Abstract
Background
Interleukin-1 receptor associated kinase-4 (IRAK-4) is an effector of the Toll-like receptor and interleukin-1 receptor pathways, which plays a critical role in innate immune responses. The role of IRAK-4 in adaptive immune functions in humans is incompletely understood.
Objective
To evaluate T cell function in Interleukin-1 receptor associated kinase-4 deficient patients.
Methods
We compared upregulation of CD25 and CD69 on T cells, and production of interleukin-2, interleukin-6, and interferon gamma following stimulation of peripheral blood mononuclear cells from four IRAK-4 deficient patients and normal controls with anti-CD3 and anti-CD28.
Results
Upregulation of CD25 and CD69 on T cells and production of interleukin-6 and interferon-gamma, but not interleukin-2, was significantly reduced in IRAK-4 deficient patients.
Conclusions
IRAK-4 deficient patients have defects in T cell activation.
Clinical Implications
Defects in T cell activation may contribute to the susceptibility of IRAK-4 deficient patients to infections.
Keywords: IRAK-4, T cell, T cell receptor, cytokines, TNFα, IL-2, IL-6, IFNγ, CD25, CD69
Introduction
Toll-like receptors (TLRs) are crucial components of the innate immune system that detect pathogen associated molecular patterns, such as lipopolysaccharide, single and double stranded RNA, and hypomethylated, CpG-rich DNA, and initiate inflammatory responses to invading microbes1. Activation of TLRs leads to production of pro-inflammatory cytokines, such as IL-1, IL-6, IL-12, TNFα, and in the case of TLR3, 7, 8, and 9 type 1 interferons. Additionally, TLR activation causes upregulation of co-stimulatory molecules, such as CD40, CD80, and CD86, and enhanced antigen presentation by antigen presenting cells2, 3. The production of IL-12 and type 1 interferons, as well as upregulation of co-stimulatory molecules, influence subsequent adaptive immune responses by inducing Th1 differentiation of naive T cells4. IL-12 also induces production of IFNγ by T cells, which further enhances the anti-microbial functions of monocytes and macrophages.
IRAK-4 is an essential effector of the IL-1 receptor and all Toll-like receptors, except for TLR32. Mice that are deficient in IRAK-4 or express kinase inactive IRAK-4 have impaired TLR-induced inflammatory responses and impaired host defense against bacterial infection5. Investigation of IRAK-4 deficient patients has confirmed that IRAK-4 plays a non-redundant role in immunity against pyogenic bacterial infections6. The susceptibility of IRAK-4 deficient patients to invasive bacterial infections diminishes with age, becoming comparable to that of the normal population by roughly age 14 years. The reason for this is not understood. Maturation of the adaptive immune system has been hypothesized to compensate for impaired innate immune function caused by IRAK-4 deficiency.
IRAK-4 deficient murine T cells were shown to be deficient in TCR-induced activation of NFκB, as well as IL-2 production and proliferation7. However, these observations were not replicated in a subsequent report8. Recently, IRAK-4−/− mice were found to have reduced splenic and peripheral expansion of CD8+ T cells in response to lymphocytic choriomeningitis virus (LCMV) infection, suggesting that IRAK-4 may be required for optimal anti-viral CD8+ T cell responses in vivo9. Furthermore, T cells from IRAK-4 kinase inactive mice and T cell blasts from IRAK-4 deficient and MyD88 deficient patients were shown to secrete reduced quantities of IL-17, which plays an important role in immunity against bacterial infection9, 10. Thus, although IRAK-4 plays a crucial role in innate immunity, its role in the development of human adaptive immune responses is incompletely understood.
We describe a new patient with IRAK-4 deficiency who suffered from recurrent, invasive infections with S. pneumoniae and P. aeruginosa. Analysis of T cell function revealed impaired upregulation of CD25 and CD69 and reduced production of IL-6 and IFNγ following T cell activation. Analysis of T cell function in three additional IRAK-4 deficient patients confirmed these findings. These observations provide support for a role of IRAK-4 in human T cell function.
MATERIALS AND METHODS
Subjects
Four unrelated patients with IRAK-4 deficiency with distinct molecular defects were studied6, 11, 12. All had clinical features of IRAK-4 deficiency and the diagnosis was confirmed by molecular analysis. Parents of all subjects enrolled on these studies signed informed consents that were approved by the University of Iowa Children's Hospital Institutional Review Board and Children’s Hospital, Boston and in accordance with the Declaration of Helsinki. Case reports are included in the online repository.
Reagents
TLR ligands used include PAM3CSK4 (TLR1/2), Poly I:C (TLR3), ultrapure LPS from S. minnesota (TLR4), Flagellin (TLR5), CpG DNA (ODN2216) (TLR9) all of which were obtained from Invivogen, San Diego, CA. The ligands for TLR7 (3M-2) and TLR8 (3M-13) were kind gifts of Dr. Richard Miller, 3M Pharmaceuticals.
Antibodies used include anti-CD3 (HIT3a) was from Biolegend (San Diego, CA) and anti-CD28 and conjugated mouse anti-human monoclonal antibodies, including CD4 FITC, CD69 PE, CD8 PerCP-Cy5.5, and CD25 PE were from BD Bioscience (San Jose, CA). Human IL1β, TNFα, and ELISA kits for human TNFα were obtained from Invitrogen (Carlsbad, CA). PMA and Ionomycin were obtained from Calbiochem (La Jolla, CA).
TLR stimulation of TNFα production
Peripheral blood mononuclear cells (PBMCs) were isolated and stimulated with TLR ligands or PMA plus ionomycin as previously described12. Cell culture supernatants were collected after 24 hours and TNFα was measured by ELISA.
For Western blotting, patient and control primary fibroblasts were stimulated with IL-1β (10 ng/ml) or TNFα (20 ng/ml) for the indicated times in RPMI plus L-glutamine and penicillin/streptomycin with 10% fetal calf serum (FCS). Cells were lysed in Sample Buffer (62.5 mM Tris, pH 6.8, 2% w/v SDS, 10% glycerol, 2% β-mercaptoethanol, 0.01% bromophenol blue). Proteins were resolved by 10% SDS-PAGE (BioRad, Hercules, CA) and transferred to PVDF membranes (Millipore, Billerica, MA). Western blotting with anti-phospho p38MAPK and anti-IRAK-4 (Cell Signaling, Danvers, MA) and anti-IκBα and anti-IKKγ (sc-8330) (Santa Cruz Biotechnology, Santa Cruz, CA) was performed according to the manufacturer’s recommendations.
Mutational analysis of IRAK-4
RNA from PBMCs was prepared with Trizol reagent (Invitrogen, Carlsbad, Calif), and cDNA was generated with Superscript II reverse transcriptase (Invitrogen). IRAK-4 specific primers were used to amplify the full-length message with the following primer sets: forward, 5′-TTCTTCTGTCGCCGGCTTCAG-3′; reverse, 5′-TGTCAACCATTGCTGCAAGC-3′; and forward, 5′-ATGGGAGAGGGAGGATTTGG-3′; reverse, 5′-ACGCTATGCCTTGTTAAAGG-3′. Genomic DNA from patient fibroblasts was prepared by phenol:chloroform extraction to confirm the mutations observed in exon 7. The patient's mutation was identified in genomic DNA in exon 7 of IRAK-4 using the following primers: forward, 5′-GCTATAACATCATCTTCAGTTGTTG-3′; reverse, 5′-GGATGAGTACTGGAAGTAGGTC-3′ as previously described12. The individual exon 7 alleles were isolated by TA cloning (pCR2.1-TOPO vector, Invitrogen, Carlsbad, CA). TA clones were sequenced using T3 primers. All sequencing was performed by the Molecular Genetics Core facility at Children's Hospital, Boston.
Upregulation of activation markers on T cells
PBMCs were plated at a density of 1–2 × 106/ml and incubated in tissue culture flasks at 37°C in 5% CO2 for 2 hours to remove adherent cells. Non-adherent lymphocytes were suspended in RPMI 1640 supplemented with 10% FCS, penicillin (1,000 U/ml), streptomycin (1,000 U/ml), and glutamine (20 mM), then plated onto 24 well Costar plates. Cells were incubated for 18 hours with one of the following conditions: phosphate buffered saline (PBS), plate-bound biotinylated anti-CD3 (0.5mg/ml, diluted 1:100 in 1× PBS) with or without soluble anti-CD28 (0.5 mg/ml), or Phorbol 12-Myristate 13 Acetate (PMA, 75 ng/ml) plus ionomycin (1 mM). Lymphocytes were removed from the media, washed, and resuspended in staining buffer (0.1% bovine serum albumin, PBS, 0.01% sodium azide). Cells were incubated for 30 minutes on ice with conjugated mouse anti-human monoclonal antibodies that included CD4 FITC, CD69 PE, CD8 PerCP-Cy5.5, and CD25 PE (BD Bioscience) and washed twice. Fluorescence was determined by a FACScan flow cytometer (Becton-Dickinson, San Jose, CA), and data analysis was performed using Cell Quest software (Becton-Dickinson), as previously described13.
Cytokine production in T cells
PBMCs (2.5 × 106 cells/ml) were plated in tissue culture flasks as above and non-adherent lymphocytes were incubated for 18 hours in media as described above with PBS, PMA plus ionomycin, or immobilized anti-CD3 plus soluble anti-CD28 as described above. Cell culture supernatants were harvested, diluted 1:3 with RPMI, and analyzed in triplicate by Bioplex assays (Bio-Rad Laboratories, Hercules, CA, USA), according to the manufacturer’s protocol using a Luminex 200 multiplexing system.
RESULTS
Impaired Toll-like receptor and IL-1 receptor signaling in a compound heterozygous IRAK-4 deficient patient
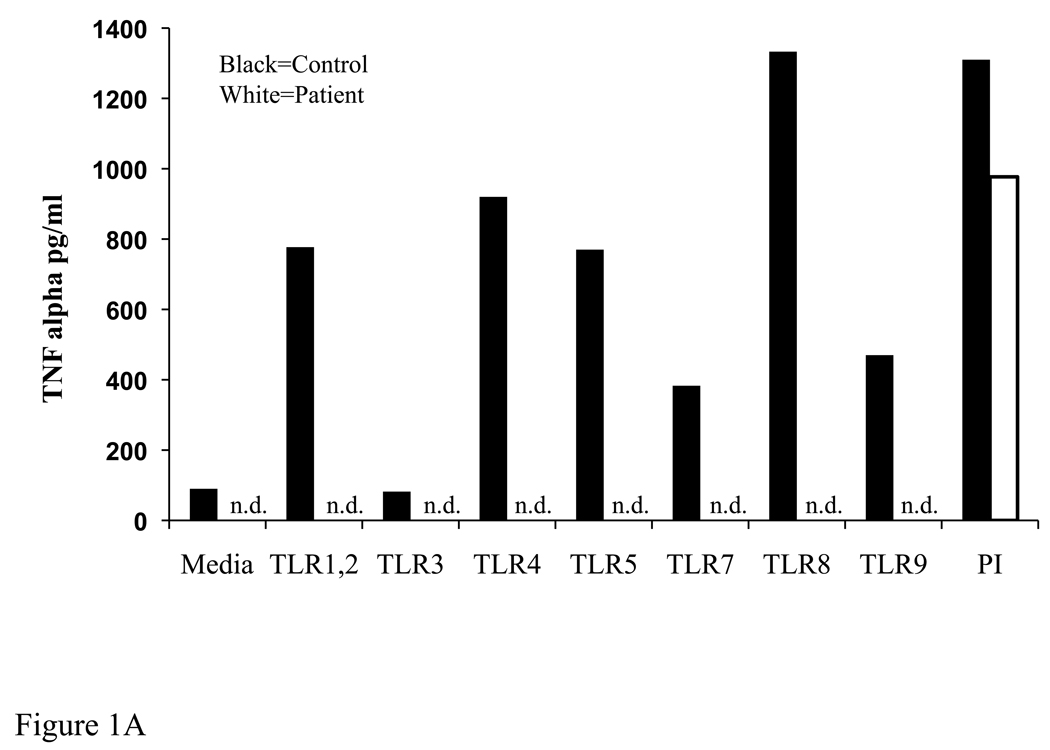
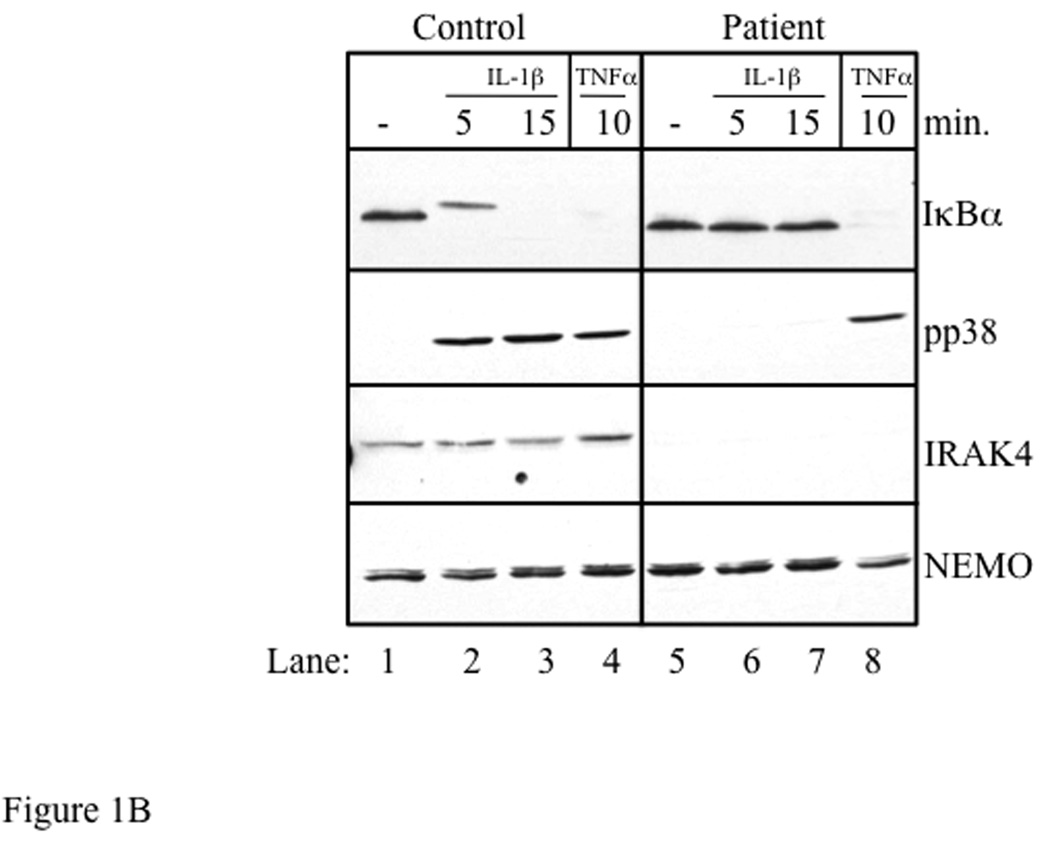
The clinical history of the index patient is detailed in the Online Repository. Severe and recurrent pyogenic infections and a diminished febrile response to invasive bacterial infections suggested a defect in innate immunity. Stimulation of the patient’s blood cells with TLR ligands failed to elicit production of TNFα (Fig 1A). TLRs and IL-1 receptor use the adaptor MyD88 and IRAK-4 to activate IκB kinase (IKK), which phosphorylates the NFκBα inhibitor IκBα. Stimulation of the patient’s fibroblasts with IL-1β for 5 and 15 minutes failed to induce phosphorylation and degradation of IκBα, as demonstrated by the absence of a mobility shift and disappearance of IκBα on Western blot respectively. IL-1β also failed to cause phosphorylation of p38 MAPK (Fig. 1B, compare lanes 2 and 3 to lanes 6 and 7). In contrast, stimulation of the patient’s fibroblasts with TNFα induced complete degradation of IκBα and normal p38 phosphorylation (Fig 1B, lane 8), ruling out a defect in the IKK subunits IKKα, IKKβ, IKKγ or in IκBα.
Fig 1. Defective TLR induced cytokine production and absent IL-1 induced signaling associated with IRAK-4 deficiency.
A) TNFα production in PBMCs in response to TLR ligands or PMA + ionomycin. n.d.=not detected B) Control and patient fibroblasts were stimulated for the times shown with IL-1β (10 ng/ml) or TNFα (20 ng/ml). Lysates were analyzed by Western blot with anti-IκBα, anti-phospho p38 MAPK, anti-IRAK-4, and anti-NEMO (loading control). Data are representative of 2 independent experiments.
The absence of TLR-induced cytokine production coupled with absent IL-1β-induced IκBα phosphorylation and degradation and p38 MAPK activation suggested a proximal defect in IL-1/TLR pathways. Western blot of the patient’s fibroblast lysates revealed absence of IRAK-4 protein, consistent with IRAK-4 deficiency (Fig 1B, lanes 5 through 8). IRAK-4 was sequenced from cDNA derived from the patient’s PBMCs, revealing compound heterozygous mutations within exon 7. The mutant alleles were confirmed by amplifying exon 7 from genomic DNA generated from the patient’s fibroblasts, followed by T-A cloning to isolate clones derived from both alleles. One mutation consisted of a novel 17 bp deletion (870-887del) that results in a premature stop codon, and was found to be inherited from the father. The other mutation consisted of the previously described C to T transition at codon 877 (C877T), which also results in a premature stop codon (Q293X), and was found to be inherited from the mother (Table 1). The patient’s healthy sister is heterozygous for the C877T mutation.
Table 1.
| Patient | Patient Mutation | Protein | Ref. |
|---|---|---|---|
| 1. | C877T/870-887del | N.D. | this report |
| 2. | 631del_G/C144G | N.D. | 6 |
| 3. | C877T/G893A | N.D. | 11 |
| 4. | C877T/C877T | N.D. | 12 |
N.D. – none detected
Impaired uprgeulation of activation markers on activated T cells from IRAK-4 deficient patients
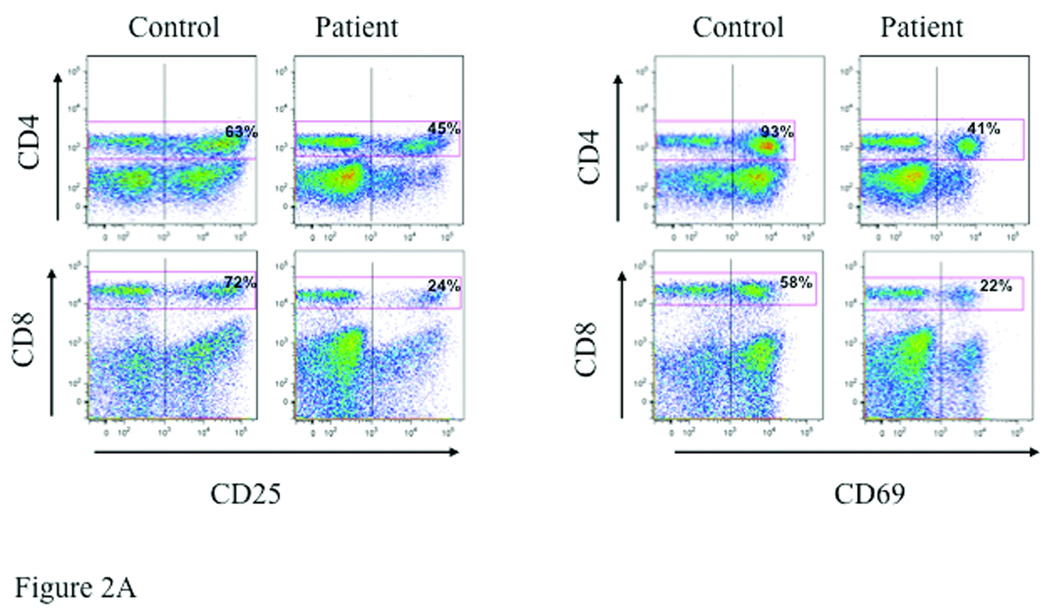
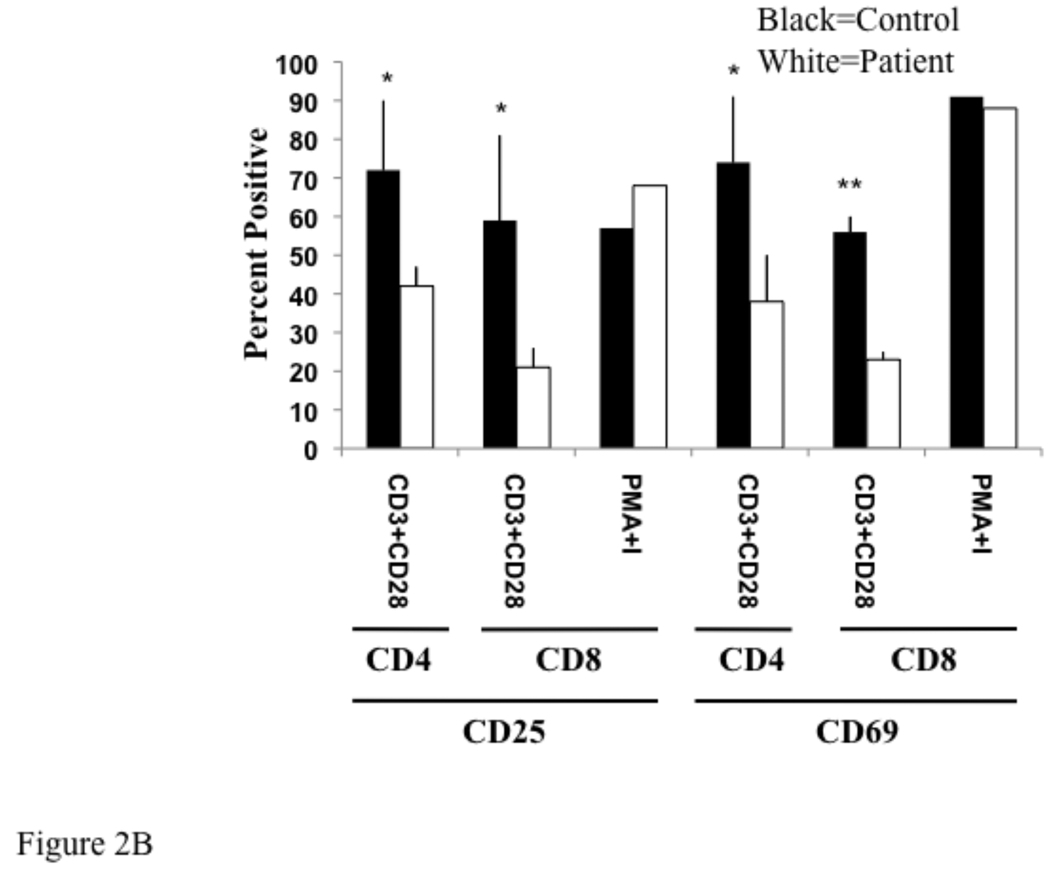
T cells were activated by cross-linking with anti-CD3 and anti-CD28 antibodies, and evaluated 18 hours later for upregulation of the activation markers CD25 and CD69 by flow cytometry. Upregulation of CD25 and CD69 on both CD4+ and CD8+ T cells was impaired in the patient (Fig. 2A). In three experiments, the mean percentage of CD4+/CD25+ cells in the patient was 42±5% compared to 72±18% in the control (p=0.049), and the mean percentage of CD8+/CD25+ cells in the patient was 21±5% compared to 59±22% in the control (p=0.043). In the same experiments the mean percentage of CD4+/CD69+ in the patient was 38±12% compared to 74±17% in the control (p=0.04), and the mean percentage of CD8+/CD69+ in the patient was 23±2% compared to 56±4% in the control (p=0.0002). Upregulation of CD25 and CD69 expression on CD8+ T cells following stimulation with PMA and ionomycin, which bypass TCR signaling by activating protein kinase C increasing intracellular calcium concentration, respectively, was comparable in the patient and control (Fig. 2B). The effect of PMA plus ionomycin on CD25 and CD69 expression on CD4+ T cells was not examined because PMA strongly downregulates CD4 expression on T cells14.
Fig 2. Impaired expression of activation markers by T cells in an IRAK 4 deficient patient.
CD25 and CD69 expression on CD4+ and CD8+ cells in non-adherent lymphocytes from the index patient and a control following 18 hours stimulation with plate bound anti-CD3 plus soluble anti-CD28. Representative data from three experiments (A) and the mean ± SD of three experiments and plotted as percent positive (B). *p<0.05, **p<0.001 by student t test.
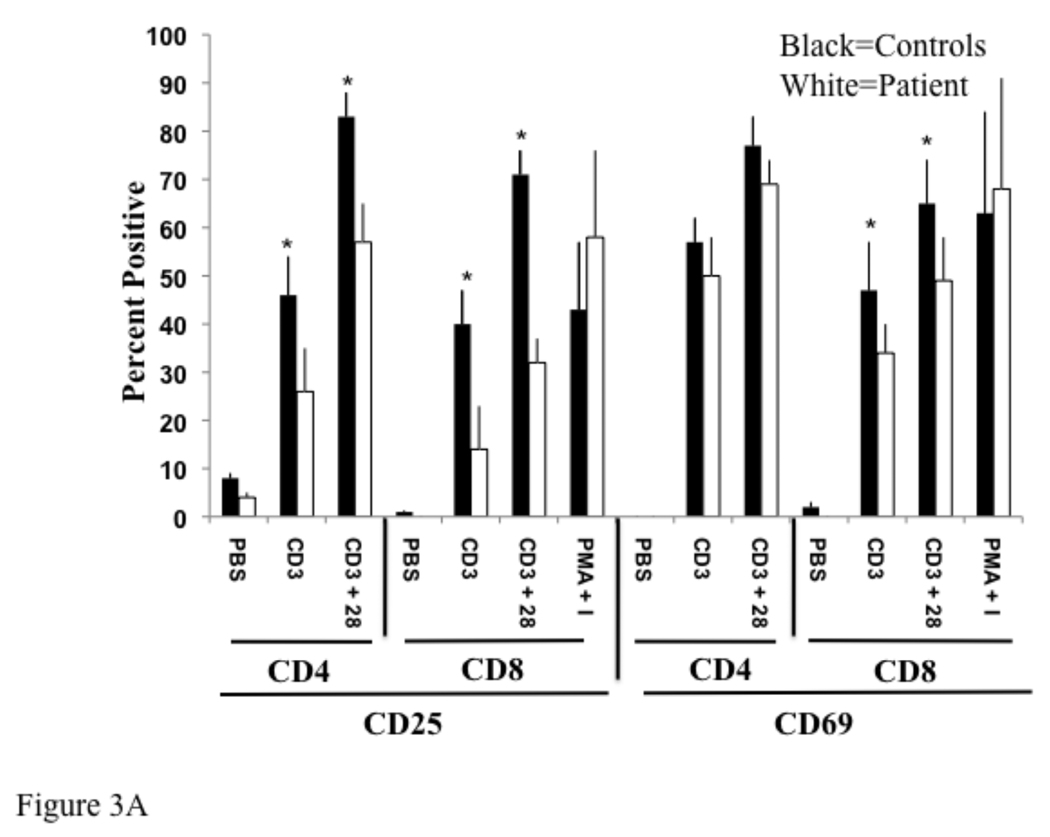
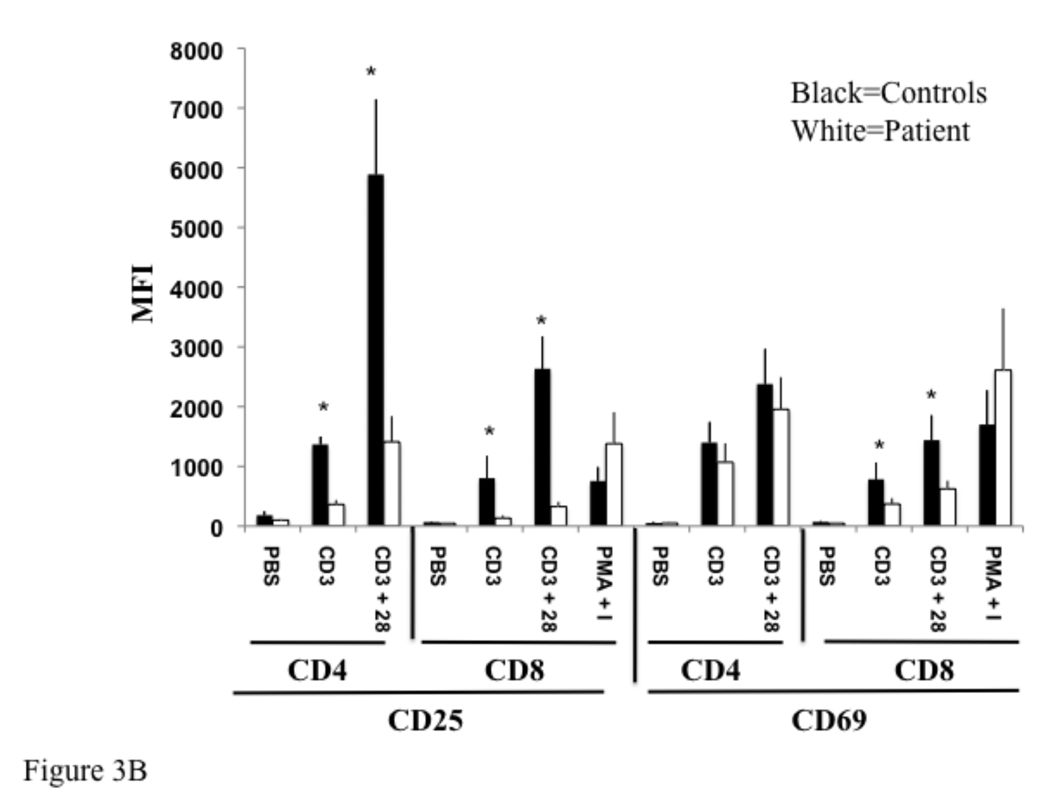
To confirm these findings, we examined the expression of T cell activation markers in three other previously characterized, unrelated IRAK-4 deficient patients (Table 1). The combined data from these three patients and our patient was pooled. Upregulation of CD25 expression on CD4+ and CD8+ T cells following cross-linking with anti-CD3 alone or anti-CD3 plus anti-CD28 was significantly impaired in IRAK-4 deficient patients relative to four healthy controls. This was evident by a decrease in the percentage of CD25+ T cells (Fig. 3A), and by decreased mean fluorescence intensity of CD25 expression by these T cells (Fig. 3B). Additionally, upregulation of CD69 following cross-linking with anti-CD3 alone or anti-CD3 plus anti-CD28 was significantly impaired in CD8+ T cells in IRAK-4 deficient patients. Upregulation of CD69 in CD4+ T cells in IRAK-4 deficient patients was reduced, but not significantly (Fig. 3A and B). Upregulation of CD25 and CD69 expression on CD8+ T cells following stimulation with PMA and ionomycin, which bypass TCR signaling, was comparable in patients and controls.
Fig 3. Reduced expression of activation markers by T cells from IRAK-4 deficient patients.
Control and patient non-adherent lymphocytes were cultured with PBS (Ø), immobilized anti-CD3 (CD3), immobilized anti-CD3 plus soluble anti-CD28 (CD3/28), or PMA and ionomycin and stained as in Fig 2. Data from four patients and controls was averaged and plotted as percent positive (A), and mean fluorescence intensity (MFI) (B). *p < 0.05 by student t test.
Impaired secretion of IL-6 and IFNγ by activated T cells from IRAK-4 deficient patients
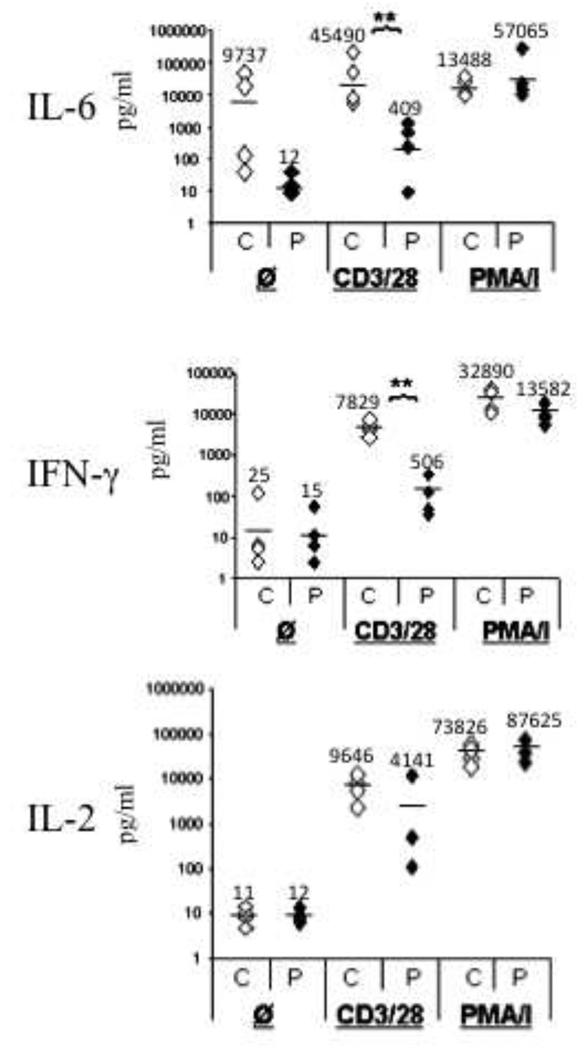
Production of IL-6 and IFNγ by T cells following cross-linking of CD3 plus CD28 was significantly impaired in IRAK-4 deficient patients (Fig. 4). For IL-6 the mean was 409 pg/ml for the patients compared to 45490 pg/ml for the controls (p=0.006). For IFNγ the mean was 506 pg/ml for the patients compared to 7829 pg/ml for the controls (p=0.02). Decreased production of IL-6 and IFNγ in the patients was not due to decreased percentages of T cells as all four patients had normal populations of circulating CD4+ and CD8+ cells (supplemental Table 1). Furthermore, IL-6 and IFNγ production following stimulation with PMA and ionomycin, which bypass TCR signaling, was comparable in patients and controls. IL-2 production by T cells following cross-linking of CD3 plus CD28 was not significantly different in patients and controls (4141 pg/ml in patients versus 9646 pg/ml in controls, p=0.14).
Fig 4. Impaired production of IL-6, and IFNγ by activated T cells of IRAK-4 deficient patients.
IL-2, IL-6, and IFNγ production in four controls (C) and patients (P) non-adherent lymphocytes stimulated with PBS ( Ø ), immobilized anti-CD3 plus soluble anti-CD28 (CD3/28), or PMA plus ionomycin (PMA/I). Measurements were performed in triplicate. Average values are indicated by a horizontal line and listed above each data set. **p<0.05 by student t test.
DISCUSSION
The results of this study support a role for IRAK-4 in T cell activation. Stimulation of T cells through the TCR leads to upregulation of CD69 and CD25, which are early markers of T cell activation15, 16. Upregulation of the activation markers CD25 and CD69 following TCR ligation and ligation of both the TCR and CD28 was impaired in all four IRAK-4 deficient patients analyzed. CD69 is the earliest marker of lymphocyte activation and may be involved in lymphocyte proliferation. CD25 is a component of the high affinity IL-2 receptor required for T cell responsiveness to IL-2. Reduced IL-2 responsiveness resulting from reduced TCR-induced upregulation of CD25 may underlie the observed reduction in splenic and peripheral expansion of CD8+ T cells in LCMV-infected IRAK-4−/− mice9.
Production of IL-6 and IFNγ following T cell activation by anti-CD3 and anti-CD28 was significantly lower in IRAK-4 deficient patients compared to normal controls, consistent with reduced LCMV-induced pro-inflammatory cytokine production observed in T cells from IRAK-4−/− mice9. IL-6 is a pro-inflammatory cytokine that has been shown to play a critical role in resistance against S. pneumoniae17. TLR-induced IL-6 production in IRAK-4 deficient monocytes, macrophages, and dendritic cells is virtually absent18. Our observation that TCR/CD28 induced IL-6 production is impaired in T cells from IRAK-4 deficient patients is novel and may contribute the susceptibility of these patients to S. pneumoniae. Specific antibody responses to polysaccharide antigens, such as those contained in the 23-valent pneumococcal vaccine (Pneumovax), are variably impaired in IRAK-4 deficient patients and this was observed in two out of four of the IRAK-4 deficient patients analyzed in this study. Although IL-6 augments production of immunoglobulins by B cells19, it is unknown whether decreased IL-6 production by IRAK-4 deficient patients contributes to impaired specific antibody responses to pneumococcus. A defect in specific antibody production in response to Pneumovax that has been observed in TLR2 and/or TLR4 deficient mice has been hypothesized to result from a deficient of response to small amounts of TLR ligands found in the 23-valent pneumococcal vaccine20. A similar mechanism could operate in IRAK-4 deficient patients.
Additionally, IL-6 is critical for the development of IL-17 and IL-22 producing T cells9, 21–23. IL-17 contributes to host defense against bacterial infections. In mouse models IL-17 plays a critical role in the clearance of nasophayngeal colonization with S. pneumoniae24. IL-17 promotes the recruitment of monocytes and macrophages to the nasopharyngeal mucosa that are responsible for phagocytosis and clearance of pneumococcus. Additionally, IL-17 contributes to host defense against invasive infections with Salmonella and P. aeruginosa, which have been observed in many IRAK-4 deficient patients25. Antigen-stimulated T cells from mice with an inactivating mutation in IRAK-4 kinase secrete reduced amounts of IL-1726. T cell blasts from patients with IRAK-4 and MyD88 deficiency have been shown to secrete reduced quantities of IL-17 when cultured in the presence of IL-1β, presumably due to a lack of responsiveness to IL-110. Reduced TCR/CD28-driven IL-6 production, in addition to reduced TLR- and IL-1-driven IL-6 production, may contribute to impaired development of Th17 T cells in IRAK-4 deficient patients. Reduced IL-17 production may contribute to the infectious susceptibility of these patients.
IFNγ produced by T cells plays a critical role in enhancing antimicrobial activity of macrophages and monocytes, recruiting inflammatory cells to sites of infection, and also in increasing antigen presentation by antigen presenting cells27. Thus, the crosstalk between T cells and monocytes and macrophages that augments antimicrobial activity may be diminished in IRAK-4 deficient patients as a result of reduced IFNγ production by T cells.
At present, it is unclear how IRAK-4 promotes T cell activation. It is not known whether IRAK-4 kinase activity plays an essential role or whether IRAK-4 functions as a scaffolding protein in the TCR signaling pathway of humans. The observation that antigen induced activation of CD8+ T cells in mice with an inactivating mutation in IRAK-4 kinase is impaired suggests that IRAK-4 kinase activity may be required9. Longitudinal studies in IRAK-4 deficiency are needed to determine whether the defective T cell functions that we have characterized in these patients will improve as they reach adolescence and become less susceptible to infections.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health grant 5KO8AI76625 (DRM) and National Institutes of Health grant PO1AI035714 (RSG).
Abbreviations
- IRAK-4
Interleukin-1 receptor-associated kinase-4
- IL-1
interleukin-1
- IL-2
interleukin-2
- IL-6
interleukin-6
- IFNγ
interferon gamma
- LCMV
lymphocytic choriomeningitis virus
- TNFα
tumor necrosis factor alpha
- TCR
T cell receptor
- PBMC
peripheral blood mononuclear cell
- TLR
Toll-like receptor
- ELISA
enzyme-linked immunosorbent assay
- PMA
phorbol 12-myristate 13-acetate
- PI
PMA plus Ionomycin
- NEMO
NFκB Essential Modifier
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of conflicts of interests: The authors have declared that they have no conflicts of interest.
References
- 1.Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill LA. Signal transduction pathways activated by the IL-1 receptor/toll-like receptor superfamily. Curr Top Microbiol Immunol. 2002;270:47–61. [PubMed] [Google Scholar]
- 3.Takeuchi O, Akira S. Toll-like receptors; their physiological role and signal transduction system. Int Immunopharmacol. 2001;1:625–635. doi: 10.1016/s1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 4.Sporri R, Reis e, Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 5.Kim TW, Staschke K, Bulek K, Yao J, Peters K, Oh KH, et al. A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J Exp Med. 2007;204:1025–1036. doi: 10.1084/jem.20061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ku CL, von Bernuth H, Picard C, Zhang SY, Chang HH, Yang K, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007;204:2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki N, Suzuki S, Millar DG, Unno M, Hara H, Calzascia T, et al. A critical role for the innate immune signaling molecule IRAK-4 in T cell activation. Science. 2006;311:1927–1932. doi: 10.1126/science.1124256. [DOI] [PubMed] [Google Scholar]
- 8.Kawagoe T, Sato S, Jung A, Yamamoto M, Matsui K, Kato H, et al. Essential role of IRAK-4 protein and its kinase activity in Toll-like receptor-mediated immune responses but not in TCR signaling. J Exp Med. 2007;204:1013–1024. doi: 10.1084/jem.20061523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lye E, Dhanji S, Calzascia T. Elford AR, Ohashi PS. IRAK-4 kinase activity is required for IRAK-4-dependent innate and adaptive immune responses. Eur J Immunol. 2008;38:870–876. doi: 10.1002/eji.200737429. [DOI] [PubMed] [Google Scholar]
- 10.de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouma G, Doffinger R, Patel SY, Peskett E, Sinclair JC, Barcenas-Morales G, et al. Impaired neutrophil migration and phagocytosis in IRAK-4 deficiency. Br J Haematol. 2009;147:153–156. doi: 10.1111/j.1365-2141.2009.07838.x. [DOI] [PubMed] [Google Scholar]
- 12.McDonald DR, Brown D, Bonilla FA, Geha RS. Interleukin receptor-associated kinase-4 deficiency impairs Toll-like receptor-dependent innate antiviral immune responses. J Allergy Clin Immunol. 2006;118:1357–1362. doi: 10.1016/j.jaci.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Goldman FD, Vibhakar R, Puck JM, Straus SE, Ballas ZK, Hollenback C, et al. Aberrant T-cell antigen receptor-mediated responses in autoimmune lymphoproliferative syndrome. Clin Immunol. 2002;104:31–39. doi: 10.1006/clim.2002.5249. [DOI] [PubMed] [Google Scholar]
- 14.Bigby M, Wang P, Fierro JF, Sy MS. Phorbol myristate acetate-induced down-modulation of CD4 is dependent on calmodulin and intracellular calcium. J Immunol. 1990;144:3111–3116. [PubMed] [Google Scholar]
- 15.Lopez-Cabrera M, Santis AG, Fernandez-Ruiz E, Blacher R, Esch F, Sanchez-Mateos P, et al. Molecular cloning, expression, and chromosomal localization of the human earliest lymphocyte activation antigen AIM/CD69, a new member of the C-type animal lectin superfamily of signal-transmitting receptors. J Exp Med. 1993;178:537–547. doi: 10.1084/jem.178.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Lier RA, Brouwer M, Rebel VI, van Noesel CJ, Aarden LA. Immobilized anti-CD3 monoclonal antibodies induce accessory cell-independent lymphokine production, proliferation and helper activity in human T lymphocytes. Immunology. 1989;68:45–50. [PMC free article] [PubMed] [Google Scholar]
- 17.van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis. 1997;176:439–444. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- 18.Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 19.Burdin N, Van Kooten C, Galibert L, Abrams JS, Wijdenes J, Banchereau J, et al. Endogenous IL-6 and IL-10 contribute to the differentiation of CD40-activated human B lymphocytes. J Immunol. 1995;154:2533–2544. [PubMed] [Google Scholar]
- 20.Sen G, Khan AQ, Chen Q, Snapper CM. In vivo humoral immune responses to isolated pneumococcal polysaccharides are dependent on the presence of associated TLR ligands. J Immunol. 2005;175:3084–3091. doi: 10.4049/jimmunol.175.5.3084. [DOI] [PubMed] [Google Scholar]
- 21.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 22.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009;119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–407. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staschke KA, Dong S, Saha J, Zhao J, Brooks NA, Hepburn DL, et al. IRAK4 kinase activity is required for Th17 differentiation and Th17-mediated disease. J Immunol. 2009;183:568–577. doi: 10.4049/jimmunol.0802361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.