Abstract
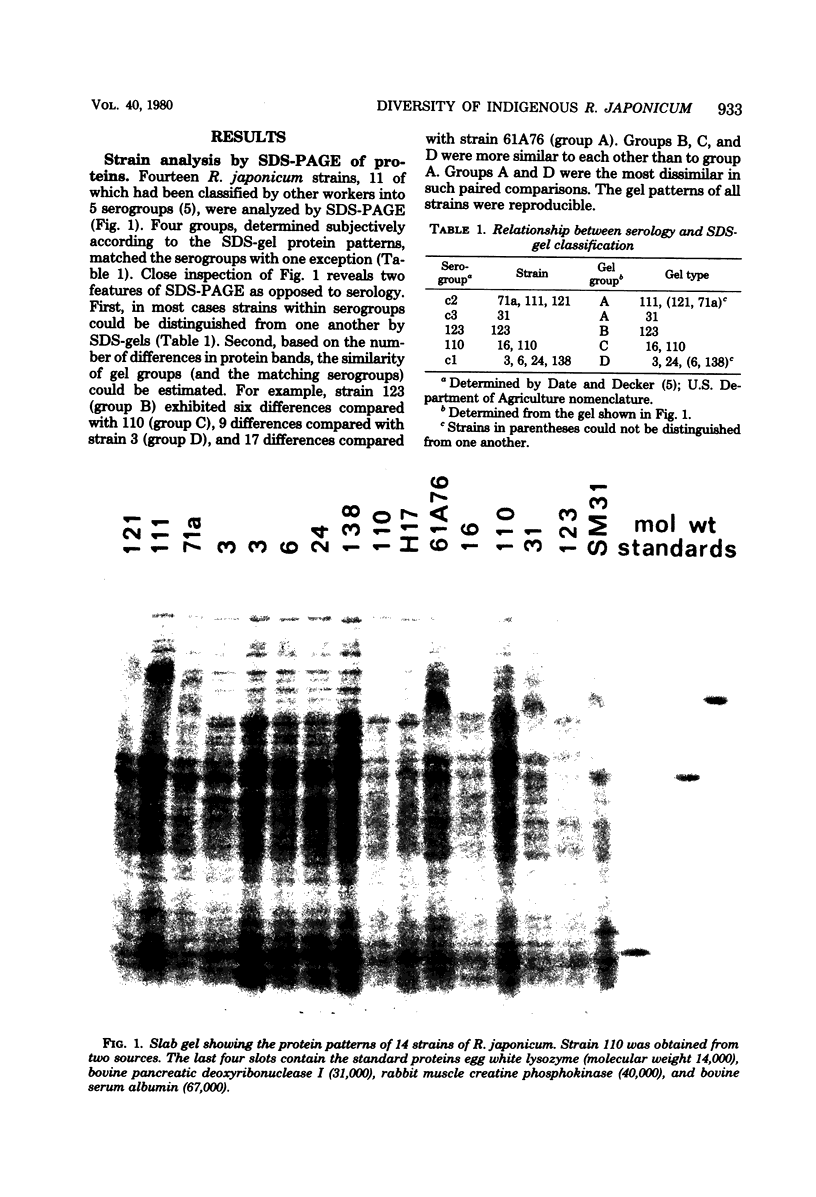
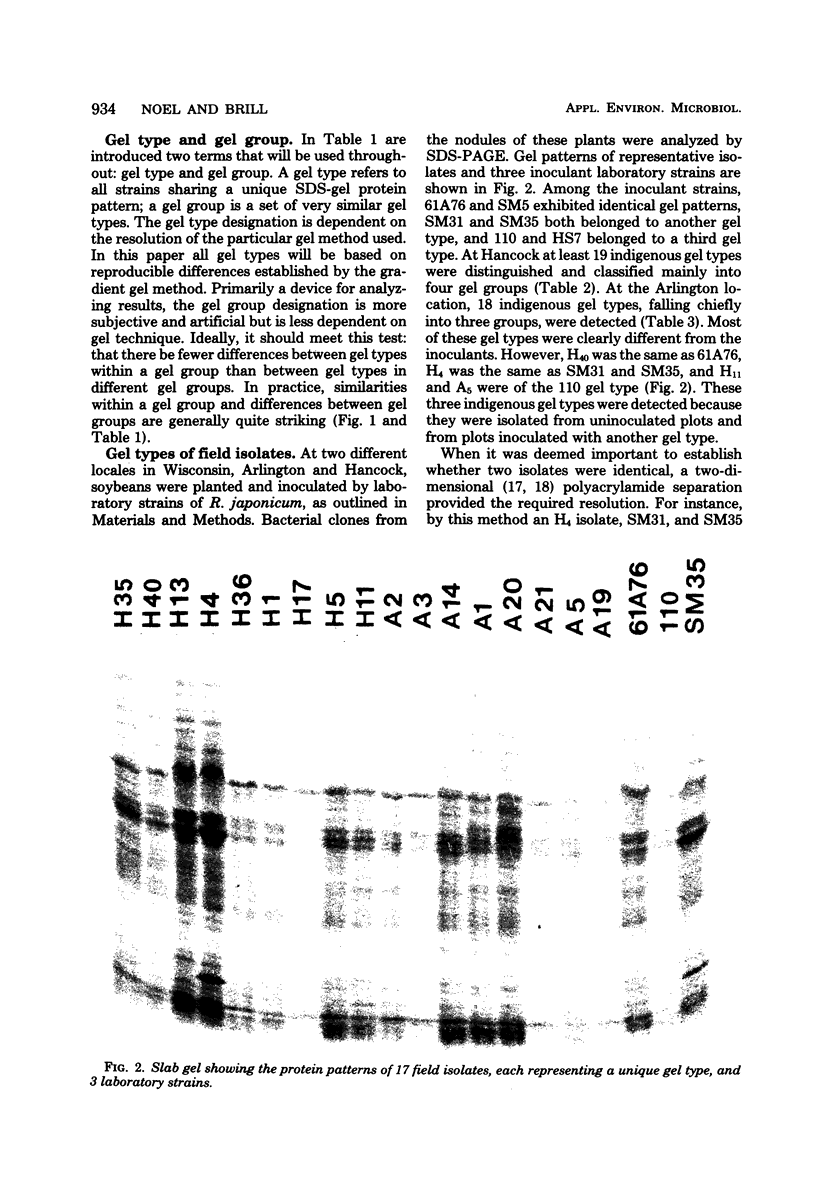
A simple method, based upon the separation of cellular proteins by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, has been devised for distinguishing between isolates of Rhizobium japonicum. Eleven laboratory strains, previously classified into five serogroups, were analyzed by gel electrophoresis. Groups determined subjectively according to protein patterns matched the serogroups, with one exception. Most strains within serogroups could be distinguished from one another. For studying the ecology of Rhizobium, an important advantage of this technique compared with serology or phage typing is that it discriminates among previously unencountered indigenous bacterial isolates as well as among known laboratory strains. SDS-gels were used to analyze the Rhizobium population of 500 nodules, sampled throughout the growing season, from soybeans at two different Wisconsin localities. Although the soybeans had been inoculated with laboratory strains of R. japonicum, indigenous R. japonicum predominated. At one location, 19 indigenous gel types were distinguished and classified mainly into four groups. At the other location, 18 gel types, falling mainly into three groups, were detected. The predominance of a particular group varied, in some cases dramatically, depending upon the time and depth of nodule formation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DATE R. A., DECKER A. M. MINIMAL ANTIGENIC CONSTITUTION OF 28 STRAINS OF RHIZOBIUM JAPONICUM. Can J Microbiol. 1965 Feb;11:1–8. doi: 10.1139/m65-001. [DOI] [PubMed] [Google Scholar]
- Kersters K., De Ley J. Identification and grouping of bacteria by numerical analysis of their electrophoretic protein patterns. J Gen Microbiol. 1975 Apr;87(2):333–342. doi: 10.1099/00221287-87-2-333. [DOI] [PubMed] [Google Scholar]
- Kuykendall L. D., Weber D. F. Genetically marked Rhizobium identifiable as inoculum strain in nodules of soybean plants grown in fields populated with Rhizobium japonicum. Appl Environ Microbiol. 1978 Dec;36(6):915–919. doi: 10.1128/aem.36.6.915-919.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MEANS U. M., JOHNSON H. W., DATE R. A. QUICK SEROLOGICAL METHOD OF CLASSIFYING STRAINS OF RHIZOBIUM JAPONICUM IN NODULES. J Bacteriol. 1964 Mar;87:547–553. doi: 10.1128/jb.87.3.547-553.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Ineffective and non-nodulating mutant strains of Rhizobium japonicum. J Bacteriol. 1976 Aug;127(2):763–769. doi: 10.1128/jb.127.2.763-769.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Mutant Strains of Rhizobium japonicum with Increased Ability to Fix Nitrogen for Soybean. Science. 1978 Aug 4;201(4354):448–450. doi: 10.1126/science.201.4354.448. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P., Leps W. T., Silver L. E., Brill W. J. Use of two-dimensional polyacrylamide gel electrophoresis to identify and classify Rhizobium strains. Appl Environ Microbiol. 1980 Feb;39(2):414–422. doi: 10.1128/aem.39.2.414-422.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semu E., Hume D. J., Corke C. T. Influence of soybean inoculation and nitrogen levels on populations and serogroups of Rhizobium japonicum in Ontario. Can J Microbiol. 1979 Jun;25(6):739–745. doi: 10.1139/m79-107. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]