Abstract
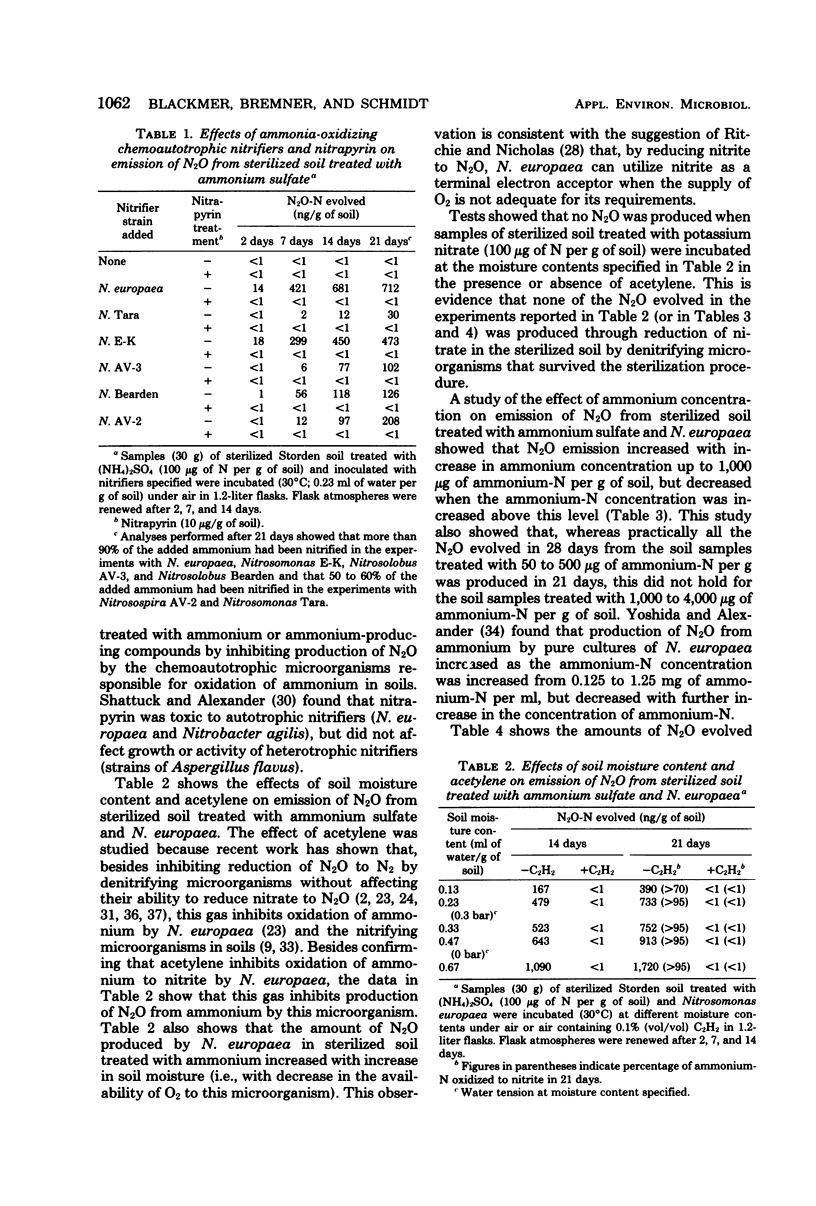
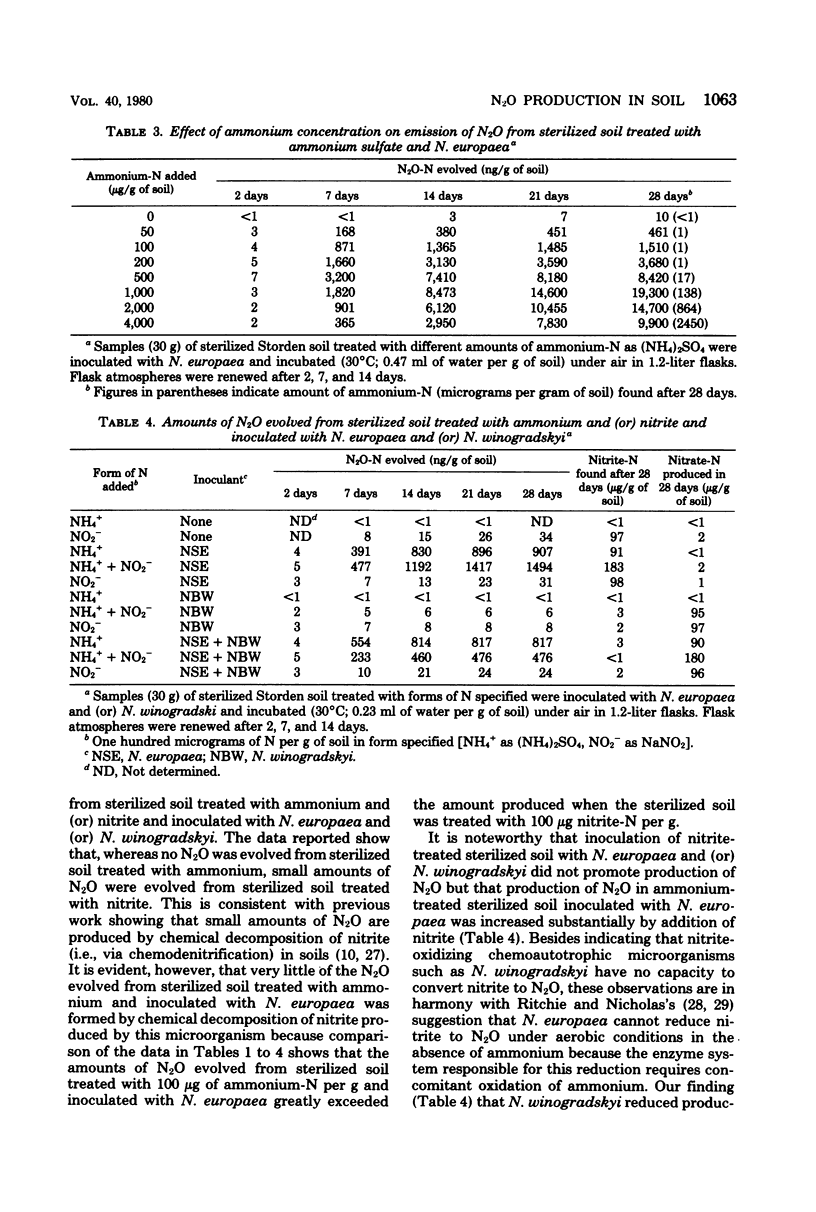
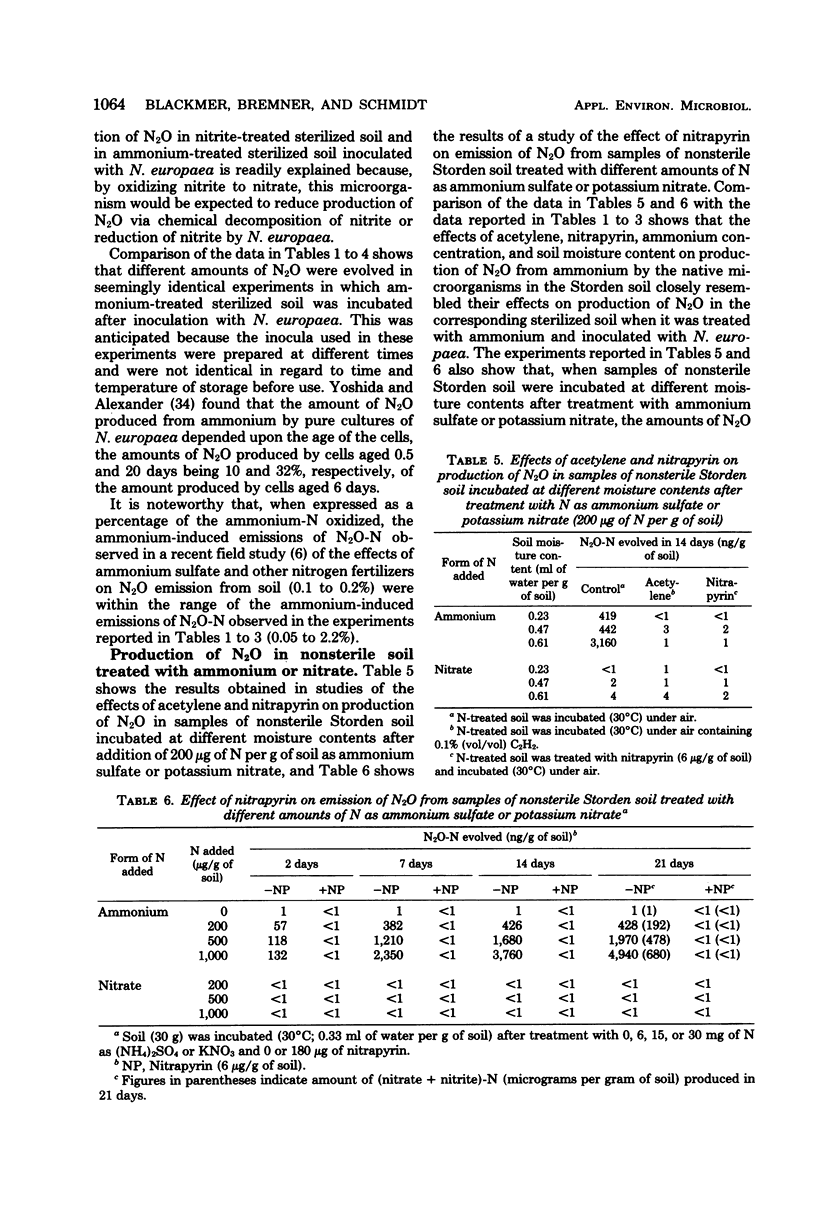
Gas chromatographic studies showed that nitrous oxide was produced in each instance when sterilized (autoclaved) soil was incubated after treatment with ammonium sulfate and inoculation with pure cultures of ammonia-oxidizing chemoautotrophic microorganisms (strains of Nitrosomonas, Nitrosospira, and Nitrosolobus). Production of N2O in ammonium-treated sterilized soil inoculated with Nitrosomonas europaea increased with the concentration of ammonium and the moisture content of the soil and was completely inhibited by both nitrapyrin and acetylene. Similar effects of nitrapyrin, acetylene, ammonium concentration, and soil moisture content were observed in studies of factors affecting N2O production in nonsterile soil treated with ammonium sulfate. These observations support the conclusion that, at least under some conditions, most of the N2O evolved from soils treated with ammonium or ammonium-producing fertilizers is generated by chemoautotrophic nitrifying microorganisms during oxidation of ammonium to nitrite.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balderston W. L., Sherr B., Payne W. J. Blockage by acetylene of nitrous oxide reduction in Pseudomonas perfectomarinus. Appl Environ Microbiol. 1976 Apr;31(4):504–508. doi: 10.1128/aem.31.4.504-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser L. W., Schmidt E. L. Serological diversity within a terrestrial ammonia-oxidizing population. Appl Environ Microbiol. 1978 Oct;36(4):589–593. doi: 10.1128/aem.36.4.589-593.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. M., Blackmer A. M. Nitrous oxide: emission from soils during nitrification of fertilizer nitrogen. Science. 1978 Jan 20;199(4326):295–296. doi: 10.1126/science.199.4326.295. [DOI] [PubMed] [Google Scholar]
- Fliermans C. B., Bohlool B. B., Schmidt E. L. Autecological study of the chemoautotroph Nitrobacter by immunofluorescence. Appl Microbiol. 1974 Jan;27(1):124–129. doi: 10.1128/am.27.1.124-129.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henniger N. M., Bollag J. M. Effect of chemicals used as nitrification inhibitors on the denitrification process. Can J Microbiol. 1976 May;22(5):668–672. doi: 10.1139/m76-098. [DOI] [PubMed] [Google Scholar]
- Ritchie G. A., Nicholas D. J. Identification of the sources of nitrous oxide produced by oxidative and reductive processes in Nitrosomonas europaea. Biochem J. 1972 Mar;126(5):1181–1191. doi: 10.1042/bj1261181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie G. A., Nicholas D. J. The partial characterization of purified nitrite reductase and hydroxylamine oxidase from Nitrosomonas europaea. Biochem J. 1974 Mar;138(3):471–480. doi: 10.1042/bj1380471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano S., Walker N. Isolation of ammonia-oxidizing autotrophic bacteria. J Appl Bacteriol. 1968 Dec;31(4):493–497. doi: 10.1111/j.1365-2672.1968.tb00397.x. [DOI] [PubMed] [Google Scholar]
- Yoshinari T., Knowles R. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem Biophys Res Commun. 1976 Apr 5;69(3):705–710. doi: 10.1016/0006-291x(76)90932-3. [DOI] [PubMed] [Google Scholar]


