epithelial cell membrane is largely uncharacterized. A recent description of the micronemal proteome of ookinetes (Lal et al., 2009) identified more than 50 putatively secreted proteins of unknown function, of which at least some are most likely important for midgut epithelial cell invasion. A similar description of the micronemal proteome of midgut sporozoites would be highly desirable. However, preparation of enough material for these studies is severely hampered by the lack of culturing methods to produce sporozoites in vitro. Third, the mechanism of the SRPN6-dependent common epithelial immune response against ookinete and sporozoite invasion of mosquito epithelia is unknown. Its manipulation could simultaneously affect the two bottlenecks of parasite development in the mosquito, which makes it an ideal tool for creating refractory mosquitoes.
Overall, the study of Plasmodium parasite-mosquito salivary gland interactions is severely limited by the lack of experimental ex vivo or in vitro systems. The development of salivary gland epithelial cell lines or robust organ culturing systems as well as culturing techniques for the mosquito stages of malaria parasites should be research priorities and would greatly facilitate the description of the molecular parasite-vector interface.
Acknowledgments
The work in the authors’ laboratory is supported by grants of the Medical Faculty of Heidelberg University (Germany) and the EXIST-Forschungstransfer (BMWI) to A.-K.M. and NIH grant 3P20RR017708-07S1 and P20RR017686 subawards to K. M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham EG, Islam S, Srinivasan P, Ghosh AK, Valenzuela JG, Ribeiro JM, Kafatos FC, Dimopoulos G, Jacobs-Lorena M. Analysis of the Plasmodium and Anopheles transcriptional repertoire during ookinete development and midgut invasion. J Biol Chem. 2004;279:5573–5580. doi: 10.1074/jbc.M307582200. [DOI] [PubMed] [Google Scholar]
- Abraham EG, Pinto SB, Ghosh A, Vanlandingham DL, Budd A, Higgs S, Kafatos FC, Jacobs-Lorena M, Michel K. An immune-responsive serpin, SRPN6, mediates mosquito defense against malaria parasites. Proc Natl Acad Sci U S A. 2005;102:16327–16332. doi: 10.1073/pnas.0508335102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaki M, Dvorak JA. A chemotactic response facilitates mosquito salivary gland infection by malaria sporozoites. J Exp Biol. 2005;208:3211–3218. doi: 10.1242/jeb.01756. [DOI] [PubMed] [Google Scholar]
- Andersen JF, Hinnebusch BJ, Lucas DA, Conrads TP, Veenstra TD, Pham VM, Ribeiro JM. An insight into the sialome of the oriental rat flea, Xenopsylla cheopis (Rots) BMC Genomics. 2007;8:102. doi: 10.1186/1471-2164-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JF, Pham VM, Meng Z, Champagne DE, Ribeiro JM. Insight into the sialome of the Black Fly, Simulium vittatum. J Proteome Res. 2009;8:1474–1488. doi: 10.1021/pr8008429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Kuraishi K, Nishikubo K, Asami T, Waidhet-Kouadio P, Matsuoka H, Chinzei Y. Sporozoite invasion of Plasmodium berghei, rodent malaria parasite, to the salivary glands of the vector mosquito, Anopheles stephensi: A electron microscopy study. Jpn, J Trop Med Hyg. 1999;27:7–12. [Google Scholar]
- Arca B, Lombardo F, Valenzuela JG, Francischetti IM, Marinotti O, Coluzzi M, Ribeiro JM. An updated catalogue of salivary gland transcripts in the adult female mosquito, Anopheles gambiae. J Exp Biol. 2005;208:3971–3986. doi: 10.1242/jeb.01849. [DOI] [PubMed] [Google Scholar]
- Arca B, Lombardo F, Francischetti IM, Pham VM, Mestres-Simon M, Andersen JF, Ribeiro JM. An insight into the sialome of the adult female mosquito Aedes albopictus. Insect Biochem Mol Biol. 2007;37:107–127. doi: 10.1016/j.ibmb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Baker RP, Wijetilaka R, Urban S. Two Plasmodium rhomboid proteases preferentially cleave different adhesins implicated in all invasive stages of malaria. PLoS Pathog. 2006;2:e113. doi: 10.1371/journal.ppat.0020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau C, Touray M, Pimenta PF, Miller LH, Vernick KD. Plasmodium gallinaceum: sporozoite invasion of Aedes aegypti salivary glands is inhibited by anti-gland antibodies and by lectins. Exp Parasitol. 1995;81:332–343. doi: 10.1006/expr.1995.1124. [DOI] [PubMed] [Google Scholar]
- Baton LA, Ranford-Cartwright LC. Plasmodium falciparum ookinete invasion of the midgut epithelium of Anopheles stephensi is consistent with the Time Bomb model. Parasitology. 2004;129:663–676. doi: 10.1017/s0031182004005979. [DOI] [PubMed] [Google Scholar]
- Baton LA, Ranford-Cartwright LC. How do malaria ookinetes cross the mosquito midgut wall? Trends Parasitol. 2005a;21:22–28. doi: 10.1016/j.pt.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Baton LA, Ranford-Cartwright LC. Spreading the seeds of million-murdering death: metamorphoses of malaria in the mosquito. Trends Parasitol. 2005b;21:573–580. doi: 10.1016/j.pt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Baum J, Richard D, Healer J, Rug M, Krnajski Z, Gilberger TW, Green JL, Holder AA, Cowman AF. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem. 2006;281:5197–5208. doi: 10.1074/jbc.M509807200. [DOI] [PubMed] [Google Scholar]
- Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, Levashina EA. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- Brennan JD, Kent M, Dhar R, Fujioka H, Kumar N. Anopheles gambiae salivary gland proteins as putative targets for blocking transmission of malaria parasites. Proc Natl Acad Sci U S A. 2000;97:13859–13864. doi: 10.1073/pnas.250472597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo E, Andersen J, Francischetti IM, de LCM, deBianchi AG, James AA, Ribeiro JM, Marinotti O. The transcriptome of adult female Anopheles darlingi salivary glands. Insect Mol Biol. 2004;13:73–88. doi: 10.1111/j.1365-2583.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- Calvo E, Dao A, Pham VM, Ribeiro JM. An insight into the sialome of Anopheles funestus reveals an emerging pattern in anopheline salivary protein families. Insect Biochem Mol Biol. 2007;37:164–175. doi: 10.1016/j.ibmb.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo E, Sanchez-Vargas I, Favreau AJ, Barbian KD, Pham VM, Olson KE, Ribeiro JM. An insight into the sialotranscriptome of the West Nile mosquito vector, Culex tarsalis. BMC Genomics. 2010;11:51. doi: 10.1186/1471-2164-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combe A, Moreira C, Ackerman S, Thiberge S, Templeton TJ, Menard R. TREP, a novel protein necessary for gliding motility of the malaria sporozoite. Int J Parasitol. 2009;39:489–496. doi: 10.1016/j.ijpara.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Coutinho-Abreu IV, Ramalho-Ortigao M. Transmission blocking vaccines to control insect-borne diseases: a review. Mem Inst Oswaldo Cruz. 2010;105:1–12. doi: 10.1590/s0074-02762010000100001. [DOI] [PubMed] [Google Scholar]
- Dessens JT, Beetsma AL, Dimopoulos G, Wengelnik K, Crisanti A, Kafatos FC, Sinden RE. CTRP is essential for mosquito infection by malaria ookinetes. Embo J. 1999;18:6221–6227. doi: 10.1093/emboj/18.22.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos G, Seeley D, Wolf A, Kafatos FC. Malaria infection of the mosquito Anopheles gambiae activates immune-responsive genes during critical transition stages of the parasite life cycle. Embo J. 1998;17:6115–6123. doi: 10.1093/emboj/17.21.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue CG, Carruthers VB, Gilk SD, Ward GE. The Toxoplasma homolog of Plasmodium apical membrane antigen-1 (AMA-1) is a microneme protein secreted in response to elevated intracellular calcium levels. Mol Biochem Parasitol. 2000;111:15–30. doi: 10.1016/s0166-6851(00)00289-9. [DOI] [PubMed] [Google Scholar]
- Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, Dimopoulos G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM, My Pham V, Mans BJ, Andersen JF, Mather TN, Lane RS, Ribeiro JM. The transcriptome of the salivary glands of the female western black-legged tick Ixodes pacificus (Acari: Ixodidae) Insect Biochem Mol Biol. 2005;35:1142–1161. doi: 10.1016/j.ibmb.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert U, Sinnis P, Cerami C, Shreffler W, Takacs B, Nussenzweig V. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J Exp Med. 1993;177:1287–1298. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischknecht F, Baldacci P, Martin B, Zimmer C, Thiberge S, Olivo-Marin JC, Shorte SL, Menard R. Imaging movement of malaria parasites during transmission by Anopheles mosquitoes. Cell Microbiol. 2004;6:687–694. doi: 10.1111/j.1462-5822.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- Garnham PC. Comments on ultrastructure. Mil Med. 1966;131(Suppl):1011–1012. [PubMed] [Google Scholar]
- Ghosh AK, Ribolla PE, Jacobs-Lorena M. Targeting Plasmodium ligands on mosquito salivary glands and midgut with a phage display peptide library. Proc Natl Acad Sci U S A. 2001;98:13278–13281. doi: 10.1073/pnas.241491198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, Devenport M, Jethwaney D, Kalume DE, Pandey A, Anderson VE, Sultan AA, Kumar N, Jacobs-Lorena M. Malaria parasite invasion of the mosquito salivary gland requires interaction between the Plasmodium TRAP and the Anopheles saglin proteins. PLoS Pathog. 2009;5:e1000265. doi: 10.1371/journal.ppat.1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenda CF, Starkweather WH, Wirtz RA. The distribution of circumsporozoite protein (CS) in Anopheles stephensi mosquitoes infected with Plasmodium falciparum malaria. J Histochem Cytochem. 1990;38:475–481. doi: 10.1177/38.4.2181019. [DOI] [PubMed] [Google Scholar]
- Gupta L, Kumar S, Han YS, Pimenta PF, Barillas-Mury C. Midgut epithelial responses of different mosquito-Plasmodium combinations: the actin cone zipper repair mechanism in Aedes aegypti. Proc Natl Acad Sci U S A. 2005;102:4010–4015. doi: 10.1073/pnas.0409642102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YS, Thompson J, Kafatos FC, Barillas-Mury C. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. Embo J. 2000;19:6030–6040. doi: 10.1093/emboj/19.22.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YS, Barillas-Mury C. Implications of Time Bomb model of ookinete invasion of midgut cells. Insect Biochem Mol Biol. 2002;32:1311–1316. doi: 10.1016/s0965-1748(02)00093-0. [DOI] [PubMed] [Google Scholar]
- Hegge S, Munter S, Steinbuchel M, Heiss K, Engel U, Matuschewski K, Frischknecht F. Multistep adhesion of Plasmodium sporozoites. FASEB J. 2010 doi: 10.1096/fj.09-148700. in press. [DOI] [PubMed] [Google Scholar]
- Heiss K, Nie H, Kumar S, Daly TM, Bergman LW, Matuschewski K. Functional characterization of a redundant Plasmodium TRAP family invasin, TRAP-like protein, by aldolase binding and a genetic complementation test. Eukaryot Cell. 2008;7:1062–1070. doi: 10.1128/EC.00089-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyer JF, Barreau C, Vernick KD. Efficiency of salivary gland invasion by malaria sporozoites is controlled by rapid sporozoite destruction in the mosquito haemocoel. Int J Parasitol. 2007;37:673–681. doi: 10.1016/j.ijpara.2006.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Cabib E, Miller LH. Malaria parasite chitinase and penetration of the mosquito peritrophic membrane. Proc Natl Acad Sci U S A. 1991;88:2807–2810. doi: 10.1073/pnas.88.7.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser K, Matuschewski K, Camargo N, Ross J, Kappe SH. Differential transcriptome profiling identifies Plasmodium genes encoding pre-erythrocytic stage-specific proteins. Mol Microbiol. 2004;51:1221–1232. doi: 10.1046/j.1365-2958.2003.03909.x. [DOI] [PubMed] [Google Scholar]
- Kappe S, Bruderer T, Gantt S, Fujioka H, Nussenzweig V, Menard R. Conservation of a gliding motility and cell invasion machinery in Apicomplexan parasites. J Cell Biol. 1999;147:937–944. doi: 10.1083/jcb.147.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariu T, Yuda M, Yano K, Chinzei Y. MAEBL is essential for malarial sporozoite infection of the mosquito salivary gland. J Exp Med. 2002;195:1317–1323. doi: 10.1084/jem.20011876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley A, Soldati D. The glideosome: a molecular machine powering motility and host-cell invasion by Apicomplexa. Trends Cell Biol. 2004;14:528–532. doi: 10.1016/j.tcb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Korochkina S, Barreau C, Pradel G, Jeffery E, Li J, Natarajan R, Shabanowitz J, Hunt D, Frevert U, Vernick KD. A mosquito-specific protein family includes candidate receptors for malaria sporozoite invasion of salivary glands. Cell Microbiol. 2006;8:163–175. doi: 10.1111/j.1462-5822.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Barillas-Mury C. Ookinete-induced midgut peroxidases detonate the time bomb in anopheline mosquitoes. Insect Biochem Mol Biol. 2005;35:721–727. doi: 10.1016/j.ibmb.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Lal K, Prieto JH, Bromley E, Sanderson SJ, Yates JR, 3rd, Wastling JM, Tomley FM, Sinden RE. Characterisation of Plasmodium invasive organelles; an ookinete microneme proteome. Proteomics. 2009;9:1142–1151. doi: 10.1002/pmic.200800404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingelbach K, Joiner KA. The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: an unusual compartment in infected cells. J Cell Sci. 1998;111:1467–1475. doi: 10.1242/jcs.111.11.1467. [DOI] [PubMed] [Google Scholar]
- Mans BJ, Andersen JF, Schwan TG, Ribeiro JM. Characterization of anti-hemostatic factors in the argasid, Argas monolakensis: implications for the evolution of blood-feeding in the soft tick family. Insect Biochem Mol Biol. 2008;38:22–41. doi: 10.1016/j.ibmb.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuschewski K, Nunes AC, Nussenzweig V, Menard R. Plasmodium sporozoite invasion into insect and mammalian cells is directed by the same dual binding system. EMBO J. 2002a;21:1597–1606. doi: 10.1093/emboj/21.7.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuschewski K, Ross J, Brown SM, Kaiser K, Nussenzweig V, Kappe SH. Infectivity-associated changes in the transcriptional repertoire of the malaria parasite sporozoite stage. J Biol Chem. 2002b;277:41948–41953. doi: 10.1074/jbc.M207315200. [DOI] [PubMed] [Google Scholar]
- Meis JF, Wismans PG, Jap PH, Lensen AH, Ponnudurai T. A scanning electron microscopic study of the sporogonic development of Plasmodium falciparum in Anopheles stephensi. Acta Trop. 1992;50:227–236. doi: 10.1016/0001-706x(92)90079-d. [DOI] [PubMed] [Google Scholar]
- Menard R, Sultan AA, Cortes C, Altszuler R, van Dijk MR, Janse CJ, Waters AP, Nussenzweig RS, Nussenzweig V. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature. 1997;385:336–340. doi: 10.1038/385336a0. [DOI] [PubMed] [Google Scholar]
- Mikolajczak SA, Silva-Rivera H, Peng X, Tarun AS, Camargo N, Jacobs-Lorena V, Daly TM, Bergman LW, de la Vega P, Williams J, Aly AS, Kappe SH. Distinct malaria parasite sporozoites reveal transcriptional changes that cause differential tissue infection competence in the mosquito vector and mammalian host. Mol Cell Biol. 2008;28:6196–6207. doi: 10.1128/MCB.00553-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed HA, Ingram GA. Salivary gland surface carbohydrate variations in three species of the Anopheles gambiae complex. Ann Soc Belg Med Trop. 1993;73:197–207. [PubMed] [Google Scholar]
- Molyneux DH, Okolo CJ, Lines JD, Kamhawi M. Variation in fluorescein-labelled lectin staining of salivary glands in the Anopheles gambiae complex. Med Vet Entomol. 1990;4:459–462. doi: 10.1111/j.1365-2915.1990.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Moreira CK, Templeton TJ, Lavazec C, Hayward RE, Hobbs CV, Kroeze H, Janse CJ, Waters AP, Sinnis P, Coppi A. The Plasmodium TRAP/MIC2 family member, TRAP-Like Protein (TLP), is involved in tissue traversal by sporozoites. Cell Microbiol. 2008;10:1505–1516. doi: 10.1111/j.1462-5822.2008.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung JM, Marshall P, Sinnis P. The Plasmodium circumsporozoite protein is involved in mosquito salivary gland invasion by sporozoites. Mol Biochem Parasitol. 2004;133:53–59. doi: 10.1016/j.molbiopara.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Okulate MA, Kalume DE, Reddy R, Kristiansen T, Bhattacharyya M, Chaerkady R, Pandey A, Kumar N. Identification and molecular characterization of a novel protein Saglin as a target of monoclonal antibodies affecting salivary gland infectivity of Plasmodium sporozoites. Insect Mol Biol. 2007;16:711–722. doi: 10.1111/j.1365-2583.2007.00765.x. [DOI] [PubMed] [Google Scholar]
- Pimenta PF, Touray M, Miller L. The journey of malaria sporozoites in the mosquito salivary gland. J Eukaryot Microbiol. 1994;41:608–624. doi: 10.1111/j.1550-7408.1994.tb01523.x. [DOI] [PubMed] [Google Scholar]
- Pinto SB, Kafatos FC, Michel K. The parasite invasion marker SRPN6 reduces sporozoite numbers in salivary glands of Anopheles gambiae. Cell Microbiol. 2008;10:891–898. doi: 10.1111/j.1462-5822.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- Pinzon-Ortiz C, Friedman J, Esko J, Sinnis P. The binding of the circumsporozoite protein to cell surface heparan sulfate proteoglycans is required for Plasmodium sporozoite attachment to target cells. J Biol Chem. 2001;276:26784–26791. doi: 10.1074/jbc.M104038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore D, McCutchan TF, Garboczi DN, Toida T, Hernaiz MJ, LeBrun LA, Lang SC, Linhardt RJ. Direct measurement of the interactions of glycosaminoglycans and a heparin decasaccharide with the malaria circumsporozoite protein. Biochemistry. 2001;40:11518–11524. doi: 10.1021/bi0105476. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Endris TM, Endris R. Saliva of the soft tick, Ornithodoros moubata, contains anti-platelet and apyrase activities. Comp Biochem Physiol A Comp Physiol. 1991;100:109–112. doi: 10.1016/0300-9629(91)90190-n. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Rowton ED, Charlab R. Salivary amylase activity of the phlebotomine sand fly, Lutzomyia longipalpis. Insect Biochem Mol Biol. 2000;30:271–277. doi: 10.1016/s0965-1748(99)00119-8. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Andersen J, Silva-Neto MA, Pham VM, Garfield MK, Valenzuela JG. Exploring the sialome of the blood-sucking bug Rhodnius prolixus. Insect Biochem Mol Biol. 2004a;34:61–79. doi: 10.1016/j.ibmb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Charlab R, Pham VM, Garfield M, Valenzuela JG. An insight into the salivary transcriptome and proteome of the adult female mosquito Culex pipiens quinquefasciatus. Insect Biochem Mol Biol. 2004b;34:543–563. doi: 10.1016/j.ibmb.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Rodriguez MH, Hernandez-Hernandez Fde L. Insect-malaria parasites interactions: the salivary gland. Insect Biochem Mol Biol. 2004;34:615–624. doi: 10.1016/j.ibmb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Rosinski-Chupin I, Briolay J, Brouilly P, Perrot S, Gomez SM, Chertemps T, Roth CW, Keime C, Gandrillon O, Couble P, Brey PT. SAGE analysis of mosquito salivary gland transcriptomes during Plasmodium invasion. Cell Microbiol. 2007;9:708–724. doi: 10.1111/j.1462-5822.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- Saenz FE, Balu B, Smith J, Mendonca SR, Adams JH. The transmembrane isoform of Plasmodium falciparum MAEBL is essential for the invasion of Anopheles salivary glands. PLoS One. 2008;3:e2287. doi: 10.1371/journal.pone.0002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos IK, Valenzuela JG, Ribeiro JM, de Castro M, Costa JN, Costa AM, da Silva ER, Neto OB, Rocha C, Daffre S, Ferreira BR, da Silva JS, Szabo MP, Bechara GH. Gene discovery in Boophilus microplus, the cattle tick: the transcriptomes of ovaries, salivary glands, and hemocytes. Ann N Y Acad Sci. 2004;1026:242–246. doi: 10.1196/annals.1307.037. [DOI] [PubMed] [Google Scholar]
- Sidjanski SP, Vanderberg JP, Sinnis P. Anopheles stephensi salivary glands bear receptors for region I of the circumsporozoite protein of Plasmodium falciparum. Mol Biochem Parasitol. 1997;90:33–41. doi: 10.1016/s0166-6851(97)00124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber KP, Huber M, Kaslow D, Banks SM, Torii M, Aikawa M, Miller LH. The peritrophic membrane as a barrier: its penetration by Plasmodium gallinaceum and the effect of a monoclonal antibody to ookinetes. Exp Parasitol. 1991;72:145–156. doi: 10.1016/0014-4894(91)90132-g. [DOI] [PubMed] [Google Scholar]
- Singh N, Preiser P, Renia L, Balu B, Barnwell J, Blair P, Jarra W, Voza T, Landau I, Adams JH. Conservation and developmental control of alternative splicing in maebl among malaria parasites. J Mol Biol. 2004;343:589–599. doi: 10.1016/j.jmb.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Sinnis P, Clavijo P, Fenyo D, Chait BT, Cerami C, Nussenzweig V. Structural and functional properties of region II-plus of the malaria circumsporozoite protein. J Exp Med. 1994;180:297–306. doi: 10.1084/jem.180.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnis P, Coppi A, Toida T, Toyoda H, Kinoshita-Toyoda A, Xie J, Kemp MM, Linhardt RJ. Mosquito heparan sulfate and its potential role in malaria infection and transmission. J Biol Chem. 2007;282:25376–25384. doi: 10.1074/jbc.M704698200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuechel M, Matuschewski K. Role for the Plasmodium sporozoite-specific transmembrane protein S6 in parasite motility and efficient malaria transmission. Cell Microbiol. 2009;11:279–288. doi: 10.1111/j.1462-5822.2008.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling CR, Aikawa M, Vanderberg JP. The passage of Plasmodium berghei sporozoites through the salivary glands of Anopheles stephensi: an electron microscope study. J Parasitol. 1973;59:593–605. [PubMed] [Google Scholar]
- Sultan AA, Thathy V, Frevert U, Robson KJ, Crisanti A, Nussenzweig V, Nussenzweig RS, Menard R. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- Tarun AS, Peng X, Dumpit RF, Ogata Y, Silva-Rivera H, Camargo N, Daly TM, Bergman LW, Kappe SH. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci U S A. 2008;105:305–310. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Cooke RE, Moore S, Anderson LF, Janse CJ, Waters AP. PTRAMP; a conserved Plasmodium thrombospondin-related apical merozoite protein. Mol Biochem Parasitol. 2004;134:225–232. doi: 10.1016/j.molbiopara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Thompson J, Fernandez-Reyes D, Sharling L, Moore SG, Eling WM, Kyes SA, Newbold CI, Kafatos FC, Janse CJ, Waters AP. Plasmodium cysteine repeat modular proteins 1–4: complex proteins with roles throughout the malaria parasite life cycle. Cell Microbiol. 2007;9:1466–1480. doi: 10.1111/j.1462-5822.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Trager W. Acquired Immunity to Ticks. J Parasitol. 1939;25:57–81. [Google Scholar]
- Tsai YL, Hayward RE, Langer RC, Fidock DA, Vinetz JM. Disruption of Plasmodium falciparum chitinase markedly impairs parasite invasion of mosquito midgut. Infect Immun. 2001;69:4048–4054. doi: 10.1128/IAI.69.6.4048-4054.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela JG, Pham VM, Garfield MK, Francischetti IM, Ribeiro JM. Toward a description of the sialome of the adult female mosquito Aedes aegypti. Insect Biochem Mol Biol. 2002;32:1101–1122. doi: 10.1016/s0965-1748(02)00047-4. [DOI] [PubMed] [Google Scholar]
- Valenzuela JG, Francischetti IM, Pham VM, Garfield MK, Ribeiro JM. Exploring the salivary gland transcriptome and proteome of the Anopheles stephensi mosquito. Insect Biochem Mol Biol. 2003;33:717–732. doi: 10.1016/s0965-1748(03)00067-5. [DOI] [PubMed] [Google Scholar]
- Vernick KD, Fujioka H, Seeley DC, Tandler B, Aikawa M, Miller LH. Plasmodium gallinaceum: a refractory mechanism of ookinete killing in the mosquito, Anopheles gambiae. Exp Parasitol. 1995;80:583–595. doi: 10.1006/expr.1995.1074. [DOI] [PubMed] [Google Scholar]
- Vinetz JM. Plasmodium ookinete invasion of the mosquito midgut. Curr Top Microbiol Immunol. 2005;295:357–382. doi: 10.1007/3-540-29088-5_14. [DOI] [PubMed] [Google Scholar]
- Vlachou D, Zimmermann T, Cantera R, Janse CJ, Waters AP, Kafatos FC. Real-time, in vivo analysis of malaria ookinete locomotion and mosquito midgut invasion. Cell Microbiol. 2004;6:671–685. doi: 10.1111/j.1462-5822.2004.00394.x. [DOI] [PubMed] [Google Scholar]
- Vlachou D, Schlegelmilch T, Christophides GK, Kafatos FC. Functional genomic analysis of midgut epithelial responses in Anopheles during Plasmodium invasion. Current Biology. 2005;15:1–11. doi: 10.1016/j.cub.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Vlachou D, Schlegelmilch T, Runn E, Mendes A, Kafatos FC. The developmental migration of Plasmodium in mosquitoes. Curr Opin Genet Dev. 2006;16:384–391. doi: 10.1016/j.gde.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Weathersby AB. The role of the stomach wall in the exogenous development of Plasmodium gallinaceum as studied by means of haemocoel injections of susceptible and refractory mosquitoes. J Infect Dis. 1952;91:198–205. doi: 10.1093/infdis/91.2.198. [DOI] [PubMed] [Google Scholar]
- Whitten MM, Shiao SH, Levashina EA. Mosquito midguts and malaria: cell biology, compartmentalization and immunology. Parasite Immunol. 2006;28:121–130. doi: 10.1111/j.1365-3024.2006.00804.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Dong Y, Abraham EG, Kocan A, Srinivasan P, Ghosh AK, Sinden RE, Ribeiro JM, Jacobs-Lorena M, Kafatos FC, Dimopoulos G. Transcriptome analysis of Anopheles stephensi-Plasmodium berghei interactions. Mol Biochem Parasitol. 2005;142:76–87. doi: 10.1016/j.molbiopara.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Ying P, Shakibaei M, Patankar MS, Clavijo P, Beavis RC, Clark GF, Frevert U. The malaria circumsporozoite protein: interaction of the conserved regions I and II-plus with heparin-like oligosaccharides in heparan sulfate. Exp Parasitol. 1997;85:168–182. doi: 10.1006/expr.1996.4134. [DOI] [PubMed] [Google Scholar]