Abstract
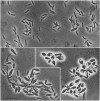
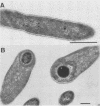
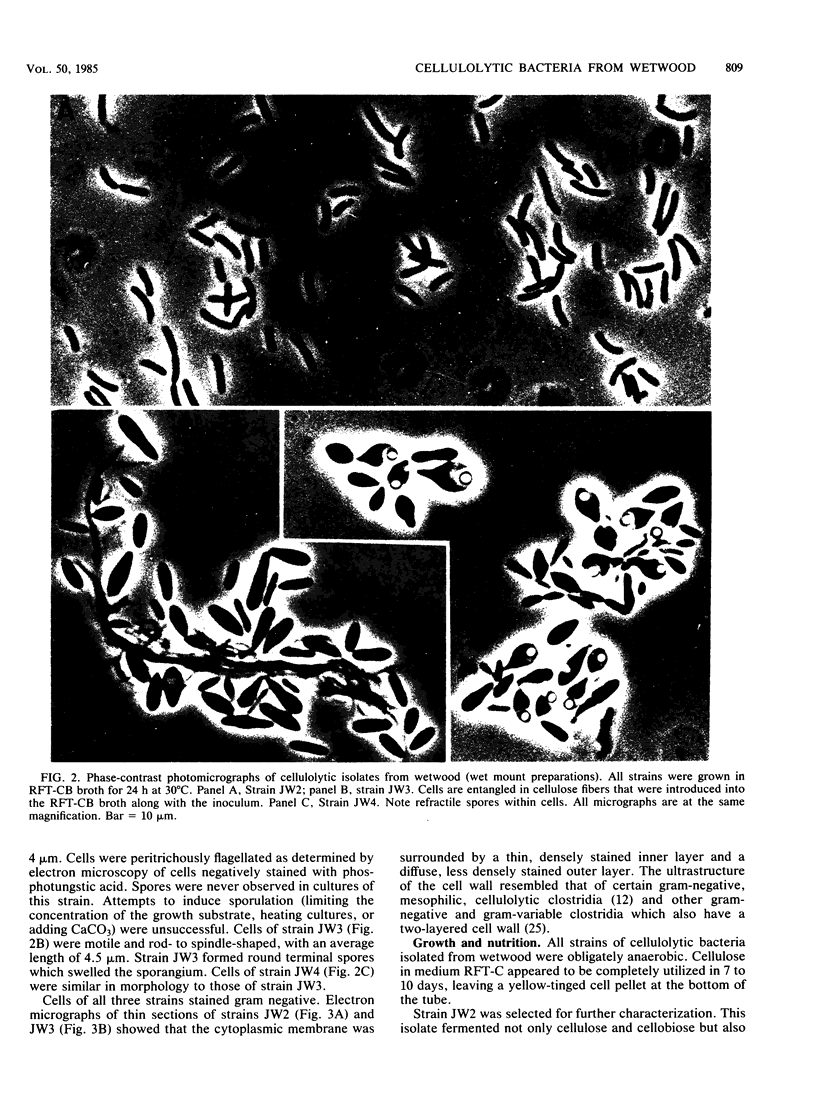
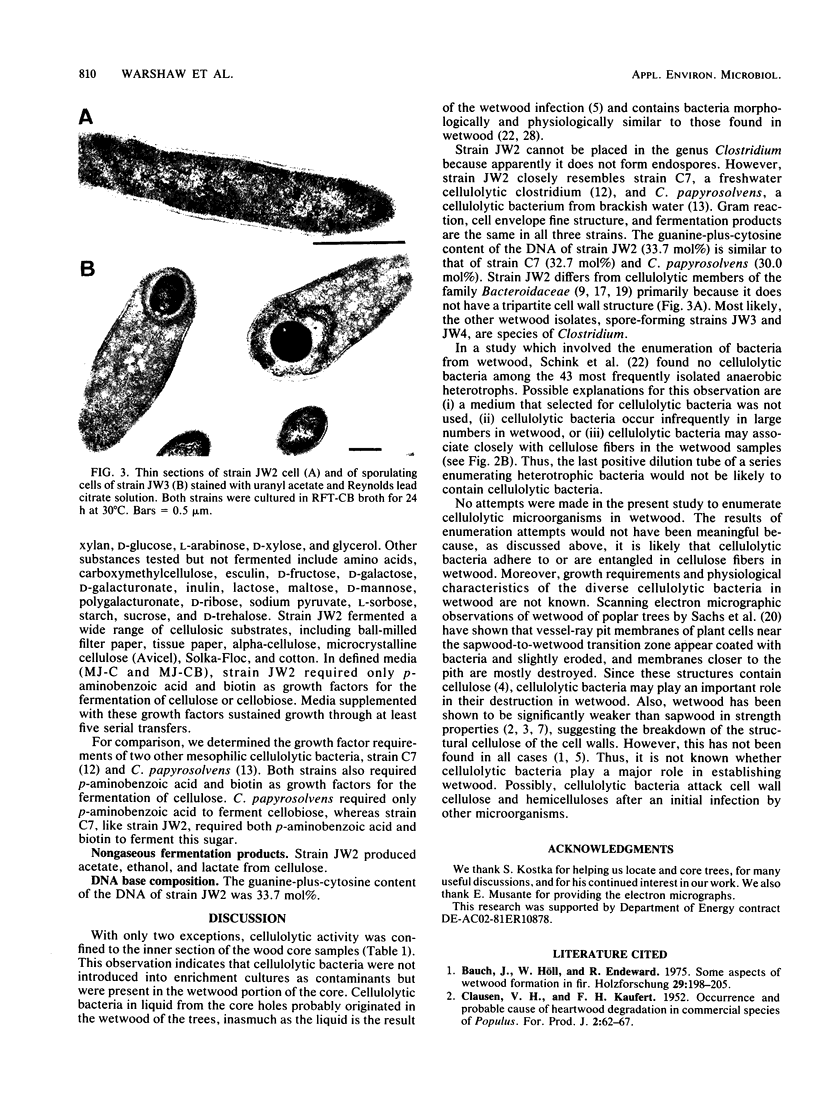
Obligately anaerobic, mesophilic, cellulolytic bacteria were isolated from the wetwood of elm and maple trees. The isolation of these bacteria involved inoculation of selective enrichment cultures with increment cores taken from trees showing evidence of wetwood. Cellulolytic bacteria were present in the cores from seven of nine trees sampled, as indicated by the disappearance of cellulose from enrichment cultures. With two exceptions, cellulolytic activity was confined to the darker, wetter, inner section of the cores. Cellulolytic bacteria were also present in the fluid from core holes. The cellulolytic isolates were motile rods that stained gram negative. Endospores were formed by some strains. The physiology of one of the cellulolytic isolates (strain JW2) was studied in detail. Strain JW2 fermented cellobiose, d-glucose, glycerol, l-arabinose, d-xylose, and xylan in addition to cellulose. In a defined medium, p-aminobenzoic acid and biotin were the only exogenous growth factors required by strain JW2 for the fermentation of cellobiose or cellulose. Acetate and ethanol were the major nongaseous end products of cellulose fermentation. The guanine-plus-cytosine content of the DNA of strain JW2 was 33.7 mol%. Cellulolytic bacteria have not previously been reported to occur in wetwood. The isolation of such bacteria indicates that cellulolytic bacteria are inhabitants of wetwood environments and suggests that they may be involved in wetwood development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Harwood C. S., Canale-Parola E. Branched-chain amino acid fermentation by a marine spirochete: strategy for starvation survival. J Bacteriol. 1981 Oct;148(1):109–116. doi: 10.1128/jb.148.1.109-116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Madia A., Demain A. L. Chemically Defined Minimal Medium for Growth of the Anaerobic Cellulolytic Thermophile Clostridium thermocellum. Appl Environ Microbiol. 1981 Apr;41(4):1060–1062. doi: 10.1128/aem.41.4.1060-1062.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschine S. B., Canale-Parola E. Mesophilic cellulolytic clostridia from freshwater environments. Appl Environ Microbiol. 1983 Sep;46(3):728–737. doi: 10.1128/aem.46.3.728-737.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster B. J., Canale-Parola E. Physiological diversity of rumen spirochetes. Appl Environ Microbiol. 1982 Mar;43(3):686–693. doi: 10.1128/aem.43.3.686-693.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schink B., Ward J. C., Zeikus J. G. Microbiology of wetwood: importance of pectin degradation and clostridium species in living trees. Appl Environ Microbiol. 1981 Sep;42(3):526–532. doi: 10.1128/aem.42.3.526-532.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Glauert A. M. Ultrastructure of the cell walls of two closely related clostridia that possess different regular arrays of surface subunits. J Bacteriol. 1976 May;126(2):869–882. doi: 10.1128/jb.126.2.869-882.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton T. B., Canale-Parola E. Enumeration and selective isolation of rumen spirochetes. Appl Environ Microbiol. 1979 Nov;38(5):965–973. doi: 10.1128/aem.38.5.965-973.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Henning D. L. Methanobacterium arbophilicum sp.nov. An obligate anaerobe isolated from wetwood of living trees. Antonie Van Leeuwenhoek. 1975;41(4):543–552. doi: 10.1007/BF02565096. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Ward J. C. Methane formation in living trees: a microbial origin. Science. 1974 Jun 14;184(4142):1181–1183. doi: 10.1126/science.184.4142.1181. [DOI] [PubMed] [Google Scholar]