Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that is characterized by the degeneration of dopamine (DA) and non-DA neurons, the almost uniform presence of Lewy bodies, and motor deficits. Although the majority of PD is sporadic, specific genetic defects in rare familial cases have provided unique insights into the pathogenesis of PD. Through the creation of animal and cellular models of mutations in LRRK2 and α-synuclein, which are linked to autosomal dominant PD, and mutations in parkin, DJ-1, and PINK1, which are responsible for autosomal recessive PD, insight into the molecular mechanisms of this disorder are leading to new ideas about the pathogenesis of PD. In this review, we discuss the animal models for these genetic causes of PD, their limitations and value. Moreover, we discuss future directions and potential strategies for optimization of the genetic models.
Introduction
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder (Lees et al., 2009). It is chronic and relentlessly progressive and affects more than 1% of the population over age 60. A distinct anatomical circuit, the nigrostriatal pathway is preferentially damaged in PD, which is important for the fine modulation of motor function. Lewy body inclusions and relative selective loss of dopamine (DA) neurons in the substantia nigra pars compacta are the pathologic and anatomical hallmarks of PD. However there are some cases of genetic PD that do not have Lewy bodies. Currently, no proven treatment can halt or even slow the progression of this disease. Although dopamine supplementation is currently used for PD as a means to replace the diminished tone of dopaminergic output from substantia nigra pars compacta, it only alleviates the symptomatic motor dysfunction and its effectiveness declines as the disease progresses. Therefore, it is essential to develop suitable animal models, which can be used for the screening and testing of new therapeutic strategies targeting the actual pathogenic process as opposed to merely developing symptomatic therapies (Savitt et al., 2006).
Genes linked to rare forms of PD or the processes that they regulate are potential therapeutic targets (Gasser, 2009; Lees et al., 2009). One can attempt to model PD in animals or cellular systems using genetic manipulation, that is transgenic overexpression of mutant genes for autosomal dominant genes such as α--synuclein and leucine rich repeat kinase 2 (LRRK2) and knockout or knockdown models for autosomal recessive genes, such as Parkin, DJ-1, phosphatase and tensin homolog (PTEN)-induced novel kinase 1 (PINK1). Other mutations in genes are also causal for PD including glucocerebrosidase (GBA) and PD syndromes such as ATP13A2, but animal models for these mutations have not been described or characterized. Many different organisms and cellular models are useful for genetic manipulation and many of these models have been especially helpful in dissecting the pathogenic mechanisms of PD.
Genetic models of neurodegenerative diseases have in general been quite instructive in understanding the pathogenesis of a variety of neurodegenerative disorders. Often the initial models only replicate a portion of the disease in humans, but as models are refined key features of most diseases can be replicated. In amyotrophic lateral sclerosis (ALS), there was instance success. Overexpression of the G93A mutation in superoxide dismutase (SOD) created a mouse model of ALS, which replicated key features of the illness in humans (Gurney et al., 1994). In Alzheimer’s disease (AD), initial animal models suffered from the lack of manifesting the full spectrum of disease (Kokjohn and Roher, 2009). However, refinement and optimization of the models such as the “triple” transgenic model expressing the APP Swedish mutation K670N/M671L, the presenilin-1 M146V mutation, and the tau P301L mutation, as well as other models provided useful tools for enhancing the molecular understanding of AD pathogenesis (Kokjohn and Roher, 2009). Similar examples on either spectrum of modeling human pathogenesis exist in other neurodegenerative diseases. Overall modeling human neurodegenerative diseases has been fruitful and yielded many fresh insights into the pathogenesis of these disorders. In particular, these models have been instructive for modeling different stages of the corresponding illness ranging from insights into the early to late stage manifestations of the disease. These models have also served as platforms to test novel therapeutics. In this review we attempt to synthesize what has been gleaned from the study of PD models. In addition we discuss controversies, future directions and further work that needs to be done to potentially optimize existing models.
PD Animal Models
Modeling PD in animals, particularly rodent models using genetics has been viewed as difficult. This is, in part, due to the prevailing concept that any “reasonable” model of PD should have progressive loss of DA neurons (Chesselet et al., 2008). None of the current PD models completely recapitulate key clinical and neuropathologic features of PD. What features should be present in an ideal model of PD? Models should be age-dependent and progressive since degeneration usually begins in late adulthood in PD. In addition to the loss of DA neurons, there should be motor dysfunction including slowness of movement, rigidity, rest tremor and postural instability that is responsive to DA replacement therapy. Another key determinant of this disorder is the presence of Lewy bodies and Lewy neurites that contain α-synuclein and ubiquitin-proteasomal proteins amongst others. However, some genetic forms of PD do not have Lewy bodies including some patients with parkin and LRRK2 mutations. These cases may represent the exceptions that prove the rule since most cases of LRRK2 have α-synuclein inclusions (Ross et al., 2006) and almost as many cases of parkin mutations that have been examined have Lewy bodies as those that do not (for review see (West et al., 2007a)). No neuropathologic studies have been reported for patients with PINK1, DJ-1 and ATP13A2 mutations, whereas mutations in GBA are associated with Lewy bodies (Clark et al., 2009). Whether there are distinct mechanisms that lead to neurodegeneration in the various genetic causes of PD requires further study. The different mutations is PD associated genes may act in series and/or in parallel pathways. However all genetic causes of PD ultimately lead to loss of DA neurons in the nigrostriatal pathway and as such there are likely to be some common final molecular mechanisms.
Extensive neuropathologic studies clearly indicate that PD is a global nervous system disorder with degeneration throughout the central and peripheral nervous system (Braak et al., 2006; Jellinger, 2009). Some neuropathologists suggest that PD begins in the lower brainstem and olfactory bulb with the substantia nigra only becoming affected during the middle stages of the disease (Braak et al., 2006). Moreover, there are numerous clinical features of PD that are not attributable to the degeneration of DA neurons (Langston, 2006). PD affects many areas of the central nervous system including the hypothalamus, nucleus basalis of Meynert and the dorsal motor nucleus of the vagus, as well as limbic and cortical areas. These non-dopaminergic features of PD are often the most disabling as current treatment inadequately addresses these symptoms (Savitt et al., 2006).
Currently there are many animal models of PD due to genetic causes in different model organisms including mice, drosophila melanogaster and Caenorhabditis elegans. There are a variety of cellular models that have used to glean insight into how mutations in the various genetic causes of PD lead to neuronal dysfunction. In addition, there are several intoxication models that are based on selective killing of DA neurons. Several excellent reviews on these animal and cellular models exist (see (Bove et al., 2005; Chesselet et al., 2008; Gupta et al., 2008; Lim and Ng, 2009; Moore et al., 2005; Pallanck and Whitworth, 2007; Wong, 2007)).
Cellular models offer the advantage of dissecting the molecular function of the genes and proteins implicated in PD. Cellular studies can be done relatively quickly and robustly using molecular, biochemical and pharmacologic approaches. Many insights into the function of the proteins implicated in PD have initially stemmed from cellular models. However, one needs to interpret cellular studies with caution since they are often prone to artifact and/or misinterpretation. Animal models provide the means to study a cellular process in the context of functional neuronal circuits in an unbiased way serving as a validation or reality check on cellular assays. Due to the ease of genetic manipulation (transgenics and knockouts), the mouse has become the preferred system to model neurodegenerative diseases, such as PD. The mouse has the relatively short life-span of 2 years, which renders it less then ideal for modeling a disorder like PD that takes five to seven decades or longer to manifest in humans. Thus, with autosomal dominant disorders, approaches have focused on overexpression in an attempt to shorten the time to disease manifestation in mice. Since most PD associated genes are not overexpressed to a great extent, one needs to interpret mouse transgenic studies with a bit of a caution as well. Validation of findings in transgenic models has relied on the use of human postmortem tissue. Although human postmortem analysis often reflects end-stage illness, footprints of early disease pathology often remain and it is the gold standard by which to measure the ability of animal models to reflect actual pathogenic processes. Occasionally animal models provide insight into the human condition that was not apparent from routine neuropathological assessments. C. elegans and drosophila are powerful models to rapidly screen for pharmacologic and genetic interventions that may modify neurodegeneration in these models. They have some shortcomings in modeling PD, including the lack of expressing α-synuclein and a limited repertoire of cell death effectors. These models offer the advantage of identifying evolutionarily conserved pathways, but the challenge in using these models is to verify that potential modifiers occur in human PD.
Although genetics has generated tremendous excitement and new energy in PD research, it is important to realize that only about 10 to 20% of PD is due to genetic causes. Sporadic PD is characterized by mitochondrial dysfunction and oxidative and nitrosative stress (Banerjee et al., 2009). Inhibition of complex I of the mitochondrial electron transport chain is selectively dysfunctional in DA neurons in sporadic PD (Dawson and Dawson, 2003). DA neurons are particularly sensitive to the toxic effects of mitochondrial complex I toxins, such as rotenone or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Banerjee et al., 2009; Dauer and Przedborski, 2003). There are a variety of intoxication models of PD and there use has provided the framework for improvements in symptomatic medical and surgical therapies for PD. How these toxins cause selective loss of DA neurons is reviewed elsewhere (Banerjee et al., 2009; Dauer and Przedborski, 2003). Unfortunately, no neuroregenerative or neuroprotective agent identified from studies in the PD intoxication models has been shown to be efficacious in PD patients. The lack of the predictive value of the PD-intoxication models may be due to the acuteness of these models and their failure to replicate all the features of PD. As such, there is tremendous hope that models based on gene mutations that are linked to PD may provide the proper context by which to ultimately develop therapies that are proven to be neuroprotective or neuroregenerative in humans with PD.
Models of Autosomal Dominant PD
Currently there are two autosomal dominantly inherited causes of PD. Mutations in α-synuclein cause one form of autosomal dominant PD. The identification of mutations in α-synuclein, the first gene linked to familial PD, changed the nature and scope of PD research. It catalyzed the identification of several other PD-linked genes and fueled research in PD. α-Synuclein’s presence in Lewy bodies and neurites, two key pathologic hallmarks of PD, illustrates the intimate relationship between familial and sporadic PD and how studying familial PD leads to fresh insights into sporadic PD. It was the genetic discovery of α-synuclein linkage to PD that led to the discovery that α-synuclein was the major structural moiety of Lewy bodies and neurites (Goedert, 2001). Mutations in LRRK2 are the most frequent genetic cause of autosomal dominant PD (Paisan-Ruiz et al., 2004; Zimprich et al., 2004). It also appears to play a prominent role in sporadic PD as well, since mutations in LRRK2 account for up to 4% of sporadic PD (Albrecht, 2005). Several animal and cellular models of α-synuclein and LRRK2 have been reported and they have provided important insight into how mutations in α-synuclein and LRRK2 may lead to neurodegeneration in PD (Table 1). Central to the utility and predictive value of these models is whether they can replicate some of the key feature of PD including age-dependent neurodegeneration, the presence of Lewy bodies, DA neuronal death, mitochondrial dysfunction and other features of PD.
Table 1.
Key Features of Autosomal Dominant (α-synuclein and LRRK2) Models*
| Model | Neuronal Loss Dopamine (DA) Non-Dopamine (Non-DA) |
L-DOPA Responsive Motor Deficits |
Mitochondrial Dysfunction |
Lewy Body – Like Inclusions |
Suitability for Testing Disease Modifying Therapy |
|---|---|---|---|---|---|
| α-synuclein | |||||
|
Drosophila (WT, A30P, A53T) |
DA + Non-DA − |
+ | ND | + | + |
| Mouse Thy1 (WT, A30P, A53T) |
DA − Non-DA + |
ND | ND | - (nonfibrillar α-synuclein inclusions) |
+ (A30P, A53T) |
| Mouse PrP (WT, A30P, A53T) |
DA − Non-DA + |
ND | + | + | + (A53T) |
| Rat AAV (WT, A53T) |
DA + Non-DA − |
+ | ND | - (nonfibrillar α-synuclein inclusions) |
+ (A53T) |
|
| |||||
| LRRK2 | |||||
|
Drosophila (WT, I1122V, Y1699C, G2019S, I2020T, G2385R) |
DA + Non-DA − |
+ | ND | − | + |
| Mouse BAC (R1441G) |
DA − (↓TH) Non-DA − |
+ | ND | − | Maybe |
Autosomal dominant models of PD with evidence of progressive neurodegeneration in either dopamine (DA) or non-dopaminergic (Non-DA) systems are highlighted. Features common to sporadic PD are indicated including age-dependent, progressive degeneration of DA and non-DA systems, levodopa (L-dopa) responsive motor deficits, the presence of Lewy body inclusions that contain filamentous α-synuclein and mitochondrial dysfunction (see text). The suitability of the various models for testing disease modifying therapy is also indicated.
Not all animal models are listed for α-synuclein and LRRK2.
Three point mutations in α-synuclein (A53T, A30P and E46K) cause familial PD (Figure 1) (Gasser, 2009; Lees et al., 2009). Mutations in α-synuclein are a 100% penetrant and are a rare cause of PD. In addition, simple duplication or triplication of α-synuclein itself is sufficient to cause PD, suggesting the level of expression of α-synuclein is a critical determinant of PD progression (Singleton, 2005; Singleton et al., 2003). Indeed, it seems that one’s relative risk for developing PD is determined by polymorphisms in the promoter or 3′-UTR, which determine the level of α-synuclein. Individuals with a high level of α-synuclein have a greater risk of developing PD, than those with a lower level of α-synuclein (Winkler et al., 2007). Recent genome wide association studies further support the notion that α-synuclein is critically linked to PD, as there is an association between PD and α-synuclein (Sutherland et al., 2009).
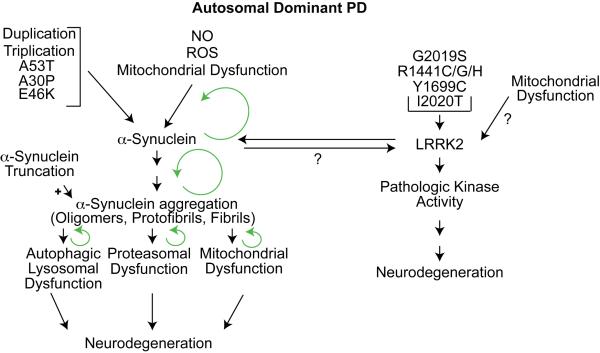
Figure 1. Mechanisms of Autosomal Dominant PD.
Mutations (A53T, A30P, E46K) or increased levels (triplication or duplication) of α-synuclein lead to aggregation and fibrillization. Features common to sporadic PD such as nitric oxide (NO), reactive oxygen species (ROS), and mitochondrial dysfunction also leads to α-synuclein aggregation. Agents or genetic modifications that inhibit α-synuclein aggregation are protective, whereas enhancing α-synuclein aggregation promotes neurodegeneration. Truncation of α-synuclein accelerates aggregation and participates in the degenerative process. α-Synuclein is degraded by both the ubiquitin proteasome system and the autophagic/lysosomal system. Mutant and aggregated forms of α-synuclein inhibit the UPS and the autophagic/lysosomal system as well as the mitochondria setting in motion feed forward cycles (indicated by green circular arrows) of enhanced accumulation and aggregation of α-synuclein (see text). LRRK2 mutations ( G2019S, R1441C/G/H, Y1699C and I2020T) lead to neurodegeneration that is kinase-dependent and GTP-binding dependent (not shown). LRRK2 shares some pathogenic mechanisms with PD due to α-synuclein since a consistent pathologic feature of patients with LRRK2 mutations is the presence of α-synuclein inclusions through unclear mechanisms (?). Mitochondrial dysfunction enhances LRRK2 neurodegeneration in some models through unclear mechanisms (?). Glucocerebrosidase and ATP13A2 genetically interact with α-synuclein and also share common pathogenic mechanisms (not shown).
α-Synuclein is an abundant presynaptic phosphoprotein that normally exits in an unstructured state. It may be involved in intracellular trafficking within the endoplasmic reticulum/Golgi network since overexpression of α-synuclein in drosophila and yeast disrupts cargo trafficking in this network (Cooper et al., 2006; Gitler et al., 2008). Toxic misfolded forms of α-synuclein appear to play a prominent role in cell death (Figure 1) (for review see (Cookson, 2005; Gupta et al., 2008; Lee and Trojanowski, 2006)). Mutations in α-synuclein increase the propensity for misfolding. A number of factors that are thought to play a prominent role in sporadic PD including oxidative and nitrosative stress and mitochondrial dysfunction also increase the propensity for α-synuclein misfolding (Figure 1). α-Synuclein exists in a variety of higher ordered structures including oligomers, protofibrils, fibrils and filaments where protofibrils and fibrils seem to be the most toxic forms (Lee and Trojanowski, 2006). Stabilization of these higher ordered structures maybe central to the pathogenesis of PD. Disease severity and the propensity to form higher ordered structures correlates with truncation of α-synuclein into more toxic forms (Li et al., 2005). Misfolded α-synuclein also contributes to other neurodegenerative disorders designated α-synucleinopathies and they are broadly defined as disorders with misfolding of α-synuclein into higher ordered structures leading to α-synuclein inclusions (Lee and Trojanowski, 2006).
Due to α-synuclein’s prominent role in PD and related α-synucleinopathies, many α-synuclein models using a variety of approaches have been created and extensively characterized (Table 1). Overexpression of WT, A53T, and A30P α-synuclein in drosophila, recapitulated many characteristics of PD including age-dependent selective DA neuron loss, Lewy body-like filamentous inclusions containing α-synuclein and DA-responsive locomotor deficits (Feany and Bender, 2000). Although whether there is actual loss of DA neurons or just loss of DA markers is not clear (Pesah et al., 2005). C. elegans overexpressing α-synuclein have loss of DA neurons, but they lack significant α-synuclein inclusions and the degeneration of DA neurons is not progressive (Kuwahara et al., 2006; Lakso et al., 2003). The strength of the drosophila model is that it is the only α-synuclein animal models with possible progressive degeneration of DA neurons. Drosophila and C. elegans lack the complexity of vertebrates and they do not express α-synuclein, thus they are imperfect models of PD. Moreover, these models fail to exhibit the cardinal clinical features of PD (bradykinesia, rest tremor, rigidity and postural instability) and thus their value mainly lies in identifying genetic and pharmacologic modifiers of α-synuclein-induced neurodegeneration. As noted above, the challenge is to verify that potential modifiers uncovered in these simple model systems are evolutionarily conserved in human PD.
Knockout of α-synuclein indicates that it plays little if any role in the development or maintenance of DA neurons (Abeliovich et al., 2000; Chandra et al., 2004), thus loss of some critical function of α-synuclein is unlikely to play a role in the pathogenesis of α-synuclein-induced neurodegeneration. However, this has not been settled since α-synuclein may function as a co-chaperone as transgenic expression of α-synuclein abolishes the lethality and neurodegeneration caused by deletion of synaptic vesicle chaperone, cysteine-string protein-α (Chandra et al., 2005). Thus, α-synuclein may play a role in protecting nerve terminals from injury and sequestering α-synuclein into aggregates could eliminate this protective function.
A number of α-synuclein transgenic mice have been reported using a variety of promoters (Table 1). The phenotypic outcome of α-synuclein overexpression in mice heavily depends on the promoters used to drive transgene expression (Chesselet, 2008). None of these models accurately represent PD, in that there is no progressive loss of DA neurons. Despite the lack of overt degenerative pathology in DA neurons in the various mouse α-synuclein models there are several functional abnormalities in the nigrostriatal system, some of which are DA responsive (see (Chesselet, 2008; Chesselet et al., 2008)). Moreover most of these models are excellent models of α-synuclein-induced neurodegeneration.
The toxicity of aggregated α-synuclein seems to occur through mitochondrial dysfunction and proteasomal and lysosomal impairments and disruption of ER-Golgi trafficking, which set forth feed-forward mechanisms of further injury (Figure 1) (Cooper et al., 2006; Cuervo et al., 2004; Martin et al., 2006; Tanaka et al., 2001). The link to mitochondrial dysfunction is particularly attractive as decrements in mitochondrial complex I is a consistent feature of sporadic PD (Schapira, 2008). Moreover, inhibition of mitochondrial complex I can lead to selective DA neurodegeneration. (Banerjee et al., 2009). Although impairment of the proteasomal and lysosomal systems can lead to α-synuclein accumulation consistent with role of these systems in catabolizing α-synuclein, inhibition of the proteasomal and lysosomal systems leads to general neurodegeneration without selectivity for any particular neuronal system (Bedford et al., 2008; Komatsu et al., 2006). Thus, in contrast to mitochondrial complex I inhibition, which selectively injures DA neurons and is intimately linked to the pathogenesis of PD, impairment of the proteasomal and lysosomal systems are likely to be the consequence of the degenerative process set in motion by PD.
There appears to be an intimate relationship between α-synuclein and mitochondrial function. DA neurons in α-synuclein knockouts are resistance to the DA neurotoxin, MPTP and other mitochondrial toxins (Dauer and Przedborski, 2003; Klivenyi et al., 2006). In addition, mice overexpressing α-synuclein are more sensitive to paraquat, another mitochondrial toxin (Norris et al., 2007). Transgenic mice overexpressing human A53T α-synuclein exhibit mitochondrial abnormalities including mitochondrial DNA damage and degeneration (Martin et al., 2006). DA neurons progressively degenerate and accumulate intraneuronal inclusions that contain α-synuclein in DA specific mitochondrial transcription factor A (TFAM) knockouts (Ekstrand et al., 2007). Moreover, the substantia nigra and striatum, but not the cerebellum of PD patients accumulate α-synuclein in the setting of decreased mitochondrial complex I activity (Devi et al., 2008) and patients with mitochondrial mutations have Lewy bodies (Betts-Henderson et al., 2009). Since mitochondrial dysfunction causes α-synuclein aggregation and α-synuclein is capable of injuring the mitochondria, there is a feed-forward loop that has the potential to set in motion the progressive unrelenting degeneration of PD (Figure 1). Understanding the relationship of α-synuclein and mitochondrial dysfunction is important since it may provide insight into the causes of sporadic PD and lead to new disease modifying therapies.
Of the many vertebrate models, only the mouse prion promoter (mPrP) A53T α-synuclein transgenic mice exhibit the full range of α-synuclein pathology that is observed in humans including α-synuclein aggregation, fibrils and truncation, α-synuclein phosphorylation and ubiquitination and progressive age-dependent neurodegeneration (Chesselet, 2008; Dawson et al., 2002; Giasson et al., 2002; Lee et al., 2002). The other transgenic models exhibit gradations of α-synuclein aggregation, but they lack the characteristic α-synuclein fibrils that are present in humans with PD and related α-synucleinopathies. Studies indicate that assembly of α-synuclein into higher order structures is one of the critical events that contribute to neurodegeneration (Lee and Trojanowski, 2006). Mutant forms of α-synuclein accelerate the formation of aggregates (Conway et al., 1998). From the transgenic α-synuclein mouse models it is clear that neuronal dysfunction and degeneration is probably initiated by the prefibrillar oligomers as models exhibiting only prefibrillar oligomers display features of neurodegeneration (Chandra et al., 2005; Freichel et al., 2007; Neumann et al., 2002; Rockenstein et al., 2002; van der Putten et al., 2000). Fibrils of α-synuclein also play a role in the toxicity since the formation of α-synuclein aggregates is required for significant neurodegeneration (Giasson et al., 2002; Lee et al., 2002; Periquet et al., 2007). However, some studies suggest that sequestration of α-synuclein into fibrils may be protective (see (Forman et al., 2005)). In any event, strategies aimed at maintaining or converting α-synuclein into its monomeric state have a high potential to be therapeutic. Mouse models with prefibrillar oligomers versus fibrillar α-synuclein could be used to test the efficacy of strategies focused on converting or maintaining α-synuclein in a monomeric state. Since humans with PD express the full spectrum of α-synuclein aggregation, therapeutic trials in animal models that also display the full complement of α-synuclein pathology are likely to be the most predictive.
Several mutations in LRRK2 cause autosomal dominant PD (Figure 1). LRRK2 is a large multidomain containing protein that is localized to membranous structures (Biskup et al., 2006). Mutations that segregate with PD are concentrated in the GTPase and kinase domains (Biskup and West, 2009). The most common mutation, G2019S has a frequency of 1% of patients with sporadic PD and 4% of patients with hereditary PD (Healy et al., 2008). The risk of PD for a person with a LRRK2 G2019S mutation increases with age and it is 28% at 59 years, 51% at 69 years and 74% at 79 years (Healy et al., 2008). The majority of cases of LRRK2 related PD that have come to autopsy are characterized pathologically by the presence of α-synuclein inclusions, suggesting that LRRK2 and α-synuclein share common pathogenic mechanisms, but cases have been described with only neuronal loss or ubiquitin or tau inclusions (Ross et al., 2006). LRRK2 may play a role in neuronal outgrowth and guidance (MacLeod et al., 2006; Sakaguchi-Nakashima et al., 2007), but its normal physiologic function, phosphosubstrates, binding partners and regulators of kinase and GTPase activity have yet to be confirmed or clarified. In cellular models, overexpression of disease causing mutations of LRRK2 are toxic and the toxicity is kinase and GTP-binding dependent (Greggio et al., 2006; Smith et al., 2006; West et al., 2007b). Thus LRRK2 kinase inhibitors and modulators of GTP-binding may be therapeutic.
Overexpression of LRRK2 in drosophila leads to age-dependent DA responsive reductions in locomotor activity and loss of DA neurons (Table 1) (Liu et al., 2008; Ng et al., 2009; Venderova et al., 2009). In a similar manner overexpression of LRRK2 leads to degeneration of DA neurons in C. elegans (Saha et al., 2009). Like the Drosophila and C. elegans α-synuclein models the LRRK2 models in these simpler model systems are imperfect. Since Drosophila and C. elegans do not express α-synuclein and a prominent feature of PD caused by mutations in LRRK2 is α-synuclein pathology, the utility of studying mechanisms of LRRK2 neurodegeneration in flies and worms is potentially problematic. Their value mainly lies in identifying genetic and pharmacologic modifiers of LRRK2-induced neurodegeneration. Potential modifiers uncovered in these simple model systems will need to carefully validated to determine whether they are evolutionarily conserved in human PD.
Knockout of LRRK homologues in drosophila and C. elegans and knockout of LRRK2 in mice suggest that LRRK2 plays little if any role in the development or maintenance of DA neurons, although there are disparate results in the drosophila studies (Andres-Mateos et al., 2009; Imai et al., 2008; Lee et al., 2007; Sakaguchi-Nakashima et al., 2007; Wang et al., 2008a). In contrast to the cellular and drosophila models, current transgenic mouse models are not very robust PD models (Table 1). Bacterial artificial chromosome (BAC) transgenic mice expressing LRRK2 WT, LRRK2 R1441G, LRRK2 G2019S have minimal evidence of neurodegeneration (Li et al., 2010; Li et al., 2009). Conditional expression of LRRK2 WT and LRRK2 G2019S also failed to exhibit neurodegeneration of DA neurons, but LRRK2 was expressed at low levels in DA neurons due to the use of the calcium/calmodulin-dependent protein kinase IIα (CamKII) promoter (Lin et al., 2009; Wang et al., 2008c). When the R1441C mutation is expressed under the control of the endogenous regulatory elements, by knockin of the R1441C mutation there is no degeneration of DA neurons (Tong et al., 2009). Most of the current LRRK2 transgenic mice have abnormalities in the nigrostriatal system such as stimulated DA neurotransmission or behavioral deficits, which are DA responsive. These abnormalities probably represent some of the earliest neuronal dysfunction that is set in motion by pathogenic LRRK2 mutations. In contrast to the other LRRK2 transgenic models, the LRRK2 R1441G BAC transgenic mice have progressive age-dependent motoric deficits leading to immobility that are levodopa and apomorphine responsive, despite no substantial neurodegeneration (Li et al., 2009). LRRK2 and α-synuclein are likely to share common pathogenic mechanisms as overexpression of LRRK2 greatly enhanced and knockout of LRRK2 reduced the neuropathologic abnormalities in A53T α-synuclein transgenic mice (Lin et al., 2009).
Why the mouse LRRK2 transgenic models do not exhibit more substantial pathology is not clear. It may relate to the fact that LRRK2 mutations in humans are only partially penetrant and that there may need to be other genetic and/or environmental hits that are required for degeneration of DA neurons (see below). The BAC and knockin models express mutant LRRK2 during development and thus there may be compensatory mechanisms in the mouse that prevent loss of DA neurons (see below). The utility of the current models is likely to be focused on how mutations in LRRK2 lead to early dysfunction of the nigrostriatal DA system. It is unlikely that these models could be used to test neuroprotective agents since there is no neurodegeneration.
Models of Autosomal Recessive PD
There are currently four autosomal recessive causes of PD. Mutations in parkin were first identified as the genetic cause of autosomal recessive juvenile Parkinsonism in Japanese families (Kitada et al., 1998). More than 100 mutations in parkin have been reported, accounting for 50% of familial PD cases and at least 20% of young onset sporadic PD (Lucking et al., 2000). Mutations in PINK1, the second most common autosomal recessive mutation following parkin contributes to almost 1-7% of early onset PD (Gasser, 2009). Mutations in DJ-1 is a rare cause of PD (Heutink, 2006). A number of animal and cellular models have been described for parkin, PINK1 and DJ-1, which have led to tremendous insight into the role these proteins play in PD. In contrast, no animal models have been reported for ATP13A2, which is another rare cause of PD (Ramirez et al., 2006). Similar to the autosomal dominant models, the drosophila models of autosomal recessive PD exhibit age-dependent loss of DA neurons and other abnormalities, whereas the mouse models fail to replicate many key features of PD (Table 2).
Table 2.
Key Features of Autosomal Recessive (parkin, PINK1, DJ-1) Models*
| Model | Neuronal Loss Dopamine (DA) Non-Dopamine (Non-DA) |
L-DOPA Responsive Motor Deficits |
Mitochondrial Dysfunction |
Lewy Body – Like Inclusions |
Suitability for Testing Disease Modifying Therapy |
|---|---|---|---|---|---|
| Parkin | |||||
| Drosophila (KO) | DA + Non-DA − |
+ | + | − | + |
| Mouse (KO) | DA − Non-DA − |
ND | + | − | − |
| Mouse (BAC- DAT-Q311X) |
DA + Non-DA − |
ND | ND | + | Maybe |
|
| |||||
| PINK1 | |||||
| Drosophila (KO) | DA + Non-DA − |
+ | + | − | + |
| Mouse (KO) | DA − Non-DA − |
ND | + | − | − |
|
| |||||
| DJ-1 | |||||
| Drosophila (KO) | DA + Non-DA − |
+ | + | − | + |
| Mouse (KO) | DA − Non-DA − |
ND | + | − | − |
Autosomal recessive models of PD are highlighted. Features common to sporadic PD are indicated including age-dependent, progressive degeneration of DA and non-DA systems, levodopa (L-dopa) responsive motor deficits, the presence of Lewy body inclusions that contain filamentous α-synuclein and mitochondrial dysfunction (see text). The suitability of the various models for testing disease modifying therapy is also indicated.
Not all animal models are listed for parkin, PINK1, DJ-1.
Parkin functions as an E3 ubiquitin ligase where it participates in the ubiquitin proteasome system (Figure 2) (Shimura et al., 2000; Zhang et al., 2000). Parkin, is a huge gene with 12 exons that spans 1.3 Mb of genomic DNA encoding a 465 amino acid protein, which may account for the high degree of mutations. Penetrance appears to be complete in individuals who have two disease causing mutations in Parkin (Lucking et al., 2000). Disease causing mutations in parkin for the most part lead to a loss of its E3 ubiquitin ligase activity. Although parkin dysfunction plays a critical role in the general pathogenesis of early onset Parkinsonism, it may also play a role in sporadic PD (Dawson and Dawson, 2009). Parkin is inactivated by dopaminergic, oxidative and nitrosative stress, which play key roles in sporadic PD (Chung et al., 2004; LaVoie et al., 2005; Yao et al., 2004).
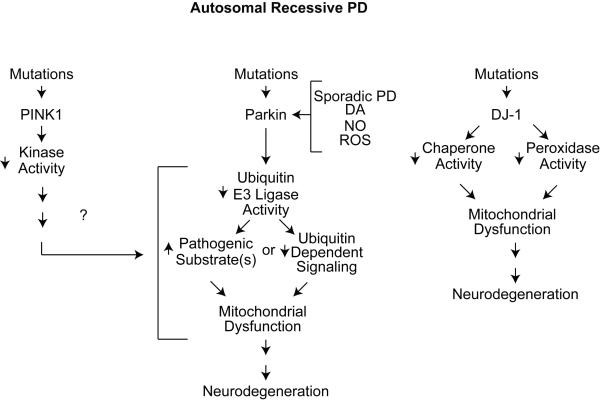
Figure 2. Mechanisms of Autosomal Recessive PD.
Loss of function mutations in PINK1, parkin and DJ-1 cause PD. PINK1 is a mitochondrial kinase in which mutations impair its kinase activity. PINK1 acts upstream of parkin to ultimately impair mitochondrial function through as yet unknown mechanisms. Parkin is inactivated by genetic mutations or nitric oxide (NO), reactive oxygen species (ROS), dopamine (DA) or in sporadic PD, which impairs parkin’s ubiquitin E3 ligase activity. This either leads to accumulation of as of yet identified pathogenic substrate or it impairs ubiquitin dependent signaling that ultimately lead to mitochondrial dysfunction and neurodegeneration. Mutations in DJ-1 lead to a loss of its chaperone activity as well as impairing its peroxidase activity among other functions, which ultimately leads to mitochondrial dysfunction and neurodegeneration.
It has been difficult to build a consensus and unifying theory on how parkin dysfunction leads to neurodegeneration in PD. Numerous putative substrates for parkin have been reported (for review see (Dawson and Dawson, 2009; West et al., 2007a)). No consensus has emerged on which of these potential parkin substrates may play a pathogenic role in parkin deficiency. Parkin is capable of modifying proteins with different ubiquitin linkages including monubiquitination and polyubiqutination using both lysine-48 and lysine-63 linkages. Monoubiquitination may be involved in receptor turnover, lysine-63 linkages may be involved with protein inclusions and lysine-48 linkages regulate protein degradation (Dawson and Dawson, 2009). Understanding these alternative ubiquitination reactions by parkin may ultimately turn out to be important in the pathogenesis of PD due to parkin inactivation.
Knockout of parkin in drosophila leads to mutant flies with reduced lifespan, male sterility and severe defects in both flight and climbing abilities (Greene et al., 2003; Whitworth et al., 2005). In addition, there are muscle and sperm mitochondrial defects that ultimately result in cell death with apoptotic morphology. Parkin mutant flies show significant indirect flight muscle degeneration with reduced muscular fibrils, reduced mitochondria with a significant loss of cristae structure. Decreased TH levels were observed in aged flies and careful examination revealed that a subset of DA neurons degenerate (Table 2). Parkin null mutants display DA responsive locomotion deficits. In contrast, none of the parkin knockout mice have any substantial dopaminergic or behavioral abnormalities (Table 2) (Goldberg et al., 2005; Itier et al., 2003; Perez and Palmiter, 2005; Von Coelln et al., 2004). Some of the parkin knockout mice have subtle abnormalities in the either the DA nigrostriatal circuit or the locus coeruleus noradrenergic system (Goldberg et al., 2005; Von Coelln et al., 2004). Interestingly overexpression of mutant human parkin in both drosophila and mice leads to progressive degeneration of DA neurons, supporting the ideas that some parkin mutants might act in a dominant negative fashion (Lu et al., 2009; Sang et al., 2007; Wang et al., 2007).
PD due to PINK1 mutations is inherited in a recessive fashion and mutations are thought to lead to a loss of function (Valente et al., 2004). Penetrance appears to be complete in individuals who have two disease causing mutations in PINK1 (Gasser, 2009). Structurally, PINK1 contains a conserved serine/threonine kinase domain with a N-terminal mitochondrial targeting motif (Figure 2). PINK1 is localized to the mitochondrial intermembrane space and membranes of the mitochondria (Silvestri et al., 2005), but a recently topology study suggests that the kinase domain of PINK1 faces the cytosol (Zhou et al., 2008). Thus, physiologic PINK1 kinase substrates may need to reside in the cytosol. PINK1 may regulate mitochondrial calcium dynamics (Gandhi et al., 2009), but additional studies are required to identify/confirm authentic physiologic and pathophysiologic PINK1 substrates and clarify the site of action of PINK1.
Drosophila lacking PINK1 exhibit male sterility, an inability to fly and a slower climbing speed (Clark et al., 2006; Park et al., 2006). Accompanying these behavioral deficits was mitochondrial degeneration leading to apoptosis in flight muscles. Similar to Drosophila lacking parkin, the mitochondria were swollen with reduced ATP levels, mitochondrial DNA, and mitochondrial proteins. DA neurons exhibited a small, but significant reduction in number with enlarged mitochondria (Table 2). Similar to parkin knockout mice, PINK1 knockout mice did not exhibit any major abnormality (Table 2) (Gautier et al., 2008; Gispert et al., 2009; Kitada et al., 2007). In particular, the number of DA neurons, the level of striatal DA, and the level of DA receptors are unchanged. Like the parkin knockouts there are deficits in nigrostriatal DA neurotransmission (Kitada et al., 2007). Both parkin and PINK1 knockouts also have mild mitochondrial defects (Gautier et al., 2008; Palacino et al., 2004).
As noted above the PINK1 drosophila mutants share marked phenotypic similarities with parkin mutant flies (Clark et al., 2006; Park et al., 2006). Transgenic expression of parkin ameliorated PINK1 loss of function phenotypes, whereas transgenic expression of PINK1 had no effect on parkin loss of function phenotypes (Clark et al., 2006; Park et al., 2006). Thus, parkin and PINK1 function in a common pathway with PINK1 functioning upstream from parkin. Additional studies suggest that parkin and PINK1 regulate mitochondrial trafficking and/or autophagy (Deng et al., 2008; Narendra et al., 2010; Poole et al., 2008; Vives-Bauza et al., 2010; Yang et al., 2008). It is likely that there is a common PINK1 and parkin substrate that is positioned between PINK1 and parkin that is important for mitochondrial function (Figure 2). Studies focused on the interplay between PINK1 and parkin and the identification of a common intermediary between PINK1 and parkin have tremendous promise to advance the understanding of how mutations in these genes cause PD.
DJ-1 is a member of the ThiJ/PfpI family of molecular chaperones (Gasser, 2009; Moore et al., 2006), and mutations in DJ-1 play a small, but important role in early-onset Parkinsonism (Bonifati et al., 2003). Penetrance appears to be complete in individuals who have two disease causing mutations in DJ-1 (Heutink, 2006). PD associated mutations in DJ-1 produce loss of function of DJ-1 by instability leading to defective dimer formation or lack of expression (Macedo et al., 2003; Moore et al., 2003). DJ-1 is normally expressed widely throughout the body and is subcellularly localized to the cytosol, mitochondrial matrix and inter-membrane space (Zhang et al., 2005). DJ-1 is a redox sensitive molecular chaperone with a variety of diverse functions (Cookson, 2005; Kahle et al., 2009; Moore et al., 2006). In cellular models it regulates redox dependent kinase signaling pathways and acts a regulator of antioxidant gene expression (for review see (Kahle et al., 2009)). DJ-1 functions in vivo as an atypical peroxiredoxin-like peroxidase where it protects against oxidative stress in mitochondria (Figure 2) (Andres-Mateos et al., 2007). DJ-1 also functions as redox-sensitive RNA binding protein(van der Brug et al., 2008). Consistent with its role as a chaperone, DJ-1 is thought to have a variety of other pleiotropic functions (for review see (Cookson, 2005; Kahle et al., 2009; Moore et al., 2006)).
In contrast to mammalian species, which contain a single DJ-1 gene, drosophila possess two orthologs of DJ-1, DJ-1a and DJ-b. Many different DJ-1 drosophila models have been generated to explore the contribution of DJ-1 dysfunction in the pathogenesis of PD (Menzies et al., 2005; Meulener et al., 2005; Park et al., 2005; Yang et al., 2005). DJ-1 double null flies for both DJ-1 homologs, DJ-1a and DJ-1b are viable, fertile, and have normal number of DA neurons and lifespan. Acute knockdown of DJ-1 by transgenic RNAi led to a robust neurodegeneration and selective transgenic targeting of DJ-1 RNAi to DA neurons led to age-dependent loss of DA neurons in the dorsomedial cluster (Table 2) (Yang et al., 2005). Similar to parkin and PINK1 knockout mice, DJ-1 knockout mice do not exhibit any major abnormality (Table 2) (Andres-Mateos et al., 2007; Chen and Feany, 2005; Goldberg et al., 2005; Kim et al., 2005). In particular, the number of DA neurons, the level of striatal DA, and the level of DA receptors are unchanged. Abnormalities in dopamine neurotransmission in the nigrostriatal circuit and mitochondrial dysfunction was observed in some DJ-1 knockout animals, similar to parkin and PINK1 knockouts (Andres-Mateos et al., 2007; Goldberg et al., 2005; Kim et al., 2005).
Since the current parkin, PINK1 or DJ-1 knockout mice models do not exhibit nigrostriatal pathology, they at the very best only represent models of the earliest changes that might occur due to parkin, PINK1 or DJ-1 deficiency. The utility of these current autosomal recessive models is how parkin, PINK1 or DJ-1 deficiency lead to early dysfunction of the nigrostriatal DA system. These models cannot be used to test neuroprotective agents since there is no neurodegeneration. Optimization of these models may be possible through conditional approaches as discussed below. The study of transgenic mice overexpressing mutant human parkin maybe an attractive alternative to parkin deficiency models for understanding neurodegeneration and therapeutic studies (Lu et al., 2009; Sang et al., 2007; Wang et al., 2007). Although the drosophila loss-of-function models of PINK1 and parkin have dramatic phenotypes, the major abnormalities reside outside the nervous system. Moreover, the DA system is only mildly affected. The only abnormalities that humans with PD share with the drosophila PINK1 and parkin loss-of-function models is the loss of DA neurons, thus these models are imperfect at best. Most of the DJ-1 drosophila deficiency models are limited as well. Thus, the utility of studying mechanisms of DA neurodegeneration in these models is constrained. Their value mainly lies in identifying genetic and pharmacologic modifiers of parkin, PINK1 or DJ-1 deficiency-induced neurodegeneration. The parkin and PINK1 deficiency models offer the ability to identify potentially evolutionarily conserved regulators of mitochondrial function. The challenge is to verify that potential modifiers uncovered in these simple model systems are evolutionarily conserved in human PD.
Why Don’t DA Neurons Degenerate in Mouse Models of PD?
Since, mutations in α-synuclein are 100% penetrant and penetrance appears to be complete in individuals who have two disease causing mutations in parkin, PINK1 or DJ-1, it is puzzling why there is no meaningful neurodegeneration of DA neurons in mouse models of PD. Attempts to generate α-synuclein transgenic mice with progressive loss of DA neurons using a variety of approaches, including transgenic mice expressing C-terminal truncated, aggregation prone, α-synuclein (Daher et al., 2009; Tofaris and Spillantini, 2005; Wakamatsu et al., 2008), conditional expression of α-synuclein (Lin et al., 2009; Nuber et al., 2008), crossing α-synuclein transgenics to parkin knockouts (von Coelln et al., 2006) and DJ-1 knockouts (Ramsey et al., 2010) and overexpressing α-synuclein with DA specific promoters have not been very fruitful (Daher et al., 2009; Matsuoka et al., 2001; Richfield et al., 2002; Thiruchelvam et al., 2004). Triple knockout of parkin, PINK1 and DJ-1 have minimal effects on DA neurons similar to the single knockout of each gene (Kitada et al., 2009). Perhaps mouse DA neurons are particularly resistant to overexpression of α-synuclein and LRRK2 and the absence of parkin, PINK1 or DJ-1 due to the expression of intrinsic protective factors or other genetic modifications that render them resistant to toxic properties of these mutations. Different mice strains do have different susceptibilities to the MPTP intoxication model of PD (Hamre et al., 1999). Thus, it is possible that the lack of degeneration of DA neurons in these different mouse models may be due to common intrinsic genetic factors in the different mouse strains utilized in these experiments. Future studies could focus on changing the genetic background of these models in an attempt to generate models with loss of DA neurons.
Genetic background may not be the sole factor accounting for the resistance of DA neurons to degeneration. For instance, overexpression of α-synuclein via viruses is capable of eliciting neurodegeneration of DA neurons (Table 1) (Kirik et al., 2003; Lo Bianco et al., 2002). What might account for the sensitivity of DA neurons to viral overexpression of α-synuclein, but resistance of DA neurons to overexpression of α-synuclein using transgenic approaches? All the promoters used to drive expression of α-synuclein primarily drive expression in neurons, with very little expression in glia (Chesselet, 2008; Chesselet et al., 2008). Adeno-associated virus, which was used in the α-synuclein viral models drives expression in both neurons and glia (Kirik et al., 2003; Lo Bianco et al., 2002). Thus, it is possible that α-synuclein may need to be expressed in both neurons and glia to elicit neurodegeneration of DA neurons, with both non-cell-autonomous and cell-autonomous processes contributing to the demise of DA neurons. Consistent with this notion is that non-cell-autonomous processes contribute to the degeneration of DA neurons in the MPTP-intoxication model through microglial derived neurotoxins (Liberatore et al., 1999). Interestingly, fetal mesencephalic DA transplants into patients with PD developed α-synuclein positive Lewy body like inclusions strongly suggesting that PD pathology was somehow transferred from the host to the transplant tissue (Kordower et al., 2008; Li et al., 2008). Whether this occurs through direct transfer of α-synuclein from affected to neighboring neurons through endocytosis (Desplats et al., 2009) or it occurs in response the surrounding activated glia require further study. However, only the transplants that had extensive microglial infiltration exhibited pathology suggesting that non-cell-autonomous processes were contributing to the pathology in the transplanted neurons (Dawson, 2008; Mendez et al., 2008). Microglia produce reactive oxygen and nitric oxide species, which are known to accelerate α-synuclein aggregation, thus activated microglia may contribute to disease progression by setting in motion a self-perpetuating cycle of α-synuclein aggregation followed by cell injury.
Since there is incomplete penetrance of LRRK2 disease causing mutations in humans the lack of substantial DA neurodegeneration in the LRRK2 mouse models, maybe due to genetic factors. Alternatively environmental factors may contribute to LRRK2-induced PD. Consistent with this notion is the observation that toxins such as the mitochondrial complex I inhibitors exacerbate neurodegeneration in drosophila and C. elegans models of LRRK2-PD (Figure 2) (Ng et al., 2009; Saha et al., 2009). Although mutations in α-synuclein are 100% penetrant and penetrance appears to be complete in individuals who have two disease causing mutations in parkin, PINK1 or DJ-1, environmental stressors may also play a role by accelerating the disease process. In addition, environmental stressors may contribute to PD in patients with polymorphisms that increase the level of α-synuclein and in patients with heterozygous mutation in parkin, PINK1 or DJ-1. Some transgenic α-synuclein mice and PINK1 or DJ-1 deficient mice DA neurons are more susceptible to the toxic effects of the DA neurotoxin, MPTP (Haque et al., 2008; Kim et al., 2005; Song et al., 2004). Interestingly parkin knockouts are equally sensitive compared to littermate controls to MPTP (Thomas et al., 2007). Exploration of different PD associated environmental stressors in the different genetic PD mouse models could provide insight into the interrelationship of genetics and environmental stressors and provide models with degeneration of DA neurons.
Arguments have been made that perhaps the reason DA neurons don’t degenerate in the various vertebrate models is that there are compensatory mechanisms that prevent the loss of DA neurons during the lifespan of the mouse. Some investigators have conceptual problems with the compensation idea. The argument is raised, why don’t humans have the same compensatory processes? Compensation in PD is a well-recognized phenomenon, as well as in most neurodegenerative disorders (for review see (Bezard et al., 2003; Palop et al., 2006)). Indeed the symptoms of PD in humans only manifest after there is substantial degeneration of DA neurons (Bezard et al., 2003). Both DA and non-DA mediated mechanisms are thought to contribute to the substantiative presymptomatic phase of PD. Perhaps one of the reasons that PD is a late adult neurodegenerative disorder is that compensatory mechanisms are active and it is the ultimate failure of these compensatory mechanisms that eventually leads to neurodegeneration and the signs and symptoms of PD. Compensation does seem to occur in mouse models of PD. For instance glutathione levels are increased in parkin knockouts (Itier et al., 2003) and mitochondrial glutathione peroxidase is increased in DJ-1 knockouts (Andres-Mateos et al., 2007) and there are substantial gene expression profile changes in α-synuclein transgenic mice. Adaptive protection is likely to begin at conception with induction of protective genes and genes with redundant function. Identifying, understanding and potentially harnessing these compensatory mechanisms is important as they may provide potential biomarkers and novel therapeutic opportunities (Palop et al., 2006).
Bypassing compensatory mechanisms by conditional overexpression or conditional knockout might provide an opportunity to create models of PD where degeneration of DA neurons occur in the lifespan of a mouse (Figure 3). Since the mouse only lives up to 2 years and it takes six to seven decades to develop PD, strategies that accelerate the phenotype are required. As mentioned above, simple overexpression of α-synuclein via viruses is capable of eliciting neurodegeneration of DA neurons (Kirik et al., 2003; Lo Bianco et al., 2002). Although viral induced overexpression of α-synuclein may induce both cell autonomous and non-cell autonomous processes, bypassing compensatory mechanisms needs to be considered as well. Almost all transgenic approaches utilized to date drive expression early in development and all knockouts are germline deletions, thus it is conceivable that the DA system compensates when genes are overexpressed or knocked out embryonically rendering DA neurons resistant to the toxic effects of overexpression of α-synuclein or LRRK2 or the absence of parkin, PINK1 or DJ-1 (Figure 3). It has been known for at least 40 years that the DA system in neonates versus adults is differentially vulnerable to the toxic effects of the neurotoxin 6-hydroxydopamine (6-OHDA) (for review see (Joyce et al., 1996)). In adult rats, 6-OHDA causes profound abnormalities including sensory neglect, aphagia, akinesia and adipsia, whereas 6-OHDA lesions in neonates induces minimal behavioral deficits. Thus, DA denervation in neonates produces long-lasting modifications in the DA system. Similar or related mechanisms accounting for this well-known differential sensitivity of the neonatal versus the adult DA system, might account for resistance of DA neurons to toxic effects of PD mutations.
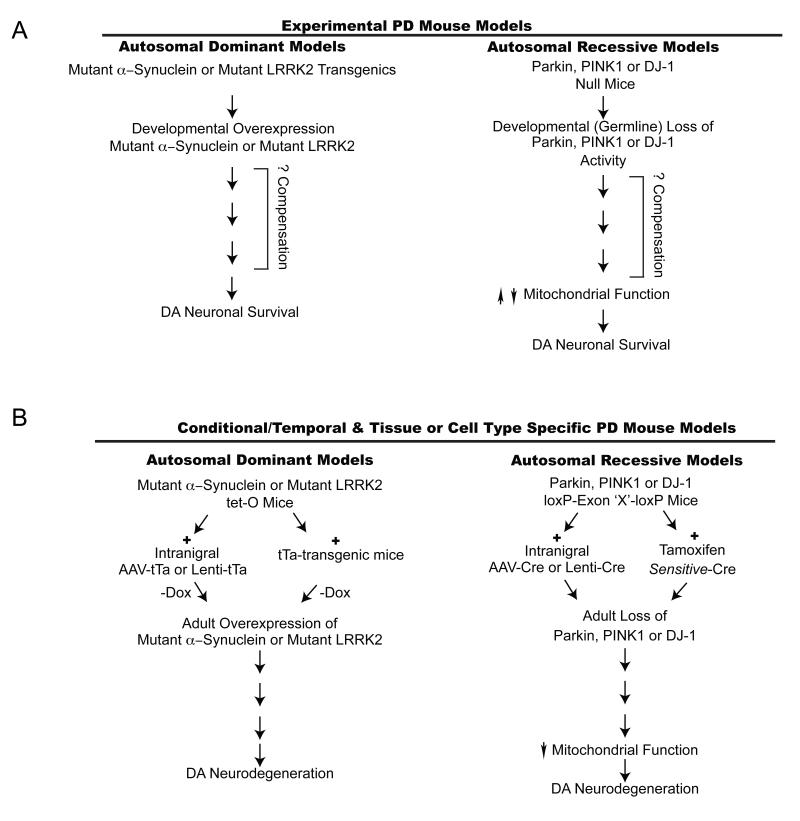
Figure 3. Current and Future Experimental PD Mouse Models.
A. Both mutant α-synuclein and mutant LRRK2 transgenic mice do not have loss of DA neurons. One possible explanation is that the transgenes are expressed early in development leading to compensatory mechanisms that prevent degeneration of DA neurons. In a similar manner, parkin, PINK1 and DJ-1 are knocked out in the germline and there may be compensatory mechanisms that also prevent degeneration of DA neurons and provide an environment that promotes DA neuronal survival (see text).
B. Strategies to potentially circumvent the compensatory mechanisms that possibly account for the survival of DA neurons in both the autosomal dominant and autosomal recessive models. For the autosomal dominant models LRRK2 and α-synuclein transgenic mice under the transcriptional control of the tetracycline operator (tetO) could be bred to different tetracycline transactivator (tTA) mice to drive expression in different neuronal populations and/or glia. Expression could be turned on in adults to avoid developmental compensation. For autosomal recessive models parkin, PINK1 or DJ-1, Cre-Lox recombination could be used to avoid developmental compensation, which may mask the true pathophysiologic action of parkin, PINK1 or DJ-1. Adeno associated virus (AAV) or lentivirus could be used to deliver Cre in adulthood and to specific neuronal populations and Tamoxifen sensitive-Cre mice could drive expression in different neuronal populations and/or glia. Expression could be turned on in adults to avoid developmental compensation.
The case for developmental plasticity of the DA system is further illustrated through generation of glial cell–line derived neurotrophic factor (GDNF) knockout mice (Pascual et al., 2008). Embryonic deletion of GDNF and its receptors has no effect on DA neurons during development or in the adult (Jain et al., 2006; Moore et al., 1996). Suppressing GDNF expression in adulthood via a conditional approach using a tamoxifen (TMX) sensitive Cre-Lox recombination, hence avoiding developmental compensation, “which masks the true physiologic action of GDNF”, leads to profound degeneration of catecholaminergic neurons (Pascual et al., 2008). In a similar manner, transgenic or knockout strategies focused on turning on transgene expression or knocking out genes in adults might avoid the developmental compensation and be very revealing (Figure 3).
Due to the success of the adult conditional knockout of GDNF and the “plasticity” of the nigrostriatal DA system, we believe that the investment in conditional approaches that enable deletion or expression in adult animals will have a high probability of success and will be very rewarding. Different TMX-sensitive Cre drivers could be used to knockout parkin, PINK1 or DJ-1 in different neuronal populations and/or glia in adult animals, thus avoiding developmental compensation. In a similar manner, LRRK2 and α-synuclein transgenic mice under the transcriptional control of the tetracycline operator (tetO) could be bred to different tetracycline transactivator (tTA) mice to drive expression in different neuronal populations and/or glia. Expression could be turned on in adults to avoid developmental compensation. If these models are successful as defined by progressive degeneration of DA neurons questions regarding selective vulnerability and cell-autonomous and non-cell autonomous mechanisms as well as the role of molecular mechanisms of compensation could begin to be answered by direct comparison between the different models. Moreover, non-DA systems that are impaired in PD could be targeted and effectively studied.
Conclusions
Although we argue above that we believe it is important to develop models with loss of DA neurons, the field has been somewhat hindered by the notion that any useful model should have degeneration of DA system. It is important to realize that it may be difficult to fully recapitulate the key neuropathologic and clinical features of PD in a single model system, but collectively many of the existing models do exhibit key features of PD. Thus, combinatorial study of these different models may provide insight into PD pathogenesis.
The rodent 6-OHDA and the non-human primate MPTP intoxication models are currently the models of choice for symptomatic therapies since they have been quite predictive. Their utility as preclinical models for neuroprotection and regeneration have been uniformly negative, although there are clinical trials currently in progress, which may change this point of view. Due to their clinically proven predictive value for symptomatic therapies, the rodent 6-OHDA and the non-human primate MPTP intoxication models will probably remain the models of choice for symptomatic therapies even when we have robust genetic models with degeneration of the nigrostriatal DA system.
The study of current parkin, PINK1 and DJ-1 knockouts may be useful to understand the earliest abnormalities in nigrostriatal DA system that occur due to these mutations. They might also be particularly useful for understanding the molecular mechanisms underlying compensation. In a similar manner, current LRRK2 transgenic models may be most useful for studying early derangements in the nigrostriatal DA system. Some transgenic α-synuclein animal models also have progressive sensorimotor anomalies that are due to dopaminergic dysfunction (Chesselet et al., 2008). Thus, many of the models are most useful for understanding processes that precede degeneration. For instance, reduction of neurotransmitter release by inhibition of synaptic vesicle reclustering after endocytosis maybe the earliest effect of increased α-synuclein expression in PD and in transgenic α-synuclein models (Nemani et al., 2010). Understanding these early processes may serve as biomarkers or targets for therapeutic intervention.
Unfortunately none of the existing vertebrate models are particularly useful for investigating the mechanisms of selective neuronal vulnerability, but as noted above conditional approaches if successful could be particularly revealing. The drosophila and C. elegans models that have degeneration of DA neurons have not been used to understand selective neuronal vulnerability. They have also failed to provide extensive phenotypic information about affected dopaminergic neurons and there has been poor characterization of the effects in non-dopaminergic neurons. Moreover, these models have not been use to study cell autonomous versus non-cell autonomous neurodegeneration. The mouse α-synuclein transgenic models with degeneration are valuable tools to study general toxicity and mechanisms of α-synuclein pathology as well as testing therapeutic strategies. Unfortunately there has been only a paucity of neuroprotection trials in the α-synuclein mice, which is difficult to understand. These studies confirm that maintaining or converting α-synuclein into its monomeric state and enhancing its degradation are therapeutic (for review see (Gupta et al., 2008)). The majority of the α-synuclein transgenic models are also useful for understanding mechanisms of degeneration in non-DA systems. The drosophila and C. elegans models are teaching us about the pathogenesis of DA neurodegeneration in flies and worms, respectively. The challenge remains to validate potential neuroprotective agents and genetic modifiers identified in these model systems in human PD.
Only a few studies have focused on assays to study non-DA systems that are affected in PD. However, due the relative lack of alterations in the nigrostriatal system, studies should be focused on non-DA neuronal dysfunction. Expanding the repertoire of investigations in PD models might be particularly revealing. For instance some of the earliest changes in PD are olfactory dysfunction, sleep disturbances and cardiac sympathetic denervation (Langston, 2006). PD patients also suffer from constipation, anxiety, depression and cognitive impairments. Thus, tests of olfaction, sleep monitoring, behavioral assessments focused on anxiety, depression and memory and assessing autonomic function in the existing different models would be important. The study of these non-motor manifestations in animal models might be rewarding, particularly if disturbances manifest early in the life of a mouse and if they were progressive. Some of the α-synuclein transgenic mice have olfactory impairments and colonic dysfunction and it likely that there are other unrecognized abnormalities (Fleming et al., 2008; Wang et al., 2008b). Understanding and study of potential abnormalities in these non-motor systems could offer new models for testing neuroprotective agents as well as provide new models for testing symptomatic therapies focused on the non-motor manifestations of PD.
ACKNOWLEDGEMENTS
This work was supported by the Morris K. Udall Parkinson’s Disease Research Center of Excellence NIH/NINDS (NS38377) and NS04826. T.M.D. is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases. Dr. T.M. Dawson is a paid consultant to Merck KGAA. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. We would like to thank Yunjong Lee and Mingyao Ying for critical comments and insights. Due to the nature of this review, we apologize to all the authors we were not able to cite regarding animal and cellular models of PD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Albrecht M. LRRK2 mutations and Parkinsonism. Lancet. 2005;365:1230. doi: 10.1016/S0140-6736(05)74810-8. [DOI] [PubMed] [Google Scholar]
- Andres-Mateos E, Mejias R, Sasaki M, Li X, Lin BM, Biskup S, Zhang L, Banerjee R, Thomas B, Yang L, et al. Unexpected lack of hypersensitivity in LRRK2 knock-out mice to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) J Neurosci. 2009;29:15846–15850. doi: 10.1523/JNEUROSCI.4357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, et al. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci U S A. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R, Starkov AA, Beal MF, Thomas B. Mitochondrial dysfunction in the limelight of Parkinson’s disease pathogenesis. Biochim Biophys Acta. 2009;1792:651–663. doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford L, Hay D, Paine S, Rezvani N, Mee M, Lowe J, Mayer RJ. Is malfunction of the ubiquitin proteasome system the primary cause of alpha-synucleinopathies and other chronic human neurodegenerative disease? Biochim Biophys Acta. 2008;1782:683–690. doi: 10.1016/j.bbadis.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Betts-Henderson J, Jaros E, Krishnan KJ, Perry RH, Reeve AK, Schaefer AM, Taylor RW, Turnbull DM. Alpha-synuclein pathology and Parkinsonism associated with POLG1 mutations and multiple mitochondrial DNA deletions. Neuropathol Appl Neurobiol. 2009;35:120–124. doi: 10.1111/j.1365-2990.2008.00981.x. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gross CE, Brotchie JM. Presymptomatic compensation in Parkinson’s disease is not dopamine-mediated. Trends Neurosci. 2003;26:215–221. doi: 10.1016/S0166-2236(03)00038-9. [DOI] [PubMed] [Google Scholar]
- Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, et al. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- Biskup S, West AB. Zeroing in on LRRK2-linked pathogenic mechanisms in Parkinson’s disease. Biochim Biophys Acta. 2009;1792:625–633. doi: 10.1016/j.bbadis.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson’s disease. NeuroRx. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord. 2006;21:2042–2051. doi: 10.1002/mds.21065. [DOI] [PubMed] [Google Scholar]
- Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, Hammer RE, Battaglia G, German DC, Castillo PE, et al. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci U S A. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- Chesselet MF. In vivo alpha-synuclein overexpression in rodents: a useful model of Parkinson’s disease? Exp Neurol. 2008;209:22–27. doi: 10.1016/j.expneurol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet MF, Fleming S, Mortazavi F, Meurers B. Strengths and limitations of genetic mouse models of Parkinson’s disease. Parkinsonism Relat Disord. 2008;14(Suppl 2):S84–87. doi: 10.1016/j.parkreldis.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin’s protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Clark LN, Kartsaklis LA, Gilbert R. Wolf, Dorado B, Ross BM, Kisselev S, Verbitsky M, Mejia-Santana H, Cote LJ, Andrews H, et al. Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch Neurol. 2009;66:578–583. doi: 10.1001/archneurol.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- Cookson MR. The biochemistry of Parkinson’s disease. Annu Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Daher JP, Ying M, Banerjee R, McDonald RS, Hahn MD, Yang L, Beal M. Flint, Thomas B, Dawson VL, Dawson TM, et al. Conditional transgenic mice expressing C-terminally truncated human alpha-synuclein (alphaSyn119) exhibit reduced striatal dopamine without loss of nigrostriatal pathway dopaminergic neurons. Mol Neurodegener. 2009;4:34. doi: 10.1186/1750-1326-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Dawson T, Mandir A, Lee M. Animal models of PD: pieces of the same puzzle? Neuron. 2002;35:219–222. doi: 10.1016/s0896-6273(02)00780-8. [DOI] [PubMed] [Google Scholar]
- Dawson TM. Non-autonomous cell death in Parkinson’s disease. Lancet Neurol. 2008;7:474–475. doi: 10.1016/S1474-4422(08)70099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. The role of parkin in familial and sporadic Parkinson’s disease. Mov Disord. 2009 doi: 10.1002/mds.22798. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, et al. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A. 2007;104:1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Tetreault NA, Mulligan CK, Hutson CB, Masliah E, Chesselet MF. Olfactory deficits in mice overexpressing human wildtype alpha-synuclein. Eur J Neurosci. 2008;28:247–256. doi: 10.1111/j.1460-9568.2008.06346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman MS, Lee VM, Trojanowski JQ. Nosology of Parkinson’s disease: looking for the way out of a quagmire. Neuron. 2005;47:479–482. doi: 10.1016/j.neuron.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Freichel C, Neumann M, Ballard T, Muller V, Woolley M, Ozmen L, Borroni E, Kretzschmar HA, Haass C, Spooren W, et al. Age-dependent cognitive decline and amygdala pathology in alpha-synuclein transgenic mice. Neurobiol Aging. 2007;28:1421–1435. doi: 10.1016/j.neurobiolaging.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, et al. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33:627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser T. Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert Rev Mol Med. 2009;11:e22. doi: 10.1017/S1462399409001148. [DOI] [PubMed] [Google Scholar]
- Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci U S A. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Gispert S, Ricciardi F, Kurz A, Azizov M, Hoepken HH, Becker D, Voos W, Leuner K, Muller WE, Kudin AP, et al. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS One. 2009;4:e5777. doi: 10.1371/journal.pone.0005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet JC, McCaffery JM, et al. The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci U S A. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, Tong Y, Martella G, Tscherter A, Martins A, et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug MP, Beilina A, Blackinton J, Thomas KJ, et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Gupta A, Dawson VL, Dawson TM. What causes cell death in Parkinson’s disease? Ann Neurol. 2008;64(Suppl 2):S3–15. doi: 10.1002/ana.21573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hamre K, Tharp R, Poon K, Xiong X, Smeyne RJ. Differential strain susceptibility following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration acts in an autosomal dominant fashion: quantitative analysis in seven strains of Mus musculus. Brain Res. 1999;828:91–103. doi: 10.1016/s0006-8993(99)01273-1. [DOI] [PubMed] [Google Scholar]
- Haque ME, Thomas KJ, D’Souza C, Callaghan S, Kitada T, Slack RS, Fraser P, Cookson MR, Tandon A, Park DS. Cytoplasmic Pink1 activity protects neurons from dopaminergic neurotoxin MPTP. Proc Natl Acad Sci U S A. 2008;105:1716–1721. doi: 10.1073/pnas.0705363105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heutink P. PINK-1 and DJ-1--new genes for autosomal recessive Parkinson’s disease. J Neural Transm Suppl. 2006:215–219. doi: 10.1007/978-3-211-45295-0_33. [DOI] [PubMed] [Google Scholar]
- Imai Y, Gehrke S, Wang HQ, Takahashi R, Hasegawa K, Oota E, Lu B. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. Embo J. 2008;27:2432–2443. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier JM, Ibanez P, Mena MA, Abbas N, Cohen-Salmon C, Bohme GA, Laville M, Pratt J, Corti O, Pradier L, et al. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet. 2003;12:2277–2291. doi: 10.1093/hmg/ddg239. [DOI] [PubMed] [Google Scholar]
- Jain S, Golden JP, Wozniak D, Pehek E, Johnson EM, Jr., Milbrandt J. RET is dispensable for maintenance of midbrain dopaminergic neurons in adult mice. J Neurosci. 2006;26:11230–11238. doi: 10.1523/JNEUROSCI.1876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA. A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders. Biochim Biophys Acta. 2009;1792:730–740. doi: 10.1016/j.bbadis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Frohna PA, Neal-Beliveau BS. Functional and molecular differentiation of the dopamine system induced by neonatal denervation. Neurosci Biobehav Rev. 1996;20:453–486. doi: 10.1016/0149-7634(95)00025-9. [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Waak J, Gasser T. DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic Biol Med. 2009;47:1354–1361. doi: 10.1016/j.freeradbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Annett LE, Burger C, Muzyczka N, Mandel RJ, Bjorklund A. Nigrostriatal alpha-synucleinopathy induced by viral vector-mediated overexpression of human alpha-synuclein: a new primate model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2003;100:2884–2889. doi: 10.1073/pnas.0536383100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Tong Y, Gautier CA, Shen J. Absence of Nigral Degeneration in Aged Parkin/DJ-1/PINK1 Triple Knockout Mice. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klivenyi P, Siwek D, Gardian G, Yang L, Starkov A, Cleren C, Ferrante RJ, Kowall NW, Abeliovich A, Beal MF. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis. 2006;21:541–548. doi: 10.1016/j.nbd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Kokjohn TA, Roher AE. Amyloid precursor protein transgenic mouse models and Alzheimer’s disease: understanding the paradigms, limitations, and contributions. Alzheimers Dement. 2009;5:340–347. doi: 10.1016/j.jalz.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Kominami E, Tanaka K. Autophagy and neurodegeneration. Autophagy. 2006;2:315–317. doi: 10.4161/auto.2974. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Kuwahara T, Koyama A, Gengyo-Ando K, Masuda M, Kowa H, Tsunoda M, Mitani S, Iwatsubo T. Familial Parkinson mutant alpha-synuclein causes dopamine neuron dysfunction in transgenic Caenorhabditis elegans. J Biol Chem. 2006;281:334–340. doi: 10.1074/jbc.M504860200. [DOI] [PubMed] [Google Scholar]
- Lakso M, Vartiainen S, Moilanen AM, Sirvio J, Thomas JH, Nass R, Blakely RD, Wong G. Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. J Neurochem. 2003;86:165–172. doi: 10.1046/j.1471-4159.2003.01809.x. [DOI] [PubMed] [Google Scholar]
- Langston JW. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ. Dopamine covalently modifies and functionally inactivates parkin. Nat Med. 2005;11:1214–1221. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Kim W, Lee S, Chung J. Loss of LRRK2/PARK8 induces degeneration of dopaminergic neurons in Drosophila. Biochem Biophys Res Commun. 2007;358:534–539. doi: 10.1016/j.bbrc.2007.04.156. [DOI] [PubMed] [Google Scholar]
- Lee VM, Trojanowski JQ. Mechanisms of Parkinson’s disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron. 2006;52:33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Li W, West N, Colla E, Pletnikova O, Troncoso JC, Marsh L, Dawson TM, Jakala P, Hartmann T, Price DL, et al. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. Proc Natl Acad Sci U S A. 2005;102:2162–2167. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Patel JC, Wang J, Avshalumov MV, Nicholson C, Buxbaum JD, Elder GA, Rice ME, Yue Z. Enhanced Striatal Dopamine Transmission and Motor Performance with LRRK2 Overexpression in Mice Is Eliminated by Familial Parkinson’s Disease Mutation G2019S. J Neurosci. 2010;30:1788–1797. doi: 10.1523/JNEUROSCI.5604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V, Zhou C, Geghman K, Bogdanov M, Przedborski S, et al. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat Neurosci. 2009;12:826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Lim KL, Ng CH. Genetic models of Parkinson disease. Biochim Biophys Acta. 2009;1792:604–615. doi: 10.1016/j.bbadis.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Lin X, Parisiadou L, Gu XL, Wang L, Shim H, Sun L, Xie C, Long CX, Yang WJ, Ding J, et al. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant alpha-synuclein. Neuron. 2009;64:807–827. doi: 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang X, Yu Y, Li X, Wang T, Jiang H, Ren Q, Jiao Y, Sawa A, Moran T, et al. A Drosophila model for LRRK2-linked parkinsonism. Proc Natl Acad Sci U S A. 2008;105:2693–2698. doi: 10.1073/pnas.0708452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Bianco C, Ridet JL, Schneider BL, Deglon N, Aebischer P. alpha - Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2002;99:10813–10818. doi: 10.1073/pnas.152339799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XH, Fleming SM, Meurers B, Ackerson LC, Mortazavi F, Lo V, Hernandez D, Sulzer D, Jackson GR, Maidment NT, et al. Bacterial artificial chromosome transgenic mice expressing a truncated mutant parkin exhibit age-dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase K-resistant alpha-synuclein. J Neurosci. 2009;29:1962–1976. doi: 10.1523/JNEUROSCI.5351-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucking CB, Durr A, Bonifati V, Vaughan J, De Michele G, Gasser T, Harhangi BS, Meco G, Denefle P, Wood NW, et al. Association between early-onset Parkinson’s disease and mutations in the parkin gene. N Engl J Med. 2000;342:1560–1567. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- Macedo MG, Anar B, Bronner IF, Cannella M, Squitieri F, Bonifati V, Hoogeveen A, Heutink P, Rizzu P. The DJ-1L166P mutant protein associated with early onset Parkinson’s disease is unstable and forms higher-order protein complexes. Hum Mol Genet. 2003;12:2807–2816. doi: 10.1093/hmg/ddg304. [DOI] [PubMed] [Google Scholar]
- MacLeod D, Dowman J, Hammond R, Leete T, Inoue K, Abeliovich A. The familial Parkinsonism gene LRRK2 regulates neurite process morphology. Neuron. 2006;52:587–593. doi: 10.1016/j.neuron.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Vila M, Lincoln S, McCormack A, Picciano M, LaFrancois J, Yu X, Dickson D, Langston WJ, McGowan E, et al. Lack of nigral pathology in transgenic mice expressing human alpha-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol Dis. 2001;8:535–539. doi: 10.1006/nbdi.2001.0392. [DOI] [PubMed] [Google Scholar]
- Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, et al. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies FM, Yenisetti SC, Min KT. Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr Biol. 2005;15:1578–1582. doi: 10.1016/j.cub.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Meulener M, Whitworth AJ, Armstrong-Gold CE, Rizzu P, Heutink P, Wes PD, Pallanck LJ, Bonini NM. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson’s disease. Curr Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Dawson VL, Dawson TM. Lessons from Drosophila models of DJ-1 deficiency. Sci Aging Knowledge Environ. 2006;2006:pe2. doi: 10.1126/sageke.2006.2.pe2. [DOI] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Zhang L, Dawson TM, Dawson VL. A missense mutation (L166P) in DJ-1, linked to familial Parkinson’s disease, confers reduced protein stability and impairs homo-oligomerization. J Neurochem. 2003;87:1558–1567. doi: 10.1111/j.1471-4159.2003.02265.x. [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. Increased Expression of alpha-Synuclein Reduces Neurotransmitter Release by Inhibiting Synaptic Vesicle Reclustering after Endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Kahle PJ, Giasson BI, Ozmen L, Borroni E, Spooren W, Muller V, Odoy S, Fujiwara H, Hasegawa M, et al. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J Clin Invest. 2002;110:1429–1439. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C-H, Mok SZS, Koh C, Ouyang X, Fivaz ML, Tan E-K, Dawson VL, Dawson TM, Yu F, Lim K-L. Parkin Protects Against LRRK2 G2019S Mutant-induced Dopaminergic Neurodegeneration in Drosophila. Journal of Neuroscience. 2009 doi: 10.1523/JNEUROSCI.2375-09.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris EH, Uryu K, Leight S, Giasson BI, Trojanowski JQ, Lee VM. Pesticide exposure exacerbates alpha-synucleinopathy in an A53T transgenic mouse model. Am J Pathol. 2007;170:658–666. doi: 10.2353/ajpath.2007.060359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuber S, Petrasch-Parwez E, Winner B, Winkler J, von Horsten S, Schmidt T, Boy J, Kuhn M, Nguyen HP, Teismann P, et al. Neurodegeneration and motor dysfunction in a conditional model of Parkinson’s disease. J Neurosci. 2008;28:2471–2484. doi: 10.1523/JNEUROSCI.3040-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, de Munain A. Lopez, Aparicio S, Gil AM, Khan N, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- Pallanck LJ, Whitworth AJ. Drosophila Models of Parkinson’s Disease. In: Dawson TM, editor. Parkinson’s Disease: Genetics and Pathogenesis. Informa Healthcare USA, Inc.; New York, NY: 2007. pp. 287–310. [Google Scholar]
- Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–773. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- Park J, Kim SY, Cha GH, Lee SB, Kim S, Chung J. Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene. 2005;361:133–139. doi: 10.1016/j.gene.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci U S A. 2005;102:2174–2179. doi: 10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periquet M, Fulga T, Myllykangas L, Schlossmacher MG, Feany MB. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007;27:3338–3346. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesah Y, Burgess H, Middlebrooks B, Ronningen K, Prosser J, Tirunagaru V, Zysk J, Mardon G. Whole-mount analysis reveals normal numbers of dopaminergic neurons following misexpression of alpha-Synuclein in Drosophila. Genesis. 2005;41:154–159. doi: 10.1002/gene.20106. [DOI] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- Ramsey CP, Tsika E, Ischiropoulos H, Giasson BI. DJ-1 deficient mice demonstrate similar vulnerability to pathogenic Ala53Thr human {alpha}-syn toxicity. Hum Mol Genet. 2010 doi: 10.1093/hmg/ddq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richfield EK, Thiruchelvam MJ, Cory-Slechta DA, Wuertzer C, Gainetdinov RR, Caron MG, Di Monte DA, Federoff HJ. Behavioral and neurochemical effects of wild-type and mutated human alpha-synuclein in transgenic mice. Exp Neurol. 2002;175:35–48. doi: 10.1006/exnr.2002.7882. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Ross OA, Toft M, Whittle AJ, Johnson JL, Papapetropoulos S, Mash DC, Litvan I, Gordon MF, Wszolek ZK, Farrer MJ, et al. Lrrk2 and Lewy body disease. Ann Neurol. 2006;59:388–393. doi: 10.1002/ana.20731. [DOI] [PubMed] [Google Scholar]
- Saha S, Guillily MD, Ferree A, Lanceta J, Chan D, Ghosh J, Hsu CH, Segal L, Raghavan K, Matsumoto K, et al. LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J Neurosci. 2009;29:9210–9218. doi: 10.1523/JNEUROSCI.2281-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi-Nakashima A, Meir JY, Jin Y, Matsumoto K, Hisamoto N. LRK-1, a C. elegans PARK8-related kinase, regulates axonal-dendritic polarity of SV proteins. Curr Biol. 2007;17:592–598. doi: 10.1016/j.cub.2007.01.074. [DOI] [PubMed] [Google Scholar]
- Sang TK, Chang HY, Lawless GM, Ratnaparkhi A, Mee L, Ackerson LC, Maidment NT, Krantz DE, Jackson GR. A Drosophila model of mutant human parkin-induced toxicity demonstrates selective loss of dopaminergic neurons and dependence on cellular dopamine. J Neurosci. 2007;27:981–992. doi: 10.1523/JNEUROSCI.4810-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitt JM, Dawson VL, Dawson TM. Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest. 2006;116:1744–1754. doi: 10.1172/JCI29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- Silvestri L, Caputo V, Bellacchio E, Atorino L, Dallapiccola B, Valente EM, Casari G. Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet. 2005;14:3477–3492. doi: 10.1093/hmg/ddi377. [DOI] [PubMed] [Google Scholar]
- Singleton AB. Altered alpha-synuclein homeostasis causing Parkinson’s disease: the potential roles of dardarin. Trends Neurosci. 2005;28:416–421. doi: 10.1016/j.tins.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9:1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- Song DD, Shults CW, Sisk A, Rockenstein E, Masliah E. Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Exp Neurol. 2004;186:158–172. doi: 10.1016/S0014-4886(03)00342-X. [DOI] [PubMed] [Google Scholar]
- Sutherland GT, Halliday GM, Silburn PA, Mastaglia FL, Rowe DB, Boyle RS, O’Sullivan JD, Ly T, Wilton SD, Mellick GD. Do polymorphisms in the familial Parkinsonism genes contribute to risk for sporadic Parkinson’s disease? Mov Disord. 2009;24:833–838. doi: 10.1002/mds.22214. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Suzuki T, Chiba T, Shimura H, Hattori N, Mizuno Y. Parkin is linked to the ubiquitin pathway. J Mol Med. 2001;79:482–494. doi: 10.1007/s001090100242. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam MJ, Powers JM, Cory-Slechta DA, Richfield EK. Risk factors for dopaminergic neuron loss in human alpha-synuclein transgenic mice. Eur J Neurosci. 2004;19:845–854. doi: 10.1111/j.0953-816x.2004.03139.x. [DOI] [PubMed] [Google Scholar]
- Thomas B, von Coelln R, Mandir AS, Trinkaus DB, Farah MH, Lim K. Leong, Calingasan NY, Beal M. Flint, Dawson VL, Dawson TM. MPTP and DSP-4 susceptibility of substantia nigra and locus coeruleus catecholaminergic neurons in mice is independent of parkin activity. Neurobiol Dis. 2007;26:312–322. doi: 10.1016/j.nbd.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofaris GK, Spillantini MG. Alpha-synuclein dysfunction in Lewy body diseases. Mov Disord. 2005;20(Suppl 12):S37–44. doi: 10.1002/mds.20538. [DOI] [PubMed] [Google Scholar]
- Tong Y, Pisani A, Martella G, Karouani M, Yamaguchi H, Pothos EN, Shen J. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci U S A. 2009;106:14622–14627. doi: 10.1073/pnas.0906334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- van der Brug MP, Blackinton J, Chandran J, Hao LY, Lal A, Mazan-Mamczarz K, Martindale J, Xie C, Ahmad R, Thomas KJ, et al. RNA binding activity of the recessive parkinsonism protein DJ-1 supports involvement in multiple cellular pathways. Proc Natl Acad Sci U S A. 2008;105:10244–10249. doi: 10.1073/pnas.0708518105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Putten H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren WP, Ruegg MA, et al. Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 2000;20:6021–6029. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venderova K, Kabbach G, Abdel-Messih E, Zhang Y, Parks RJ, Imai Y, Gehrke S, Ngsee J, Lavoie MJ, Slack R, et al. Leucine-rich repeat kinase interacts with Parkin, DJ-1 and PINK-1 in a Drosophila melanogaster model of Parkinson’s disease. Hum Mol Genet. 2009 doi: 10.1093/hmg/ddp394. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Coelln R, Thomas B, Andrabi SA, Lim KL, Savitt JM, Saffary R, Stirling W, Bruno K, Hess EJ, Lee MK, et al. Inclusion body formation and neurodegeneration are parkin independent in a mouse model of alpha-synucleinopathy. J Neurosci. 2006;26:3685–3696. doi: 10.1523/JNEUROSCI.0414-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Coelln R, Thomas B, Savitt JM, Lim KL, Sasaki M, Hess EJ, Dawson VL, Dawson TM. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc Natl Acad Sci U S A. 2004;101:10744–10749. doi: 10.1073/pnas.0401297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamatsu M, Ishii A, Iwata S, Sakagami J, Ukai Y, Ono M, Kanbe D, Muramatsu S, Kobayashi K, Iwatsubo T, et al. Selective loss of nigral dopamine neurons induced by overexpression of truncated human alpha-synuclein in mice. Neurobiol Aging. 2008;29:574–585. doi: 10.1016/j.neurobiolaging.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Wang C, Lu R, Ouyang X, Ho MW, Chia W, Yu F, Lim KL. Drosophila overexpressing parkin R275W mutant exhibits dopaminergic neuron degeneration and mitochondrial abnormalities. J Neurosci. 2007;27:8563–8570. doi: 10.1523/JNEUROSCI.0218-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Tang B, Zhao G, Pan Q, Xia K, Bodmer R, Zhang Z. Dispensable role of Drosophila ortholog of LRRK2 kinase activity in survival of dopaminergic neurons. Mol Neurodegener. 2008a;3:3. doi: 10.1186/1750-1326-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Fleming SM, Chesselet MF, Tache Y. Abnormal colonic motility in mice overexpressing human wild-type alpha-synuclein. Neuroreport. 2008b;19:873–876. doi: 10.1097/WNR.0b013e3282ffda5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xie C, Greggio E, Parisiadou L, Shim H, Sun L, Chandran J, Lin X, Lai C, Yang WJ, et al. The chaperone activity of heat shock protein 90 is critical for maintaining the stability of leucine-rich repeat kinase 2. J Neurosci. 2008c;28:3384–3391. doi: 10.1523/JNEUROSCI.0185-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB, Dawson VL, Dawson TM. The Role of Parkin in Parkinson’s Disease. In: Dawson TM, editor. Parkinson’s Disease: Genetics and Pathogenesis. Informa Healthcare USA, Inc.; 2007a. pp. 199–218. [Google Scholar]
- West AB, Moore DJ, Choi C, Andrabi SA, Li X, Dikeman D, Biskup S, Zhang Z, Lim KL, Dawson VL, et al. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet. 2007b;16:223–232. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- Whitworth AJ, Theodore DA, Greene JC, Benes H, Wes PD, Pallanck LJ. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2005;102:8024–8029. doi: 10.1073/pnas.0501078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler S, Hagenah J, Lincoln S, Heckman M, Haugarvoll K, Lohmann-Hedrich K, Kostic V, Farrer M, Klein C. alpha-Synuclein and Parkinson disease susceptibility. Neurology. 2007;69:1745–1750. doi: 10.1212/01.wnl.0000275524.15125.f4. [DOI] [PubMed] [Google Scholar]
- Wong G. Caenorhabditis elegans Models of Parkinson’s Disease. In: Dawson TM, editor. Parkinson’s Disease: Genetics and Pathogenesis. Informa Healthcare USA, Inc.; New York, NY: 2007. pp. 311–324. [Google Scholar]
- Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, Beal MF, Nishimura I, Wakamatsu K, Ito S, et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci U S A. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, Lu B. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D, Gu Z, Nakamura T, Shi ZQ, Ma Y, Gaston B, Palmer LA, Rockenstein EM, Zhang Z, Masliah E, et al. Nitrosative stress linked to sporadic Parkinson’s disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci U S A. 2004;101:10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, Marupudi NI, Torp R, Torgner IA, Ottersen OP, Dawson TM, et al. Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci U S A. 2000;97:13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Huang Y, Shao Y, May J, Prou D, Perier C, Dauer W, Schon EA, Przedborski S. The kinase domain of mitochondrial PINK1 faces the cytoplasm. Proc Natl Acad Sci U S A. 2008;105:12022–12027. doi: 10.1073/pnas.0802814105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]