Abstract
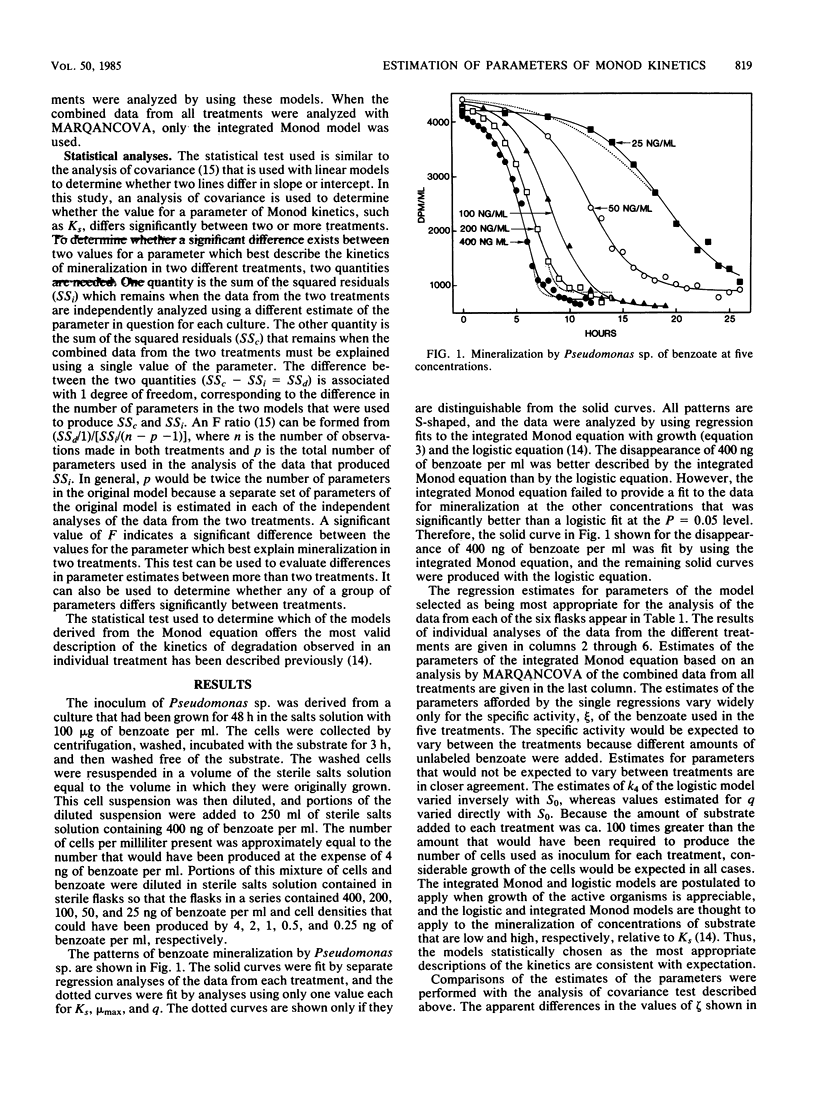
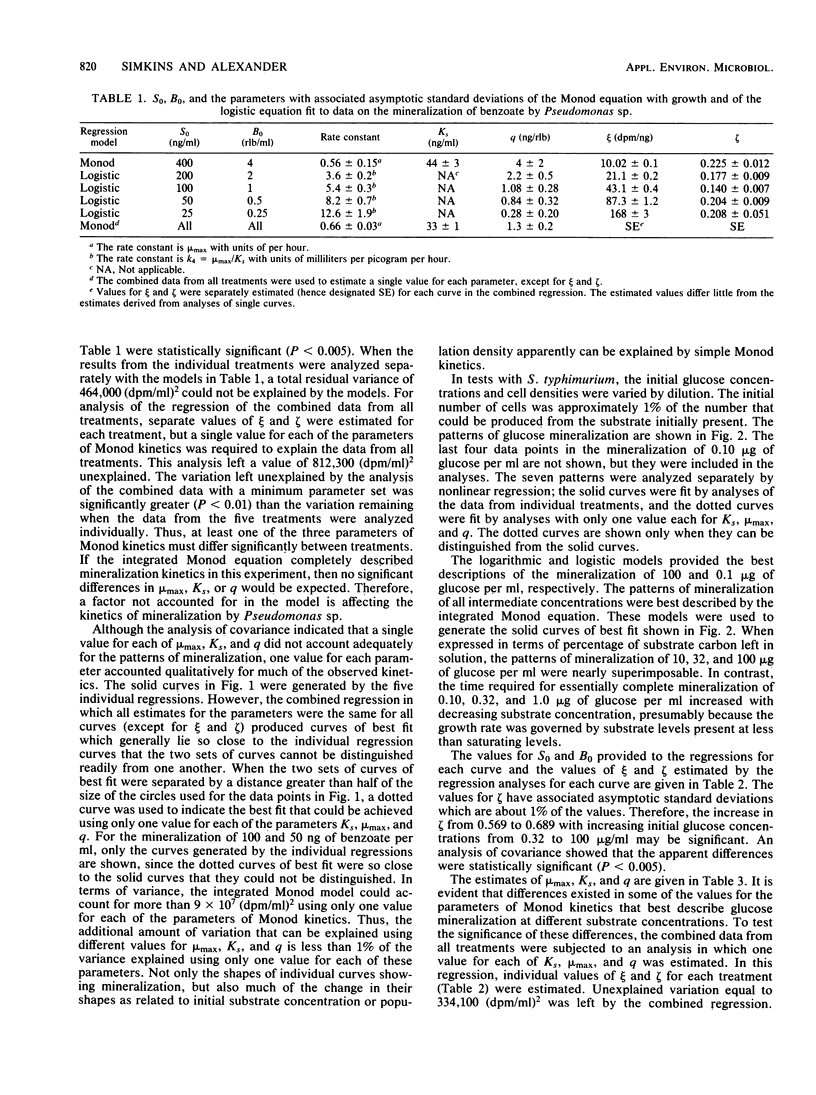
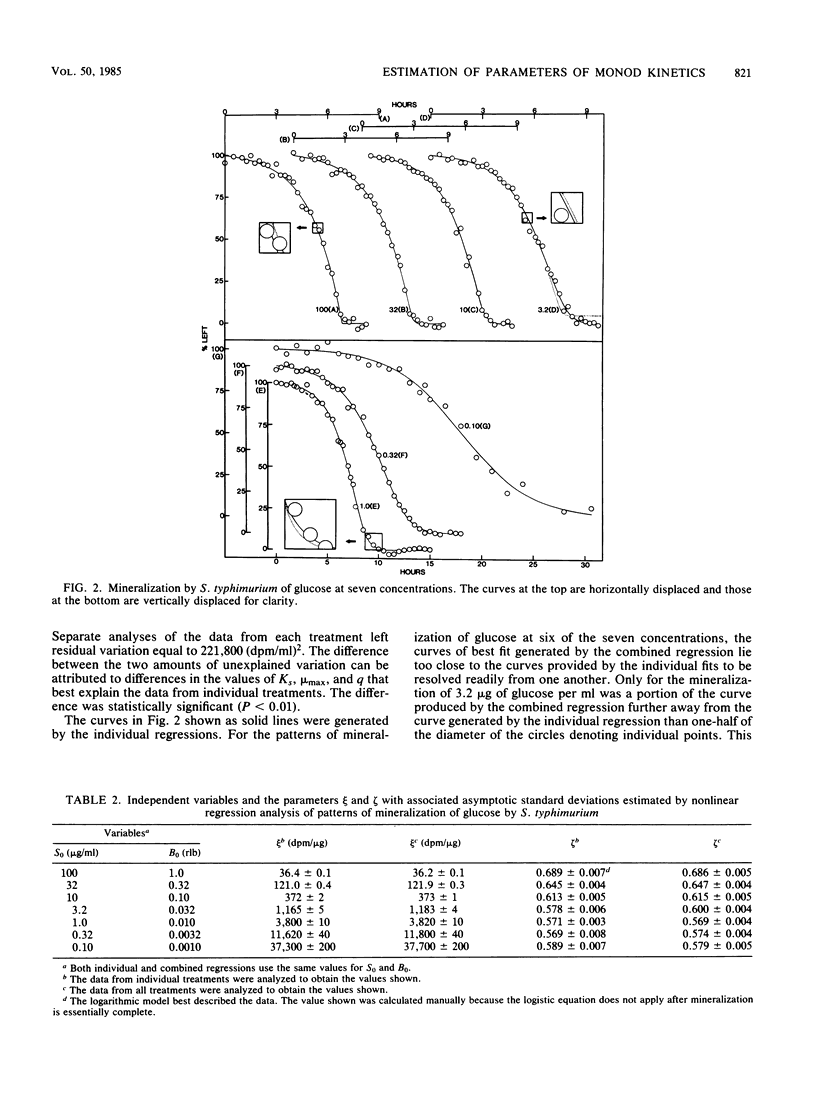
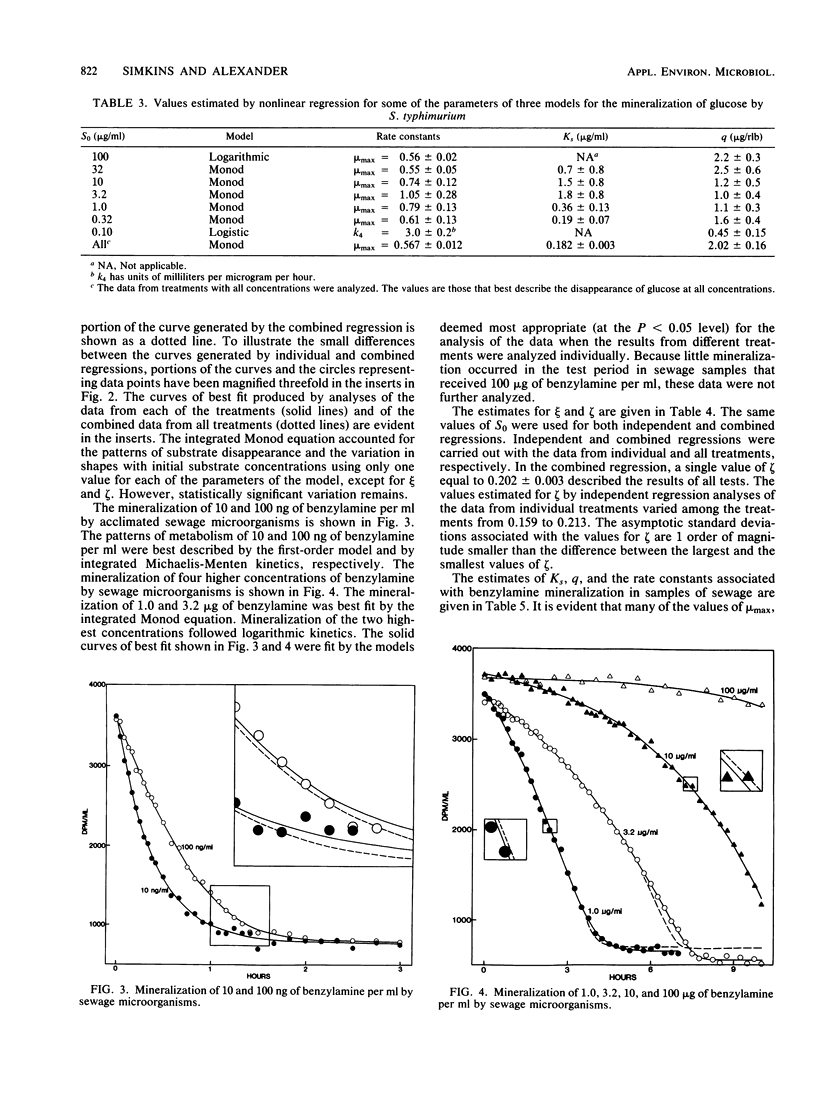
The kinetics of mineralization of a wide range of concentrations of benzoate, glucose, and benzylamine by Pseudomonas sp., Salmonella typhimurium, and microorganisms in acclimated sewage was studied. The treatment of initial substrate concentration and population density as independent variables in nonlinear regression analysis permitted the estimation of a single value for each of the parameters of Monod kinetics that best described the mineralization of substrate at each concentration by the pure cultures and the sewage microflora. One value for each of the parameters of Monod kinetics was used for each of the three compounds to produce theoretical curves which lay close to the observed data on mineralization. Statistically significant differences existed in the values of the parameters of Monod kinetics that best described mineralization in cultures differing only in initial substrate concentration and cell density. However, for the compounds tested, the variance left by analyses using one value for each parameter of Monod kinetics was less than double the unexplained variance left by individual analyses of the data from each treatment. Although significant, this increase is small compared with the amount of variance that could be explained using only one value for each parameter of Monod kinetics.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hassan G. A., Zablotowicz R. M., Focht D. D. Kinetics of denitrifying growth by fast-growing cowpea rhizobia. Appl Environ Microbiol. 1985 Mar;49(3):517–521. doi: 10.1128/aem.49.3.517-521.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E., Loveless J. E. The effect of growth conditions on respiratory activity and growth efficiency in facultative anaerobes grown in chemostat culture. J Gen Microbiol. 1971 Sep;68(1):35–43. doi: 10.1099/00221287-68-1-35. [DOI] [PubMed] [Google Scholar]
- Lopilato J. E., Garwin J. L., Emr S. D., Silhavy T. J., Beckwith J. R. D-ribose metabolism in Escherichia coli K-12: genetics, regulation, and transport. J Bacteriol. 1984 May;158(2):665–673. doi: 10.1128/jb.158.2.665-673.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Steen W. C., Baughman G. L., Barnett J. T. Second-order model to predict microbial degradation of organic compounds in natural waters. Appl Environ Microbiol. 1981 Mar;41(3):603–609. doi: 10.1128/aem.41.3.603-609.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. A., Tiedje J. M. Nonlinear estimation of Monod growth kinetic parameters from a single substrate depletion curve. Appl Environ Microbiol. 1983 May;45(5):1453–1458. doi: 10.1128/aem.45.5.1453-1458.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkins S., Alexander M. Models for mineralization kinetics with the variables of substrate concentration and population density. Appl Environ Microbiol. 1984 Jun;47(6):1299–1306. doi: 10.1128/aem.47.6.1299-1306.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. A reevaluation of the method for the determination of ATP production by measuring molar growth yields. Biochim Biophys Acta. 1973 Feb 12;301(1):53–70. doi: 10.1016/0304-4173(73)90012-8. [DOI] [PubMed] [Google Scholar]
- Stumm-Zollinger E. Effects of inhibition and repression on the utilization of substrates by heterogeneous bacterial communities. Appl Microbiol. 1966 Jul;14(4):654–664. doi: 10.1128/am.14.4.654-664.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suflita J. M., Robinson J. A., Tiedje J. M. Kinetics of microbial dehalogenation of haloaromatic substrates in methanogenic environments. Appl Environ Microbiol. 1983 May;45(5):1466–1473. doi: 10.1128/aem.45.5.1466-1473.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


