Abstract
An enormous amount of knowledge about the ovary has been generated over the last two decades, due in part to the development of strategies to genetically manipulate the mouse using embryonic stem cell technology. Our group and others have identified multiple factors that are important and essential at all stages of ovarian folliculogenesis from formation of the primordial factor to ovulation. It is obvious that an oocyte, the key cargo of the ovary, and the surrounding granulosa cells, the support cells of the follicle, entertain a dialog that is key for granulosa growth and differentiation and oocyte growth, maturation, and fertilization. In addition to the involvement of genes in these processes, small non-coding RNAs including microRNAs and siRNAs have been implicated as key regulators, especially in the oocyte. These studies have direct implications for human fertility control in the assisted reproductive technology (ART) laboratory.
Keywords: MicroRNAs, siRNAs, Ovarian folliculogenesis
1. Introduction
The first knockouts of genes in mice were achieved in the late 1980s. However, the last two decades have seen the publication of a wealth of information on the development and physiology of the mammals using the newfound technology. Included among this list are over 500 mutant mouse models that have a fertility phenotype [1,2]. In particular, our group has been focused on understanding the factors involved in intercellular communication between the oocyte and the surrounding cumulus cells in the periovulatory follicle [3]. In addition, not only are genes encoding proteins involved in this process but also small non-coding RNAs that function to regulate gene expression [4].
2. Oocyte-somatic cell communication
Beginning with formation of the primordial follicle, the oocyte and its surrounding granulosa cells communicate, a dialog that is essential for fertility. Although all of the secreted factors required in primordial follicle recruitment have not been identified, it is obvious that the oocyte plays a key role at later stages. At the transition from the primary (one layer) follicle to the secondary (two or more layers preantral) follicle, growth differentiation factor 9 (GDF9) in mouse [5] and bone morphogenetic protein 15 (BMP15) in sheep [6] are essential and critical for fertility. GDF9 and BMP15, members of the transforming growth factor β (TGFβ) superfamily that play key roles in mammals [7], are most closely homologous to each other in the family and are synthesized in oocytes beginning at primordial follicle recruitment [8–10].
The transition from preantral to antral follicles requires extragonadal signaling. The pituitary glycoprotein hormones, follicle stimulating hormone (FSH) and luteinizing hormone (LH) have distinct roles in the regulation of folliculogenesis [4]. In the absence of FSH the ovarian follicle halts at the large preantral follicle stage [11,12]. However, in the presence of FSH, many of the intercellular connections between the granulosa cells and the oocyte are disrupted, allowing an antrum to form [13], the first step in the formation of a preovulatory follicle. The preovulatory follicle separates the granulosa cell component into two parts: those lining the wall and adjacent to the basement membrane are called the mural granulosa cells whereas those adjacent to the oocyte are called the cumulus granulosa cells. LH plays a dual role in the follicle, stimulating androgen synthesis by the thecal cells and inducing ovulation of a cumulus cell-oocyte complex (COC) [4,14–16].
In addition to a role of LH in ovulation, LH works indirectly with the oocyte to induce the process of cumulus expansion, the laying down of a proteinaceous and hyaluronic acid rich matrix [1,2,4]. Multiple genes have been shown to play roles in cumulus expansion (Table 1). Oocyte secreted factors including GDF9, BMP15, and fibroblast growth factor 8 (FGF8) are required for this process, and the Eppig laboratory has elegantly demonstrated important roles of the oocyte in regulation of cumulus cell amino acid transport, glycolysis, and cholesterol biosynthesis [17–20]. Because LH receptors are not present on cumulus cells, LH acts by inducing the secretion of epidermal growth factor (EGF)-like ligands from the mural granulosa cells and subsequent synergism with the oocyte-secreted proteins to regulate cumulus cell functions [21]. These signaling pathways are important for the ART laboratory.
Table 1.
Mouse models with defects in cumulus expansion. For more detailed information, refer to reference [4].
| Gene (symbol) | Fertility status | Ref |
|---|---|---|
| Prostaglandin-endoperoxide synthase 2 (Ptgs2; Cox2) | Mostly infertile | [22,23] |
| Prostaglandin E receptor 2, subtype EP2 (Ptger2) | Subfertile | [24–26] |
| Pentraxin 3 (Ptx3) | Subfertile | [27,28] |
| Tumor necrosis factor α induced protein 6 (Tnfaip6) | Infertile | [29] |
| Sulfotransferase family 1E, member 1 (Sult1e1) | Subfertile | [30,31] |
| Alpha 1 microglobulin/bikunin (Ambp) | Subfertile | [32,33] |
| Amphiregulin (Areg) | Subfertile | [34] |
| Bone morphogenetic protein 15 (Bmp15) | Subfertile | [35] |
| Bone morphogenetic protein receptor, type IB (Bmpr1b) | Subfertile | [36] |
| Epiregulin (Eregwa2/wa2; hypomorph) | Subfertile | [34] |
| Mitogen-activated protein kinases 3 and 1 (Mapk3−/− Mapk1 cKO) | Infertile | [37] |
| Nuclear receptor subfamily 5, group 2, member 1 (Nr5a1; Sf1, steroidogenic factor 1) (cKO) | Infertile | [38] |
| Nuclear receptor subfamily 5, group 2, member 2 (Nr5a2; Lrh1, liver receptor homolog 1) (cKO) | Infertile | [39] |
cKO: conditional knockout.
3. Specific small RNAs in the ovary
MicroRNAs are small RNA molecules that are approximately 22 nucleotides in length [40]. These molecules are thought to function as repressors of gene expression by blocking mRNA translation and/or destabilizing and degrading the mRNA transcript [41]. Using mouse modeling techniques, microRNAs have been implicated to play roles in ovarian function and development. Although microRNAs and/or small interfering RNAs (siRNAs) are required in the oocyte (see next section), microRNAs likely function only through “fine tuning” of gene expression in granulosa cells. To date, most of the data involves microRNA profiling of ovarian tissues, and therefore there is only speculation of the actual function of specific microRNAs (Fig. 1) [42,43].
Fig. 1.
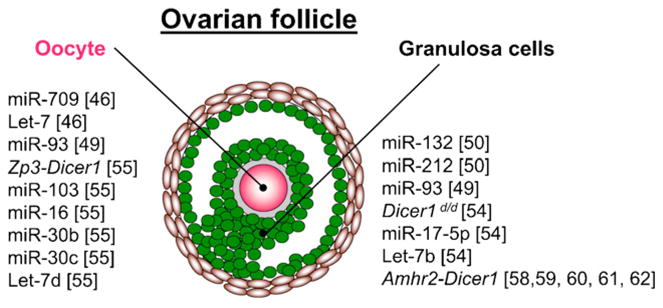
MicroRNAs play a functional role in the oocyte and the ovarian follicle. Les microARN jouent un rôle fonctionnel dans l’ovocyte et le follicule ovarien.
Nobox, newborn ovary homeobox-encoding gene, is a homeobox gene critical for oocyte development. Nobox null mice are infertile due to early ovarian insufficiency, demonstrating a block from the primordial to primary follicle stage [44,45]. Characterization of the microRNA profiles in wild type newborn ovaries revealed especially high levels of miR-709 [46], an abundant microRNA in mouse embryos [47]. Additionally let-7 microRNA family members were abundant as well in newborn ovaries. Differentially expressed microRNAs between the Nobox null and wild type mouse newborn ovaries included only modest fold changes in microRNA levels [46]. However, the functional significance of these microRNAs in the Nobox null ovaries is not known (i.e., it is not clear whether the microRNA changes are a consequence of major changes in downstream targets of Nobox, whether they are directly regulated by Nobox, and/or if the microRNA changes play any major roles in the primordial follicle).
Lhx8 is a Lim homeodomain protein important for ovarian development [48]. Similar to Nobox, Lhx8 is required for transition from the primordial to primary follicle stage and is required for fertility. In silico targeting algorithms speculate that miR-93 may target this important gene in ovarian development [49], although functional relevance of miR-93 in reproduction will have to await conditional knockout of miR-93 in oocytes.
MicroRNAs in granulosa cells are hormonally regulated. Using mouse mural granulosa cells treated with human chorionic gonadotropin (hCG), a set of hormonally responsive microRNAs was described [50]. In particular, miR-132 and miR-212 were significantly upregulated by hCG. In silico targeting speculated 77 common targets between these two microRNAs and hCG responsive genes. Specific targeting results suggest that miR-132 and miR-212 inhibit C-terminal binding protein 1 (Ctbp1) protein synthesis. However, the role of CTBP1 in ovarian function is not known.
Lastly, specific microRNA expression on steroid hormone expression from human primary granulosa cells has been described. Multiple microRNAs affected production of pro-gesterone, testosterone, and estradiol from these primary cell cultures [51]. However, they only examined 187 individual microRNAs out of the now 750 known human microRNAs, based on miRBase version 14.0, release September 2009. Therefore, the global effect of microRNAs on ovarian function is still undiscovered.
3.1. Global disruption of small RNA synthesis in various compartments of the ovary
Dicer1 is an endoribonuclease III essential for microRNA synthesis. It is responsible for cleavage of the precursor microRNA species with its stem-loop structure to the double stranded mature microRNA and corresponding star form [52]. Dicer1 is essential for mammalian development as deletion of Dicer1 through traditional knockout mouse technology has shown embryonic lethality at day e11.5 [53].
Mutant mice with a Dicer1 hypomorphic allele (Dicer1d/d) are infertile [54]. However, these mice were unable to maintain a pregnancy, presumably due to impaired corpus luteum function. Additionally, the corpora lutea of these mice had abnormal angiogenesis, likely through dysregulation of the antiangiogenic factor, tissue inhibitor of metalloproteinase 1 (Timp1). In silico microRNA targeting algorithms predict that miR-17-5p and let-7b target Timp1. Both of these microRNAs were present in wild type ovaries but not in the Dicer1 hypomorphic ovaries. Additional functional studies validated the role of miR-17-5p and let-7b on Timp1 activity and angiogenic function. Thus, miR-17-5p and let-7b play a role in angiogenesis in the mouse corpus luteum.
Dicer1 is highly expressed in the mouse oocyte and is regulated with folliculogenesis and embryogenesis [55]. Conditional deletion of Dicer1 in the oocyte using a cre recombinase driven by the zona pellucida protein 3 (Zp3) promoter, which drives cre expression in the postnatal oocyte, results in infertile mice. Oocyte growth and development and responses to gonadotropins were normal in these mice. However, the oocytes had meiotic defects due to defective spindle formation. MicroRNA profiling suggest that miR-103, miR-16, miR-30b, miR-30c, and let-7d target genes important in spindle organization. However, these defects in spindle formation may also be attributed to disruption of siRNAs as Dicer1 is also important for siRNA processing. Additionally, Dicer1 knockout oocytes have increased expression of mRNA targets of endogenous siRNAs [56,57]. Since these endogenous siRNAs appear to play a significant role in oocytes, the relative significance of microRNAs versus siRNAs in oocytes is unknown.
Dicer1 is expressed in the somatic cells of the ovary but expression does not vary with folliculogenesis. However, conditional deletion of Dicer1 in the somatic cells of the female reproductive tract using Amhr2-cre leads to infertility in female mice due mainly to the formation of oviductal diverticuli. However, unstimulated ovaries of these mice have normal histology. The presence of the oviductal diverticuli does not allow the fertilized oocytes and embryos to transit to the uterus [58–62].
Recently, the ovaries from the Amhr2-Dicer1 cKO mice were found to have a significant decrease in miR-503 expression compared to controls [62]. Additionally, this change in expression was dependent on hormonal manipulation. MiR-503 is a target of cyclin D2 (Ccnd2), which is important for proliferation during folliculogenesis. Therefore, miR-503 could play a role in folliculogenesis; however, miR-503 likely plays a minimal role functionally since the oviductal diverticuli of normally cycling Amhr2-Dicer1 cKO mice contain hundreds of oocyte remnants and embryos [58], indicating that global depletion of small RNAs in granulosa cells does not grossly disrupt normal ovarian function.
Acknowledgments
These studies are supported by grants from the National Institutes of Health (R01CA60651, R01HD32067, R37HD33438, U54HD07495, and 5K12HD050128), the Ovarian Cancer Research Fund, the Dan L. Duncan Cancer Center, and the Young Texans Against Cancer, and the Herman L. and LeNan Gardner Research Fund in Obstetrics and Gynecology.
Footnotes
Conflict of interest statement
None.
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit:
References
- 1.Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Med. 2002;8(S1):S41–9. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- 2.Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matzuk MM, Burns K, Viveiros MM, Eppig J. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–80. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 4.Edson MA, Nagaraja AK, Matzuk MM. The Mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–5. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 6.Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25:279–83. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- 7.Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian TGF-β superfamily. Endocr Rev. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- 8.McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9:131–6. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- 9.Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol. 1998;12:1809–17. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- 10.Elvin JA, Yan C, Matzuk MM. Oocyte-expressed TGF-β superfamily members in female fertility. Mol Cell Endocrinol. 2000;159:1–5. doi: 10.1016/s0303-7207(99)00185-9. [DOI] [PubMed] [Google Scholar]
- 11.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–4. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 12.Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, et al. Impairing follicle-stimulating hormone (FSH) signaling in vivo: Targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998;95:13612–7. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Combelles CM, Carabatsos MJ, Kumar TR, Matzuk MM, Albertini DF. Hormonal control of somatic cell oocyte interactions during ovarian follicle development. Mol Reprod Dev. 2004;69:347–55. doi: 10.1002/mrd.20128. [DOI] [PubMed] [Google Scholar]
- 14.Ma X, Dong Y, Matzuk MM, Kumar TR. 2004 Targeted disruption of luteinizing hormone β subunit leads to hypogonadism, defects in gonadal steroidogenesis and infertility. Proc Natl Acad Sci U S A. 2004;101:17294–9. doi: 10.1073/pnas.0404743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15:172–83. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- 16.Lei ZM, Mishra S, Zou W, Xu B, Foltz M, Li X, et al. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol. 2001;15:184–200. doi: 10.1210/mend.15.1.0586. [DOI] [PubMed] [Google Scholar]
- 17.Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK. Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biol Reprod. 2005;73:351–7. doi: 10.1095/biolreprod.105.041798. [DOI] [PubMed] [Google Scholar]
- 18.Diaz FJ, O’Brien MJ, Wigglesworth K, Eppig JJ. The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol. 2006;299:91–104. doi: 10.1016/j.ydbio.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, et al. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134:2593–603. doi: 10.1242/dev.006882. [DOI] [PubMed] [Google Scholar]
- 20.Su YQ, Sugiura K, Wigglesworth K, O’Brien MJ, Affourtit JP, Pangas SA, et al. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development. 2008;135:111–21. doi: 10.1242/dev.009068. [DOI] [PubMed] [Google Scholar]
- 21.Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–4. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 22.Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, et al. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–9. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- 23.Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, et al. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 24.Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2) Proc Natl Acad Sci U S A. 1999;96:10501–6. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy CRJ, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, et al. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5:217–20. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 26.Tilley SL, Audoly LP, Hicks EH, Kim H-S, Flannery PJ, Coffman TM, et al. Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J Clin Invest. 1999;103:1539–45. doi: 10.1172/JCI6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577–86. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- 28.Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, et al. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16:1154–67. doi: 10.1210/mend.16.6.0859. [DOI] [PubMed] [Google Scholar]
- 29.Fulop C, Szanto S, Mukhopadhyay D, Bardos T, Kamath RV, Rugg MS, et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130:2253–61. doi: 10.1242/dev.00422. [DOI] [PubMed] [Google Scholar]
- 30.Gershon E, Hourvitz A, Reikhav S, Maman E, Dekel N. Low expression of COX-2, reduced cumulus expansion, and impaired ovulation in SULT1E1-deficient mice. FASEB J. 2007;21:1893–901. doi: 10.1096/fj.06-7688com. [DOI] [PubMed] [Google Scholar]
- 31.Qian YM, Sun XJ, Tong MH, Li XP, Richa J, Song WC. Targeted disruption of the mouse estrogen sulfotransferase gene reveals a role of estrogen metabolism in intracrine and paracrine estrogen regulation. Endocrinology. 2001;142:5342–50. doi: 10.1210/endo.142.12.8540. [DOI] [PubMed] [Google Scholar]
- 32.Sato H, Kajikawa S, Kuroda S, Horisawa Y, Nakamura N, Kaga N, et al. Impaired fertility in female mice lacking urinary trypsin inhibitor. Biochem Biophys Res Commun. 2001;281:1154–60. doi: 10.1006/bbrc.2001.4475. [DOI] [PubMed] [Google Scholar]
- 33.Zhuo L, Yoneda M, Zhao M, Yingsung W, Yoshida N, Kitagawa Y, et al. Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J Biol Chem. 2001;276:7693–6. doi: 10.1074/jbc.C000899200. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, et al. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–24. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan C, Wang P, DeMayo J, DeMayo F, Elvin J, Carino C, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–66. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- 36.Yi SE, LaPolt PS, Yoon BS, Chen JY, Lu JK, Lyons KM. The type I BMP receptor BmprIB is essential for female reproductive function. Proc Natl Acad Sci U S A. 2001;98:7994–9. doi: 10.1073/pnas.141002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, et al. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–41. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelusi C, Ikeda Y, Zubair M, Parker KL. Impaired follicle development and infertility in female mice lacking steroidogenic factor 1 in ovarian granulosa cells. Biol Reprod. 2008;79:1074–83. doi: 10.1095/biolreprod.108.069435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, Auwerx J, et al. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 2008;22:1871–6. doi: 10.1101/gad.472008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development. 2005;132:4645–52. doi: 10.1242/dev.02070. [DOI] [PubMed] [Google Scholar]
- 41.Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet. 2004;5:396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- 42.Tang F, Kaneda M, O’Carroll D, Hajkova P, Barton SC, Sun YA, et al. Maternal microRNAs are essential for mouse zygotic development. Genes Dev. 2007;21:644–8. doi: 10.1101/gad.418707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ro S, Song R, Park C, Zheng H, Sanders KM, Yan W. Cloning and expression profiling of small RNAs expressed in the mouse ovary. RNA Society. 2007;13:2366–80. doi: 10.1261/rna.754207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzumori N, Yan C, Matzuk MM, Rajkovic A. Nobox is a homeobox-encoding gene preferentially expressed in primordial and growing oocytes. Mech Dev. 2002;111:137–41. doi: 10.1016/s0925-4773(01)00620-7. [DOI] [PubMed] [Google Scholar]
- 45.Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–9. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- 46.Choi Y, Qin Y, Berger MF, Ballow DJ, Bulyk ML, Rajkovic A. Microarray analyses of newborn mouse ovaries lacking Nobox. Biol Reprod. 2007;77:312–9. doi: 10.1095/biolreprod.107.060459. [DOI] [PubMed] [Google Scholar]
- 47.Mineno J, Okamoto S, Ando T, Sato M, Chono H, Izu H, et al. The expression profile of microRNAs in mouse embryos. Nucleic Acids Res. 2006;34:1765–71. doi: 10.1093/nar/gkl096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, et al. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci U S A. 2006;103:8090–5. doi: 10.1073/pnas.0601083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao H, Rajkovic A. MicroRNAs and mammalian ovarian development. Semin Reprod Med. 2008;26:461–8. doi: 10.1055/s-0028-1096126. [DOI] [PubMed] [Google Scholar]
- 50.Fiedler SD, Carletti MZ, Hong X, Christenson LK. Hormonal regulation of MicroRNA expression in periovulatory mouse mural granulosa cells. Biol Reprod. 2008;79:1030–7. doi: 10.1095/biolreprod.108.069690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sirotkin AV, Ovcharenko D, Grossmann R, Laukova M, Mlyncek M. Identification of microRNAs controlling human ovarian cell steroidogenesis via a genome-scale screen. J Cell Physiol. 2009;219:415–20. doi: 10.1002/jcp.21689. [DOI] [PubMed] [Google Scholar]
- 52.Shyu AB, Wilkinson MF, van Hoof A. Messenger RNA regulation: to translate or to degrade. EMBO J. 2008;27:471–81. doi: 10.1038/sj.emboj.7601977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, et al. Dicer is essential for mouse development. Nat Genet. 2003;35:215–7. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 54.Otsuka M, Zheng M, Hayashi M, Lee JD, Yoshino O, Lin S, et al. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest. 2008;118:1944–54. doi: 10.1172/JCI33680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murchison EP, Stein P, Xuan Z, Pan H, Zhang MQ, Schultz RM, et al. Critical roles for Dicer in the female germline. Genes Dev. 2007;21:682–93. doi: 10.1101/gad.1521307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe T, Totoki Y, Toyoda A, Kaneda M, Kuramochi-Miyagawa S, Obata Y, et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 2008;453:539–43. doi: 10.1038/nature06908. [DOI] [PubMed] [Google Scholar]
- 57.Tam OH, Aravin AA, Stein P, Girard A, Murchison EP, Cheloufi S, et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature. 2008;7194:534–8. doi: 10.1038/nature06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagaraja AK, Andreu-Vieyra C, Franco HL, Ma L, Chen R, Han DY, et al. Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol. 2008;22:2336–52. doi: 10.1210/me.2008-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pastorelli LM, Wells S, Fray M, Smith A, Hough T, Harfe BD, et al. Genetic analyses reveal a requirement for Dicer1 in the mouse urogenital tract. Mamm Genome. 2009;20:140–51. doi: 10.1007/s00335-008-9169-y. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez G, Behringer RR. Dicer is required for female reproductive tract development and fertility in the mouse. Mol Reprod Dev. 2009;76:678–88. doi: 10.1002/mrd.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong X, Luense LJ, McGinnis LK, Nothnick WB, Christenson LK. Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology. 2008;149:6207–12. doi: 10.1210/en.2008-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lei L, Jin S, Gonzalez G, Behringer RR, Woodruff TK. The regulatory role of Dicer in folliculogenesis in mice. Mol Cell Endocrinol. 2010;315:63–73. doi: 10.1016/j.mce.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


