Abstract
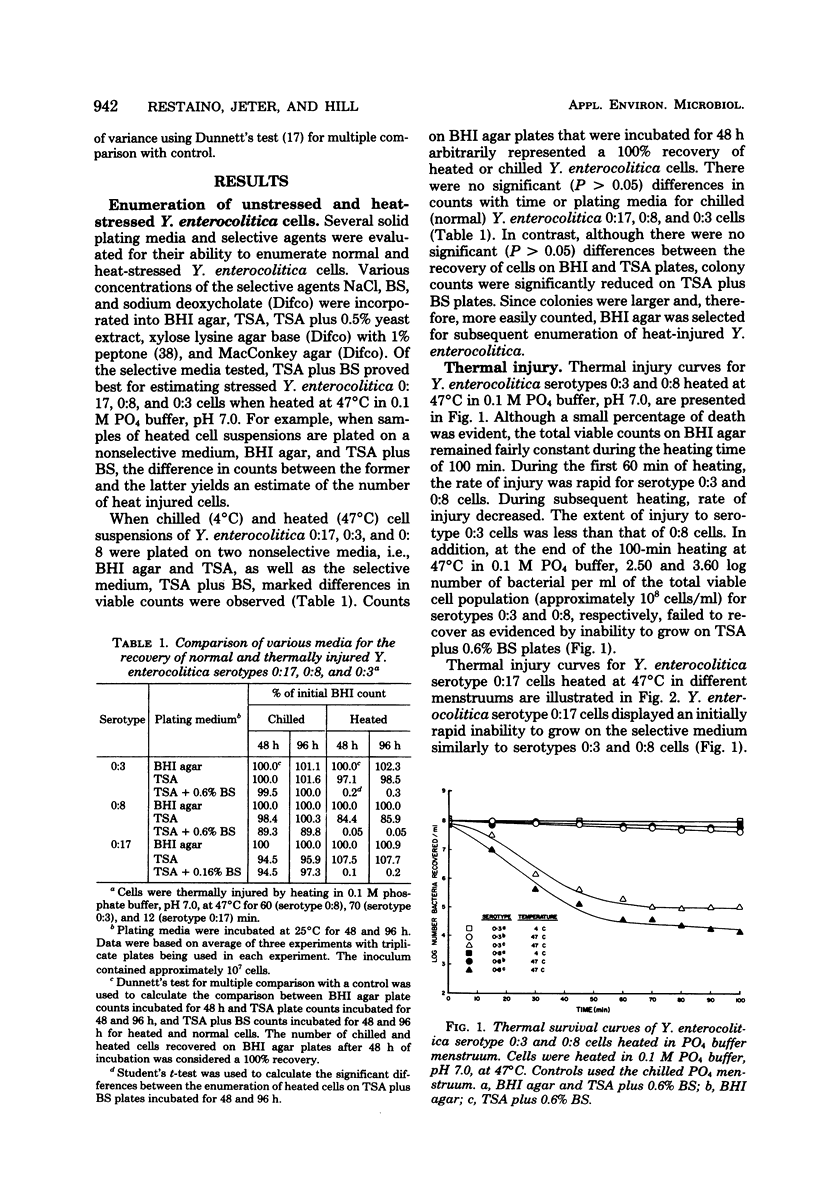
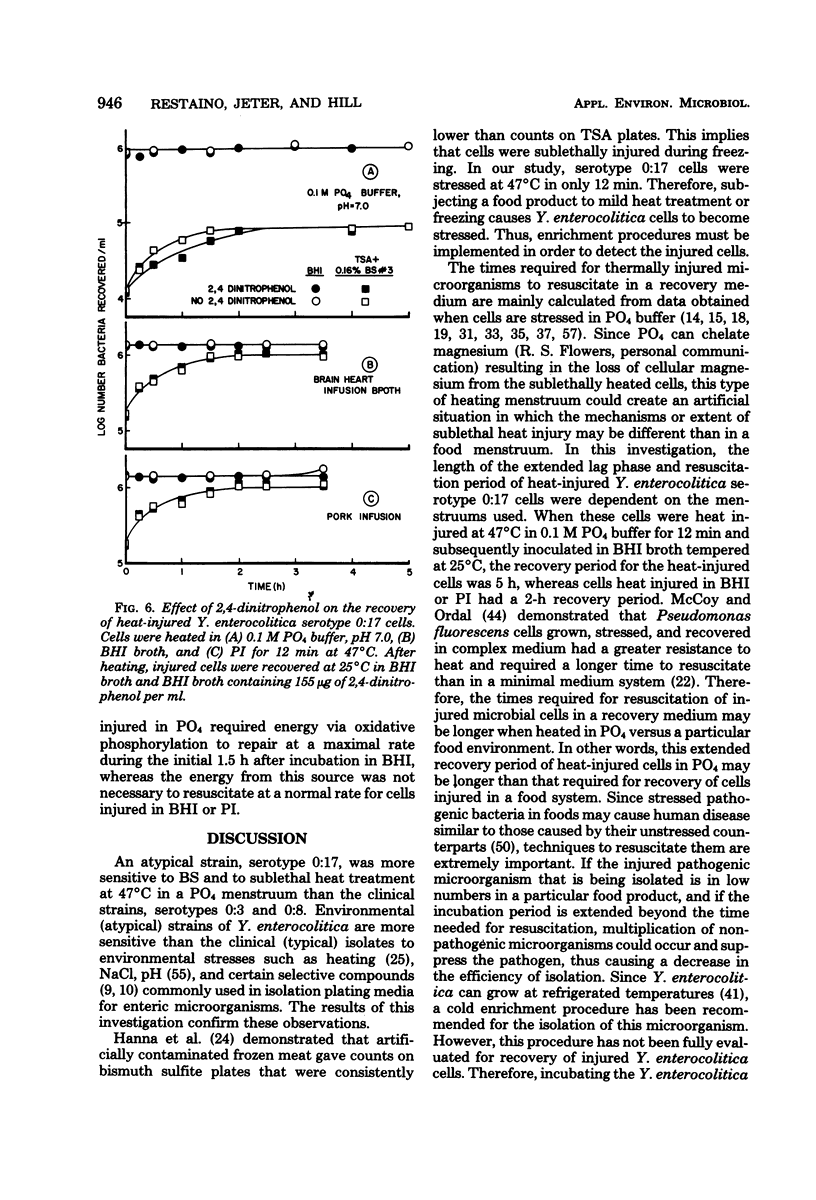
Procedures were developed to evaluate thermal injury to three strains of Yersinia enterocolitica (serotypes 0:3, 0:8, and 0:17). Serotype 0:17 (atypical strain) was more sensitive to bile salts no. 3 (BS) and to sublethal heat treatment than the typical strains, 0:3 and 0:8. When the 0:3, 0:8, and 0:17 serotypes were thermally stressed in 0.1 M PO4 buffer, pH 7.0, at 47 degrees C for 70, 60, and 12 min, respectively, greater than 99% of the total viable cell population was injured. Injury was determined by the ability of cells to form colonies on brain heart infusion (BHI) agar, but not on Trypticase soy agar (TSA) plus 0.6% BS for serotypes 0:3 and 0:8 and TSA plus 0.16% BS for 0:17. Heat injury of serotype 0:17 cells for 15 min in 0.1 M PO4 buffer caused an approximate 1,000-fold reduction in cell numbers on selective media as compared with cells heated in pork infusion (PI), BHI broth, and 10% nonfat dry milk (NFDM). The extended lag and resuscitation period in BHI broth was 2.5 times greater for 0:17 cells injured in 0.1 M PO4 than for cells injured in BHI or PI. The rate and extent of repair of Y. enterocolitica 0:17 cells in three recovery media were directly related to the heating menstruum used for injury. The use of metabolic inhibitors demonstrated that ribonucleic acid synthesis was required for repair, whereas deoxyribonucleic, cell wall, and protein synthesis were not necessary for recovery of 0:17 cells injured in 0.1 M PO4 buffer, BHI, or PI. Inhibition of respiration by 2,4-dinitrophenol slowed repair only for 0:17 cells injured in 0.1 M PO4 buffer, not for cells injured in PI or BHI.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN A. A. A rapid plate method of counting spores of Clostridium botulinum. J Bacteriol. 1951 Oct;62(4):425–432. doi: 10.1128/jb.62.4.425-432.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwood M. C., Russell A. D. Growth and metabolic activities of heat treated Staphylococcus aureus. J Appl Bacteriol. 1969 Mar;32(1):79–85. doi: 10.1111/j.1365-2672.1969.tb02191.x. [DOI] [PubMed] [Google Scholar]
- Andrews G. P., Martin S. E. Catalase activity during the recovery of heat-stressed Staphylococcus aureus MF-31. Appl Environ Microbiol. 1979 Sep;38(3):390–394. doi: 10.1128/aem.38.3.390-394.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa Y., Akahane S., Kagata N., Noguchi M., Sakazaki R. Two community outbreaks of human infection with Yersinia enterocolitica. J Hyg (Lond) 1973 Dec;71(4):715–723. doi: 10.1017/s002217240002297x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. E., Jackson R. J., Tsai T., Medvesky M., Shayegani M., Feeley J. C., MacLeod K. I., Wakelee A. M. Epidemic Yersinia enterocolitica infection due to contaminated chocolate milk. N Engl J Med. 1978 Jan 12;298(2):76–79. doi: 10.1056/NEJM197801122980204. [DOI] [PubMed] [Google Scholar]
- Bluhm L., Ordal Z. J. Effect of sublethal heat on the metabolic activity of Staphylococcus aureus. J Bacteriol. 1969 Jan;97(1):140–150. doi: 10.1128/jb.97.1.140-150.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottone E. J., Chester B., Malowany M. S., Allerhand J. Unusual Yersinia enterocolitica isolates not associated with mesenteric lymphadenitis. Appl Microbiol. 1974 May;27(5):858–861. doi: 10.1128/am.27.5.858-861.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottone E. J. Yersinia enterocolitica: a panoramic view of a charismatic microorganism. CRC Crit Rev Microbiol. 1977;5(2):211–241. doi: 10.3109/10408417709102312. [DOI] [PubMed] [Google Scholar]
- Botzler R. G., Wetzler F. T., Cowan A. B., Quan T. J. Yersiniae in pond water and snails. J Wildl Dis. 1976 Oct;12(4):492–496. doi: 10.7589/0090-3558-12.4.492. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Nyberg G., Wrethén J. Hydrogen peroxide and superoxide radical formation in anaerobic broth media exposed to atmospheric oxygen. Appl Environ Microbiol. 1978 Aug;36(2):223–229. doi: 10.1128/aem.36.2.223-229.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. W., Ordal Z. J. Thermal injury and recovery of Salmonella typhimurium and its effect on enumeration procedures. Appl Microbiol. 1969 Sep;18(3):332–336. doi: 10.1128/am.18.3.332-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. W., Witter L. D., Ordal Z. J. Thermal injury and recovery of Streptococcus faecalis. Appl Microbiol. 1968 Nov;16(11):1764–1769. doi: 10.1128/am.16.11.1764-1769.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emswiler B. S., Pierson M. D., Shoemaker S. P. Sublethal heat stress of Vibrio parahaemolyticus. Appl Environ Microbiol. 1976 Dec;32(6):792–798. doi: 10.1128/aem.32.6.792-798.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill C. O., Newton K. G. Spoilage of vacuum-packaged dark, firm, dry meat at chill temperatures. Appl Environ Microbiol. 1979 Mar;37(3):362–364. doi: 10.1128/aem.37.3.362-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R. F., Sinskey A. J., Davies R., Labuza T. P. Minimal medium recovery of heated Salmonella typhimurium LT2. J Gen Microbiol. 1973 Feb;74(2):267–274. doi: 10.1099/00221287-74-2-267. [DOI] [PubMed] [Google Scholar]
- Gray R. J., Witter L. D., Ordal Z. J. Characterization of mild thermal stress in Pseudomonas fluorescens and its repair. Appl Microbiol. 1973 Jul;26(1):78–85. doi: 10.1128/am.26.1.78-85.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S., Greenwood J. R., Pickett M. J., Mah R. A. Recovery of Yersinia enterocolitica from streams and lakes of California. Appl Environ Microbiol. 1976 Sep;32(3):352–354. doi: 10.1128/aem.32.3.352-354.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinis J. J., Beuchat L. R., Boswell F. C. Antimetabolite sensitivity and magnesium uptake by thermally stressed Vibrio parahaemolyticus. Appl Environ Microbiol. 1978 Jun;35(6):1035–1040. doi: 10.1128/aem.35.6.1035-1040.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A., Hurst A. Magnesium requirement of Staphylococcus aureus for repair from sublethal heat injury. Can J Microbiol. 1976 Aug;22(8):1202–1205. doi: 10.1139/m76-177. [DOI] [PubMed] [Google Scholar]
- Hughes D. Isolation of Yersinia enterocolitica from milk and a dairy farm in Australia. J Appl Bacteriol. 1979 Feb;46(1):125–130. doi: 10.1111/j.1365-2672.1979.tb02589.x. [DOI] [PubMed] [Google Scholar]
- Hurst A. Bacterial injury: a review. Can J Microbiol. 1977 Aug;23(8):935–944. doi: 10.1139/m77-139. [DOI] [PubMed] [Google Scholar]
- Hurst A., Hendry G. S., Hughes A., Paley B. Enumeration of sublethally heated staphylococci in some dried foods. Can J Microbiol. 1976 May;22(5):677–683. doi: 10.1139/m76-100. [DOI] [PubMed] [Google Scholar]
- Hurst A., Hughes A., Beare-Rogers J. L., Collins-Thompson D. L. Pysiological studies on the recovery of salt tolerance by Staphylococcus aureus after sublethal heating. J Bacteriol. 1973 Nov;116(2):901–907. doi: 10.1128/jb.116.2.901-907.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst A., Hughes A., Collins-Thompson D. L., Shah B. G. Relationship between loss of magnesium and loss of salt tolerance after sublethal heating of Staphylococcus aureus. Can J Microbiol. 1974 Aug;20(8):1153–1158. doi: 10.1139/m74-178. [DOI] [PubMed] [Google Scholar]
- Iandolo J. J., Ordal Z. J. Repair of thermal injury of Staphylococcus aureus. J Bacteriol. 1966 Jan;91(1):134–142. doi: 10.1128/jb.91.1.134-142.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen J. Yersinia enterocolitica in drinking-water. Scand J Infect Dis. 1972;4(2):125–127. doi: 10.3109/inf.1972.4.issue-2.11. [DOI] [PubMed] [Google Scholar]
- McCoy D. R., Ordal Z. J. Thermal stress of Pseudomonas fluorescens in complex media. Appl Environ Microbiol. 1979 Mar;37(3):443–448. doi: 10.1128/aem.37.3.443-448.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybylski K. S., Witter L. D. Injury and recovery of Escherichia coli after sublethal acidification. Appl Environ Microbiol. 1979 Feb;37(2):261–265. doi: 10.1128/aem.37.2.261-265.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B., Janssen D. W., Busta F. F. Characterization of the repair of injury induced by freezing Salmonella anatum. Appl Microbiol. 1972 Apr;23(4):803–809. doi: 10.1128/am.23.4.803-809.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B., Jezeski J. J., Busta F. F. Repair of injury in freeze-dried Salmonella anatum. Appl Microbiol. 1971 Sep;22(3):401–407. doi: 10.1128/am.22.3.401-407.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIERSON S. S., AMSTERDAM D. A simplified tube procedure for the routine determination of bacterial sensitivity to antibiotics. Am J Clin Pathol. 1959 Jan;31(1):81–86. doi: 10.1093/ajcp/31.1_ts.81. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E. EFFECT OF MAGNESIUM ON PERMEABILITY CONTROL IN CHILLED BACTERIA. Nature. 1964 Sep 19;203:1304–1305. doi: 10.1038/2031304a0. [DOI] [PubMed] [Google Scholar]
- Schiemann D. A. Association of Yersinia enterocolitica with the manufacture of cheese and occurrence in pasteurized milk. Appl Environ Microbiol. 1978 Aug;36(2):274–277. doi: 10.1128/aem.36.2.274-277.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann D. A., Toma S. Isolation of Yersinia enterocolitica from raw milk. Appl Environ Microbiol. 1978 Jan;35(1):54–58. doi: 10.1128/aem.35.1.54-58.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelye R. J., Yearbury B. J. Isolation of Yersinia enterocolitica-resembling organisms and Alteromonas putrefaciens from vacuum-packed chilled beef cuts. J Appl Bacteriol. 1979 Jun;46(3):493–499. doi: 10.1111/j.1365-2672.1979.tb00848.x. [DOI] [PubMed] [Google Scholar]
- Tomlins R. I., Ordal Z. J. Requirements of Salmonella typhimurium for recovery from thermal injury. J Bacteriol. 1971 Feb;105(2):512–518. doi: 10.1128/jb.105.2.512-518.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenyoji H., Maruyama T., Sakai S., Kimura S., Mizuno T. An outbreak of enteritis due to Yersinia enterocolitica occurring at a junior high school. Jpn J Microbiol. 1973 May;17(3):220–222. doi: 10.1111/j.1348-0421.1973.tb00730.x. [DOI] [PubMed] [Google Scholar]


