Abstract
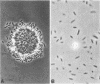
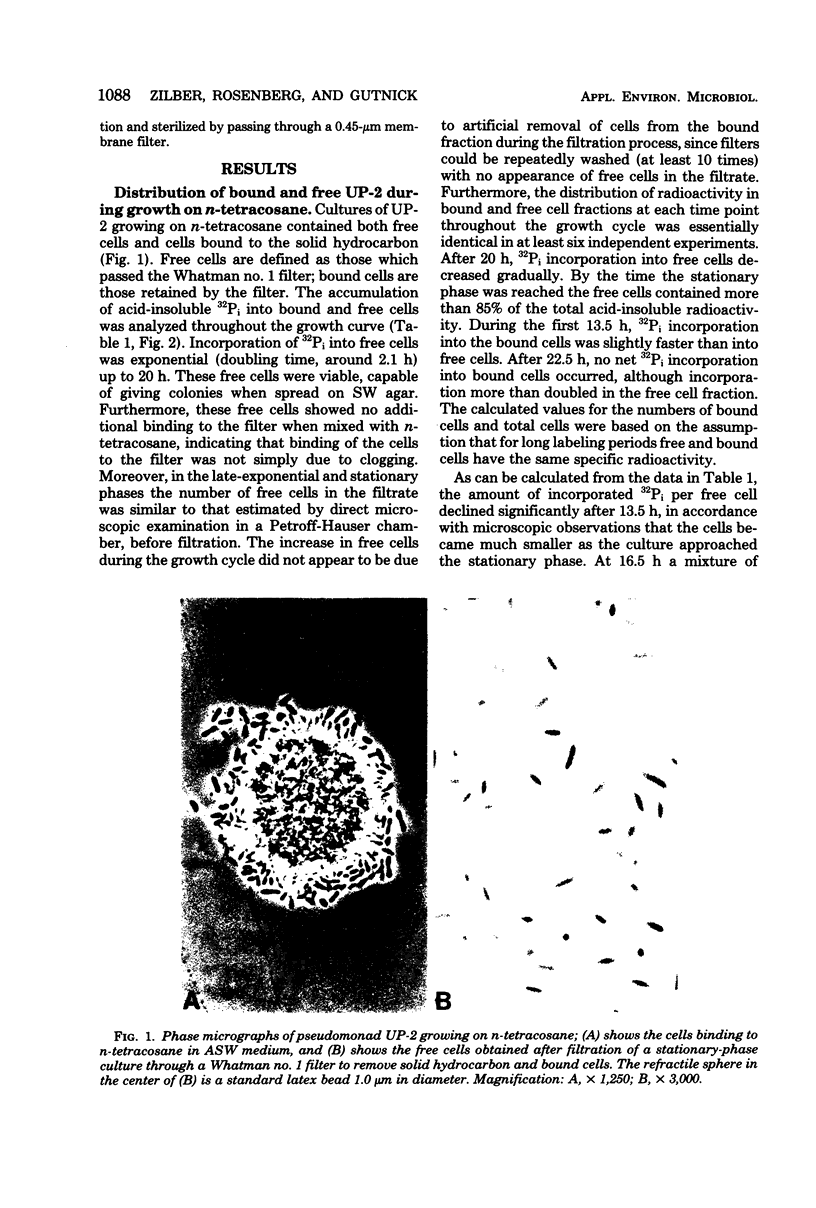
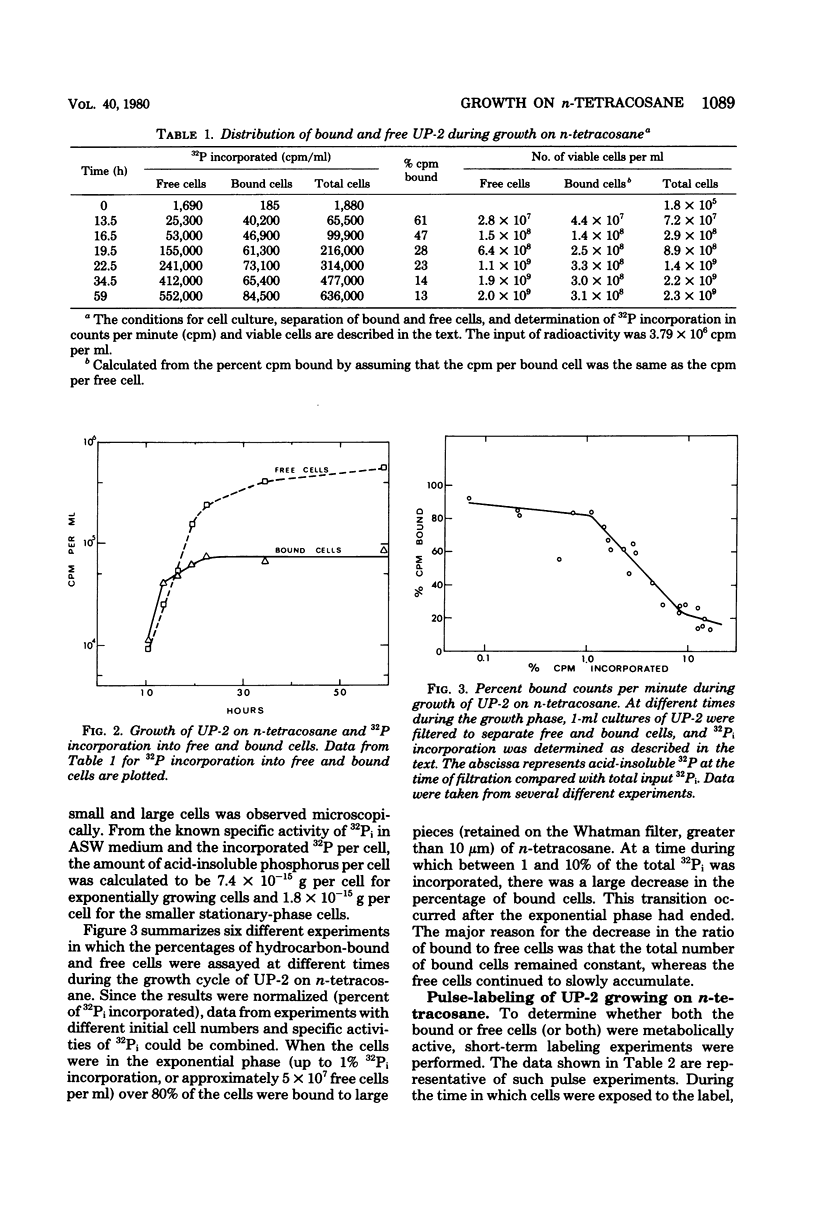
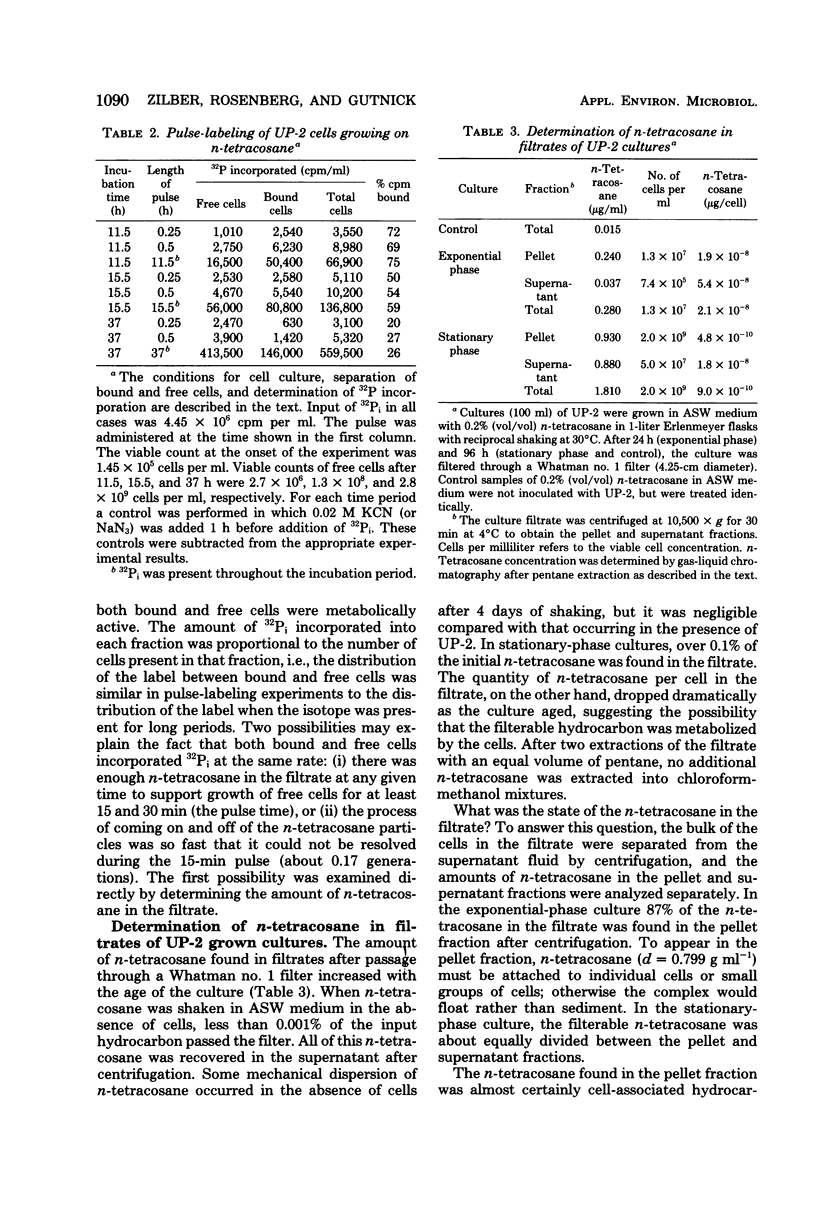
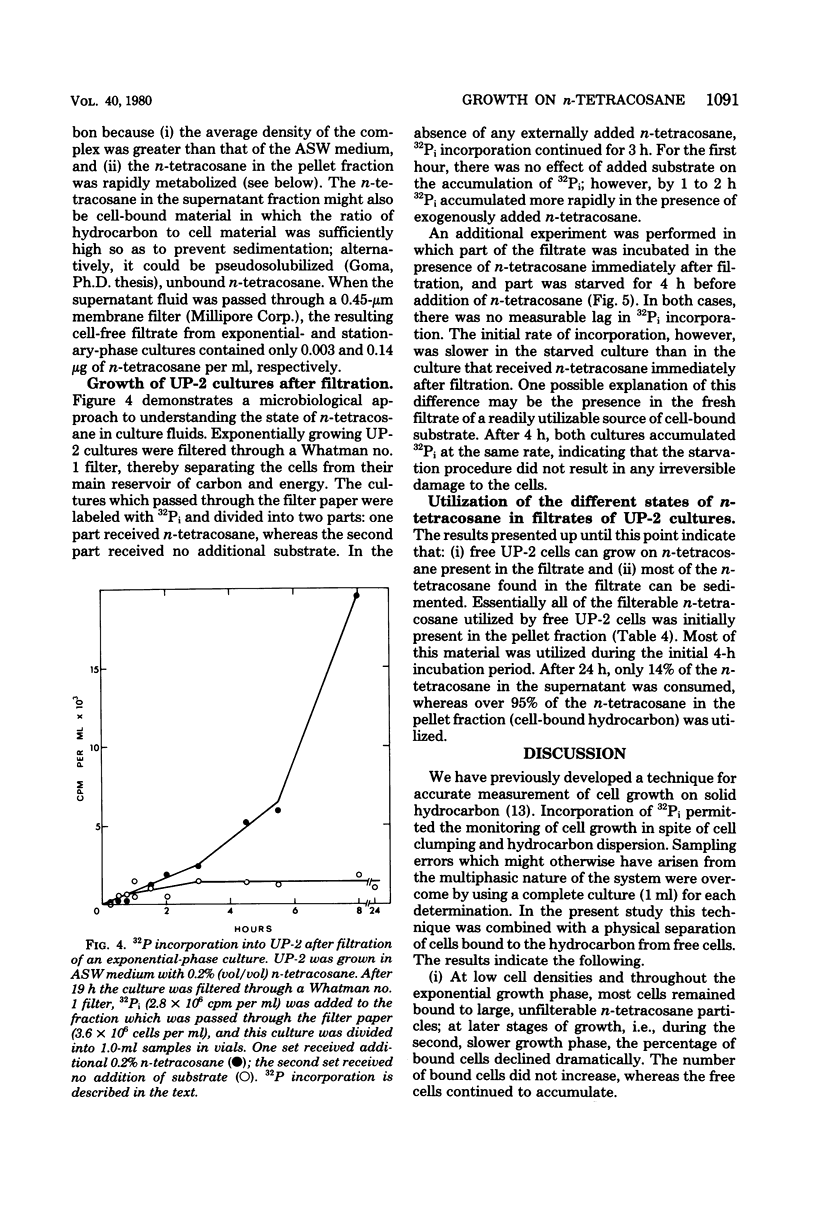
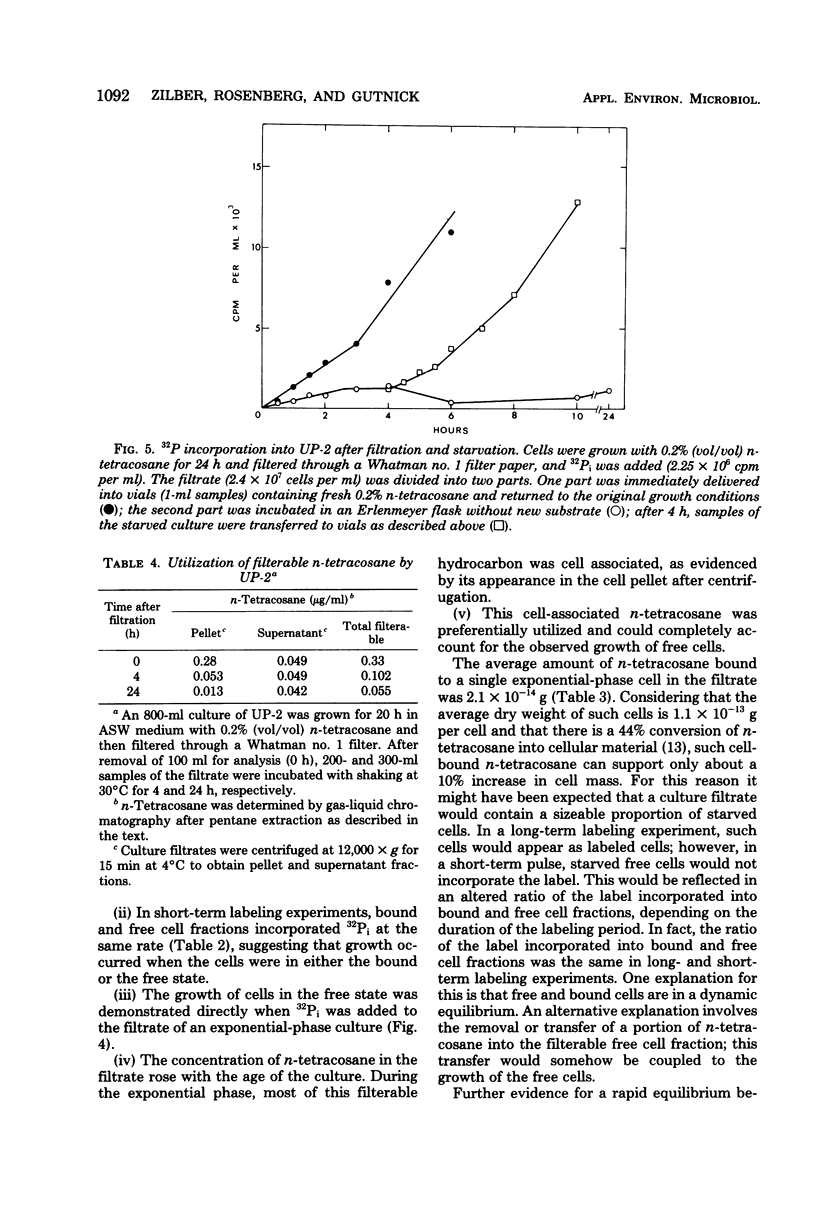
Cultures of the marine pseudomonad UP-2 growing on n-tetracosane contained both free cells and cells bound to the solid hydrocarbon. After separation by filtration through a Whatman no. 1 filter, the numbers of free and bound cells were estimated from the amount of 32P incorporated into each fraction and the determined value of 32P incorporation per viable cell in the filtrate (free cells). During the early exponential growth phase, over 80% of the cells were bound to large pieces of n-tetracosane; as the culture approached the stationary phase, the number of bound cells remained constant, whereas free cells continued to accumulate. Pulse-labeling experiments indicated that cells grew both on the surface of the solid and in the aqueous medium. During the growth cycle, a portion of the n-tetracosane which was initially nonfilterable was recovered in the filtrate in a form which was largely cell associated. This cell-associated n-tetracosane was preferentially utilized and could completely account for the observed growth of free cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott B. J., Gledhill W. E. The extracellular accumulation of metabolic products by hydrocarbon-degrading microorganisms. Adv Appl Microbiol. 1971;14:249–388. doi: 10.1016/s0065-2164(08)70546-x. [DOI] [PubMed] [Google Scholar]
- Amin P. M., Nigam J. N., Lonsane B. K., Baruah B., Singh H. D., Baruah J. N., Iyengar M. S. Microbial biomass production of solid hydrocarbons. Folia Microbiol (Praha) 1973;18(1):49–55. doi: 10.1007/BF02884249. [DOI] [PubMed] [Google Scholar]
- Beam H. W., Perry J. J. Microbial degradation and assimilation of n-alkyl-substituted cycloparaffins. J Bacteriol. 1974 May;118(2):394–399. doi: 10.1128/jb.118.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS W. C., FERNLEY H. N., GRIFFITHS E. OXIDATIVE METABOLISM OF PHENANTHRENE AND ANTHRACENE BY SOIL PSEUDOMONADS. THE RING-FISSION MECHANISM. Biochem J. 1965 Jun;95:819–831. doi: 10.1042/bj0950819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T., Mahadevan V., Davey J. F. Bacterial metabolism of para- and meta-xylene: oxidation of the aromatic ring. J Bacteriol. 1974 Sep;119(3):930–936. doi: 10.1128/jb.119.3.930-936.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grund A., Shapiro J., Fennewald M., Bacha P., Leahy J., Markbreiter K., Nieder M., Toepfer M. Regulation of alkane oxidation in Pseudomonas putida. J Bacteriol. 1975 Aug;123(2):546–556. doi: 10.1128/jb.123.2.546-556.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A., Gutnick D., Rosenberg E. Sequential growth of bacteria on crude oil. Appl Microbiol. 1975 Jul;30(1):10–19. doi: 10.1128/am.30.1.10-19.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeppeli O., Fiechter A. The mode of interaction between the substrate and cell surface of the hydrocarbon-utilizing yeast Candida tropicalis. Biotechnol Bioeng. 1976 Jul;18(7):967–974. doi: 10.1002/bit.260180709. [DOI] [PubMed] [Google Scholar]
- Kennedy R. S., Finnerty W. R. Microbial assimilation of hydrocarbons. I. The fine-structure of a hydrocarbon oxidizing Acinetobacter sp. Arch Microbiol. 1975;102(2):75–83. doi: 10.1007/BF00428349. [DOI] [PubMed] [Google Scholar]
- Käppeli O., Müller M., Fiechter A. Chemical and structural alterations at the cell surface of Candida tropicalis, induced by hydrocarbon substrate. J Bacteriol. 1978 Feb;133(2):952–958. doi: 10.1128/jb.133.2.952-958.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkins-Phillips G. J., Stewart J. E. Distribution of hydrocarbon-utilizing bacteria in Northwestern Atlantic waters and coastal sediments. Can J Microbiol. 1974 Jul;20(7):955–956. doi: 10.1139/m74-147. [DOI] [PubMed] [Google Scholar]
- Nakahara T., Erickson L. E., Gutierrez J. R. Characteristics of hydrocarbon uptake in cultures with two liquid phases. Biotechnol Bioeng. 1977 Jan;19(1):9–25. doi: 10.1002/bit.260190103. [DOI] [PubMed] [Google Scholar]
- Pirnik M. P., Atlas R. M., Bartha R. Hydrocarbon metabolism by Brevibacterium erythrogenes: normal and branched alkanes. J Bacteriol. 1974 Sep;119(3):868–878. doi: 10.1128/jb.119.3.868-878.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E., Katarski M., Gottlieb P. Deoxyribonucleic acid synthesis during exponential growth and microcyst formation in Myxococcus xanthus. J Bacteriol. 1967 Apr;93(4):1402–1408. doi: 10.1128/jb.93.4.1402-1408.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E., Zuckerberg A., Rubinovitz C., Gutnick D. L. Emulsifier of Arthrobacter RAG-1: isolation and emulsifying properties. Appl Environ Microbiol. 1979 Mar;37(3):402–408. doi: 10.1128/aem.37.3.402-408.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. C., Finnerty W. R. Characterization of intracytoplasmic hydrocarbon inclusions from the hydrocarbon-oxidizing Acinetobacter species HO1-N. J Bacteriol. 1976 Jul;127(1):481–489. doi: 10.1128/jb.127.1.481-489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]