Abstract
The steroid, 17β-oestradiol (E2) has pervasive psychological and physical effects throughout the lifespan. A question is whether there are divergent oestrogen receptor (ER)-mediated mechanisms for these effects in the central nervous system (CNS) and periphery. This review will focus on results of studies using a whole animal model (i.e. female rats and mice) to investigate the relative effects and mechanisms of oestrogens in the CNS and the periphery. By using this approach, it has been possible to differentiate E2’s enhancing effects on behavioural processes mediated by the hippocampus, such as affective behaviour, and trophic effects to increase tumourigenesis and uterine growth. Studies using pharmacological manipulations and knockout mice are reviewed that suggest that a likely mechanism underlying the beneficial effects of E2 for hippocampal function, but not proliferative effects in the body, involve actions at ERβ, changes in cell cycle/division (e.g. cyclin D1), and/or histone modifications. Thus, it may be possible to differentiate the beneficial effects of oestrogens through ERβ, particularly in the CNS, from negative proliferative effects on peripheral, E2-sensitive tissues.
Keywords: Oestradiol, anxiety, depression, tumour, conjugated equine oestrogen, proliferation
Oestrogens, such as 17β-oestradiol (E2), have pleiotropic effects throughout the body, owing, in part, to their having several sources and target tissues. E2 is primarily thought of as being produced and secreted by the ovaries, and influencing reproduction-related function. In addition to a clear ovarian source, E2 is also produced in other regions of the body, including the adrenal glands, adipose and the central nervous system (1). One challenge has been that many of these sources of oestrogens are also a main target of them for their diverse trophic effects (e.g. the brain, ovaries) throughout the lifespan. Furthermore, E2’s diverse effects are not limited to reproductive function, and can influence mood and cognition. E2’s effects are not limited to a discrete time period but have actions throughout the lifespan, from development to late age. It is beyond the scope of this review to exhaustively cover all of E2’s sources, targets, effects, and the timing of E2’s influence. Instead, this review will focus on the possibility that E2’s beneficial effects in the brain at oestrogen receptor beta (ERβ), may be distinguishable from negative proliferative effects on peripheral, ER(α)-rich tissues, such as the uterus and mammary glands. Basic science experiments performed in our laboratory and others in support of this notion will be reviewed and discussed. More recent lines of investigation to further understand the mechanisms of E2’s effects at ERs, which may involve changes in the cell cycle, will be summarized. What follows first is a brief summary of the most well-recognised physical and psychological effects of E2 throughout the adult lifespan.
General Overview of Physical and Psychological Effects of E2
Puberty
Puberty is characterised by the initiation of substantive E2 secretion from the ovaries (2). In the young adult female, well-known physical effects of E2 occur, such as growth and the emergence of the secondary sex characteristics. In some young some women, this can also be a time for changes in affective responses. For example, rates of depression are similar among adolescent males and females, but at menarche, the rate in females doubles (3, 4).
Adulthood
Among adult females, E2 secretion from the ovaries is cyclical (2) and can have physical and psychological effects. Among adult women, E2 has clear physical effects as evidenced by menstruation, the initiation of the corpus luteum formation, uterine growth and the further development of secondary sex characteristics (2). E2 has effects on metabolic functions, such as water metabolism (i.e. increase water retention), calcium metabolism, and bone growth and maintenance (2). Cyclical changes in E2 can alter cognitive functions (5, 6) In a subset of women, changes in E2 throughout the menstrual cycle (e.g. premenstrual syndrome, premenstrual dysphoric disorder) or following pregnancy (e.g. postpartum depression) is associated with changes in mood and/or cognitive functions (4, 7, 8).
Menopause
The hallmark of menopause is decline in E2 production from the ovaries. Physical and psychological symptoms of decline in E2 production and response with age and menopause uncover a role of E2 for these functions. Symptoms can include changes in cardiovascular control, hot flashes, night sweats, drying of mucosal membranes, changes in bone mass maintenance that can increase risk of osteoporosis and psychological effects, such as sleeplessness, forgetfulness, anxiety, depression, and cognitive changes. Studies demonstrate that certain symptoms of E2 decline can be alleviated with synthetic E2 therapies. However, there is considerable individual variability in these positive effects leaving many women with few physical and psychological benefits. Furthermore, in the large Women’s Health Initiative clinical trials, there were potentially deleterious side effects related of E2 therapy such as increased risk of stroke and/or reproductive tissue cancers (uterus, breast; 9, 10). These findings of a lack of effect and deleterious effects of E2 are surprising. Rodent models of menopausal decline in E2, such as aging models or ovariectomy (OVX), have demonstrated that replacing back E2 can have clear beneficial effects in stroke, neurodegeneration, cognitive, anxiety, and depression indices of females (11-14). These effects in rodent models and the clinical data may be explained by the health of the system, and regimen and dosing of E2 therapy used; however, a discussion of this is beyond the scope of this review and has been described elsewhere (15). Thus, the various effects of E2 throughout the lifespan, and in several systems that may influence disease states (e.g. stroke, cardiovascular disease, cognitive decline, neurodegeneration, mood/anxiety, osteoporosis, cancer, metabolic syndrome, etc.), support studies to investigate the mechanisms of E2’s functional effects.
Utility of an animal model to investigate E2’s effects and mechanisms
Despite the numerous effects of E2 throughout the lifespan, and clear negative effects when E2 precipitously declines at menopause for many women, the mechanisms of E2 for its functional effects are not entirely clear. Although the clinical literature has provided insights into the nature of E2 decline and its replacement, there are differences in responsiveness to E2 among women that depend on many factors (age, prior E2 experience, health, socio-economic status, social supports, etc). As such, investigating the mechanisms of E2’s effects in an animal model in which these potential factors can be controlled or manipulated is useful. Additionally, we have been interested in developing a model system in which both the psychological and physical effects of E2 can be investigated simultaneously. Described as follows are studies that we, and others, have done to investigate the effects and mechanisms of E2 in the body and brain using rodent models.
For the most part, the remainder of this review will focus on the effects of E2 with respect to the adult female rodent, and the later portion of the developmental process. Studies that characterised the nature of oestrogens’ functional effects so that potential mechanisms of these effects could be manipulated in subsequent studies will be reviewed. In these studies, effects of E2 covariation, extirpation, and replacement for anxiety-like behaviour of rodents were determined. These studies were followed up by further investigating the dose-dependent effects of E2 for behavioural processes as well as growth in peripheral E2-sensitive tissues, such as the uterus and mammary tumours. Following this overview on the nature of E2’s effects, this review will discuss the mechanisms of oestrogens’ for these effects, with a primary focus on E2’s actions through the cognate ER, of which there are two cloned types, ERα and ERβ. A focus will be on studies that have characterised the role of E2 through ERβ for beneficial psychological effects in animal models. This will be followed by a discussion of more recent studies in which the effects of E2 in the brain were compared and contrasted with the effects of E2 at ERs in peripheral tissues. Lastly, novel mechanisms of E2 as well as other potential mechanisms downstream of ERs for these effects in animal models and/or in vitro models will be addressed. Given the profound effects of E2 throughout the lifespan, it is imperative to have a greater understanding of its effects and mechanisms.
Nature of E2’s effects
Nature of E2’s effects for anxiety-like behaviour
To be able to initiate studies investigating the mechanisms of E2’s effects, it was necessary to first characterise E2’s effects in a rodent model. As spontaneously ovulating mammals, there are similarities in the endocrine cycles of women and rats. There is cyclical regulation of ovarian secretion of E2 and progesterone following pulsatile hypothalamic gonadotrophin releasing hormone and surges of pituitary follicle stimulating hormone (FSH) and luteinising hormone (LH). There are species-specific differences in the cycles of women and rats and mice. For rats and mice, the average oestrous cycle length is 4 days (2, 16, 17), whereas the average menstrual cycle length in women is 28 days (2). The oestrous cycle is divided into four phases: metoestrus, dioestrus, pro-oestrus, oestrus. Over the oestrous cycle LH and FSH levels are low and increase during pro-oestrus. E2 rises during metoestrus, peaks during pro-oestrus, and is then decreased during oestrus. Progesterone increases during metoestrus and dioestrus, peaking for a second time during late pro-oestrus. The menstrual cycle occurs in three phases: follicular, luteal, menstrual (2). During the follicular phase, LH and FSH gradually increase. E2 increases during this phase and there is a surge in LH and FSH following peaking E2 levels. During the luteal phase, progesterone levels increase and E2 levels gradually wane following a precipitous decline post-ovulation. During menstruation, levels of progesterone and E2 are low. Despite these general similarities in endocrine control of the oestrous and menstrual cycles, there are robust differences in how these cycles are altered with aging among women and rats. Menopause is characterized by changes in cyclicity followed by cessation in menstrual cycles and a decline in E2 and progesterone levels. Conversely, in rats the pattern of changes in cyclicity and E2 and progesterone secretion, and reductions in reproductive-viability (reproductive senescence, which can be referred to as “oestropause;”18) are more varied. In aged rats, there can be a pattern of persistent oestrus or persistent dioestrus. Generally, when cycling ceases among rats, E2 levels decline to steady moderate levels and then increase (19, 20), which is unlike the decline observed during menopause. Because of the similarities and differences between cyclicity and reproductive senescence in women and rats, we have utilized several approaches to determine the role of E2 for its functional effects in our rat model. Generally, the classic behavioral neuroendocrinology approach of assessing hormonal covariation, extirpation, and replacement for a functional effect was utilized. First, young cycling and older reproductively senescent rats were behaviorally assessed during different E2 (and progestin) milieu. Second, because E2 co-varies with progestins during oestrous and there are differences in E2 secretion with aging and reproductive senescence, rats were ovariectomised (OVX) and replaced back with E2 alone or not. Overall, what we have found is that physiological E2 levels in plasma (depicted with circles in Figure 1) occurred concomitant with greater anti-anxiety-like behaviour using the elevated plus maze of rats. The elevated plus maze is a well-validated bioassay of anxiety-related behaviour in rodents in which an increase in time spent on the open arms is utilised as the primary behavioural index (21). The details of the findings using this model are as follows.
Figure 1. Higher levels of estradiol (E2) across endogenous states or following extirpation and replacement increase anti-anxiety-like behaviour of rats.
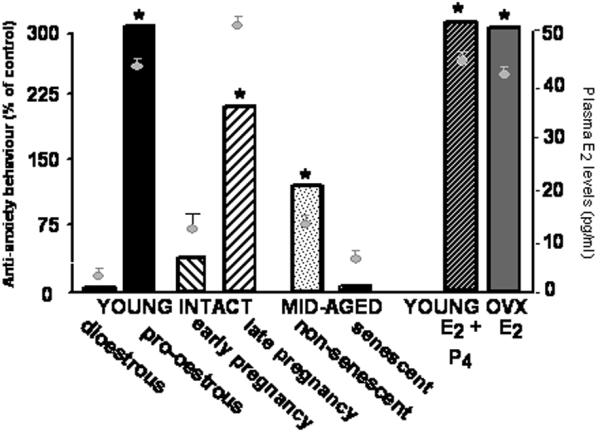
Bars depict ant-anxiety-like behavior (i.e. time spent on the open arms of the plus maze) as a percent of the ovariectomized control rat values. Adult female rats were tested in different stage of the estrous cycle (dioestrous versus pro-oestrous), pregnancy (early versus late pregnancy), and aging (mid-aged non-senescent versus reproductively senescent), or following ovariectomy (OVX) and replacement back with E2 and progesterone (P4), or E2. * p<0.05 compared to respective control groups in each comparison. Circles depict plasma E2 levels as measured by radioimmunoassay of rats in these conditions.
Role of E2 Covariation
Figure 1 depicts the effects of natural covariation in E2 levels among rats for behaviour in the elevated plus maze. Young adult rats in pro-oestrous (when naturally sexually-receptive) and in late pregnancy, spent more time in the open arms of the plus maze, compared to rats in the dioestrous stage of the oestrous cycle or in late pregnancy, respectively. Furthermore, time spent on the open arms of the plus maze decline with aging/reproductive senescence as indicated by the behavioural responses of middle-aged (12-14 months old) rats coincident with lower E2 levels in these subjects. Thus, variations in natural E2 levels can alter anxiety-like behavior of rats.
Role of E2 extirpation & replacement
To further determine the extent to which E2 alters anxiety-like behavior of rats, rats were OVX and replaced-backed with placebo vehicle or E2 regimen that mimics the oestrous cycle rise in E2. As depicted in Figure 1, OVX rats had low levels of open arm exploration, coincident with low E2 levels, similar to dioestrous rats. Replacement of E2 alone, or with progesterone (P4; which co-varies with E2 across endogenous cycles), similarly increased open arm time, compared to vehicle administration to OVX rats. Thus, these data show that E2 alone can have anti-anxiety-like effects among rats.
Nature of E2’s actions for trophic effects in the body
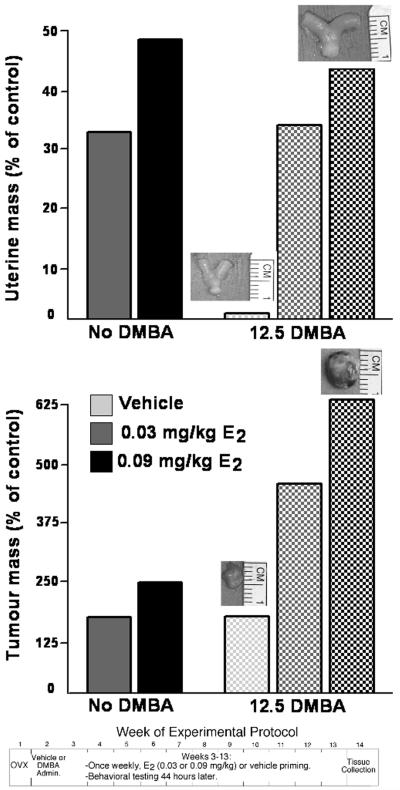
As discussed above, physiologically-relevant levels of E2 reduce anxiety-like behaviour, but the extent to which E2 has physical effects in this animal model needed to be examined. We developed a breast cancer-relevant model in which some rats were exposed to a chemical carcinogen (DMBA, which reliably induces mammary tumours in rodents), or not, and were administered weekly injections of E2 at low, dioestrous-like (0.03 mg/kg), or physiological, pro-oestrous-like (0.09 mg/kg) concentrations, or placebo vehicle. Rats were behaviourally-tested weekly, and had tumours, if present, and uteri collected and weighed at the end of the study (22,23). As depicted in Figure 2, E2, at both dosages, increased uterine weight (a typical bioassay of E2 action). E2 dose-responsively increased tumour weight, an effect potentiated with carcinogen exposure, as compared to OVX, vehicle-administered control rats (Figure 2). In these rats, we also saw a clear dose-dependent effect of E2 for depression-like behaviour. Rats administered E2 regimen that produces pro-oestrous-like E2 levels had decreased immobility in the forced swim test compared to OVX vehicle-administered rats (22). This study demonstrated the utility of simultaneously investigating E2’s effects for psychological and physical effects in a rat model.
Figure 2. Dose-dependent effects of estradiol (E2) to ovariectomised rats administered the chemical carcinogen (DMBA), or not, for uterine growth and tumourigenesis of rats.
As depicted at the bottom of the figure, rats were ovariectomised and exposed to a chemical carcinogen, or not. Rats were then primed weekly with vehicle (VEH) or one of two dosages of E2 (0.03 mg/kg, which produces low, dioestrous-like E2 levels, or 0.09 mg/kg, which produces physiological, pro-oestrous-like E2 levels), and behaviourally-tested (Walf & Frye, 2009a). At the end of the study, uteri and tumours (if present) were collected and assessed. Bars depict uterine and tumour mass as a percent of ovariectomised, vehicle-administered control rats. Pictures above bars are a representative photo of tissue in that condition.
Together, these data demonstrate that some of E2’s functional effects in E2-responsive tissues (i.e. uterus, mammary tumours, and the brain) may be dissociated from each other. Indeed, these studies supported further investigation of the mechanisms of E2’s effects using this integrated animal model that assesses peripheral trophic and central nervous system effects, described as follows (22,23).
Mechanisms of E2’s effects- Actions at ERs
General actions of ERs
The traditional view of E2’s mechanisms are akin to that of the other steroid hormones (e.g. progestins, androgens). That is, steroids act by binding to their intracellular steroid receptors, which form a steroid receptor complex that dimerises, enters the nucleus, binds DNA, alters gene expression, and, ultimately, produces changes in the cell’s (and organisms’) behaviour. In this regard, E2 is known to have effects through at least two classic ER subtypes that have been identified to date, which are referred to as ERα and ERβ. There is some indication that E2 can also regulate gene expression irrespective of actions at these receptors. A discussion of these ER-independent actions will be addressed the end of this review. Of great interest is that ERα and ERβ are widely, and differentially, distributed throughout the central nervous system and body, suggesting that some of the specificity of E2’s various actions are through binding to ERα versus ERβ.
ER distribution in the body
Generally, ERα is widely expressed throughout most E2-sensitive tissues, whereas ERβ expression is more circumscribed, in the body, which may underlie some of the physical effects of E2. It may be that increased expression of ERα in the uterus and mammary glands underlies the unwanted proliferative effects of E2 in these tissues (24,25). On the contrary, effects of ERβ in peripheral tissues may not underlie these negative proliferative effects. In support, although ERβ is typically not expressed at high levels in mammary glands, prognosis in women with breast cancer whom have greater expression of ERβ is more favorable than those with lower ERβ expression (26). As well, there is greater expression of ERβ in bone and ovaries, than ERα. Together, differences in expression in these tissues suggest a potential role of therapeutics acting at ERβ for many disease states, such as polycystic ovarian syndrome, osteoporosis, and breast cancer (24,27).
ER distribution in the brain
There are differences in distribution of ERs in the central nervous system. For example, ERs are expressed in the hypothalamus, but can have clear differences in the specific regions in which they are expressed, and the behavioural responses that may be related to E2’s actions in these regions. ERα, compared to ERβ, is highly expressed in the ventrolateral, ventral medial, and dorsal medial hypothalamus, and arcurate nucleus, and effects of E2 for sexual behaviour may be important in some of these nuclei (28). On the contrary, ERβ is more widely expressed in the paraventricular nucleus of the hypothalamus, on corticotrophin releasing factor neurons, and this pattern of expression may be to ERβ’s effects for HPA function (28,29). Results of studies comparing the expression of ER subtypes across brain regions were of interest because there was clearly greater expression of ERβ than ERα in the hippocampus (28), which is a well-known limbic stricture involved in affective and cognitive regulation. Given the clear effects of E2 for these hippocampus-mediated behaviours, we hypothesised that some of E2’s effects for anxiety and depression-like behaviour were due to actions at ERβ in the hippocampus (30). Studies addressing the role of ERβ for its functional effects through actions in the hippocampus are as follows.
The role of ERβ in the hippocampus
We conducted a series of experiments to test this hypothesis and determine the role of ERβ in the hippocampus for E2’s effects on anxiety- and/or depression-like behaviour. A summary of the major findings from these experiments is depicted in Figure 3.
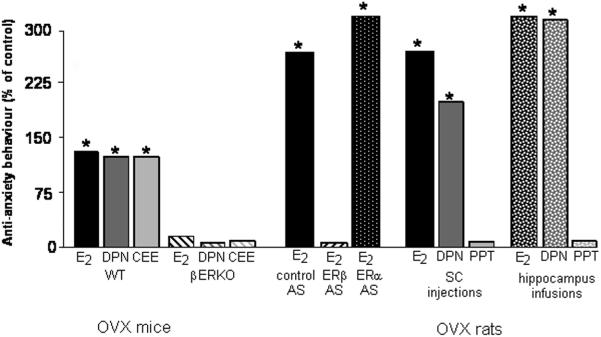
Figure 3. Anti-anxiety-like behaviour of mice and rats following treatment with estradiol (E2) or SERMs may be due to actions of oestrogen receptor β (ERβ).
Bars depict time spent on the open arms of the plus maze of adult female mice or rats as a percentage of ovariectomised controls. WT, but not ERβ knockout (βERKO), mice treated with E2, an ERβ-SERM (diarylpropionitrile - DPN), or a clinically-prescribed E2 mimetic (conjugated equine oestrogens-CEE), had increased anti-anxiety-like behaviour. Ovariectomised, E2-primed rats administered scrambled control (control AS) or antisense oligonucleotides targeted against ERα (ERα AS), but not ERβ (ERβ AS), had increased anti-anxiety-like behaviour. Subcutaneous or hippocampal administration of E2 or DPN, but not an ERα-SERM (propyl pyrazole triol - PPT), similarly increased anti-anxiety-like behaviour of ovariectomised rats. * p<0.05 compared to respective control groups in each comparison.
ERβ knockout mice
To investigate the requirement of ERβ, anxiety-like behaviour of mice lacking ERβ, compared to their wildtype counterparts, was determined. Mice were OVX and administered E2 (which acts at both ERα and ERβ similarly) or a selective oestrogen receptor modulator (SERM) that has greater affinity for ERβ than ERα, DPN, or vehicle. As depicted in Figure 3, E2 and DPN similarly increased anti-anxiety-like behaviour in the elevated plus maze of wildtype, but not βERKO, mice, compared to OVX, vehicle-administered wildtype mice (31). Additionally, we have found that wildtype, but not βERKO mice, have improved performance in cognitive or affective tasks that are mediated by the hippocampus, when in pro-oestrus (32). Although we have not investigated the effects of these manipulations for anxiety-like responding in ERα knockout mice, the literature demonstrates that ERα knockout mice would behave similarly as wildtype controls in anxiety measures (33). Indeed, studies in knockout mice suggest that knockout of ERα, rather than ERβ, may have greater effects on general arousal/motor behavior and social behaviors (34-36). These investigations lend support to findings in other laboratories that have shown the importance of ERβ for anxiety and depression responding in female mice (33, 37, 38). Indeed, in a recent report, it has been suggested that ERβ may be particularly important for the anti-anxiety effects of physiological E2, rather than the anxiogenic effects of E2 when in high and/or sustained concentrations (39). Together, these studies demonstrate that ERβ knockout in mice can regulate anxiety-like responding to oestrogens.
Recently, we have been interested in whether some of the individual differences in responses to hormone replacement therapies may be related to the mechanisms of the treatment used. We have begun investigating this by administering the most commonly-prescribed E2 therapy in the U.S., conjugated equine oestrogens (CEE) in our rodent models. CEE is composed of approximately fifty compounds, only one of which is E2, so determining its mechanisms of action to improve its efficacy can be a challenge. In vitro studies have demonstrated that there is a more robust neuroprotective effects with specific oestrogens contained in CEE than in others (40). We have found that middle-aged rats have improved cognitive, anxiety, and social behaviour when administered CEE compared to vehicle (41), but the mechanisms for this effect are not entirely understood and are presently under investigation. A recent study in our laboratory has investigated this question in OVX wildtype and βERKO mice and found that only wildtype mice had anti-anxiety-like effects of CEE (Figure 3). Thus, it may be that ERβ is important for the anti-anxiety effects of CEE and investigations are ongoing to elucidate this further.
ERβ Antisense Oligonucleotides
Although transgenic and knockout murine technologies have advanced the field of neuroendocrinology, there are always concerns about potential compensatory mechanisms and/or developmental effects of gene mutation or deletion for behavioural responses. As well, the gene is deleted throughout the brain and body. To begin to investigate the temporal- and site-specificity of the ERβ effect, we used intracerebroventicular administration of antisense oligonucleotides (AS-ODNs) targeted against ERα or ERβ, versus saline or scrambled control, AS-ODNs to OVX, E2-primed rats. We found that ERβ AS-ODNs, but not saline, or scrambled or ERα AS-ODNs, during E2-priming, attenuated anti-anxiety behaviour in the plus maze (Figure 3; 42). We also determined that this treatment reduced expression of ERβ in the hippocampus, but not ventral medial hypothalamus (control site), concomitant with this behavioural response (42). Thus, knocking down ERβ expression in the hippocampus reduces E2’s anti-anxiety-like effects among rats.
Selective estrogen receptor modulators (SERMs)
In addition to using these genetic techniques to block a behavioural response that may be related to ERβ action we and others have also determined the effects of selectively activating ERα or ERβ in the hippocampus. A summary of these data are depicted in Figure 3. We, and others, found similar effects of E2 or DPN, but not an ERα SERM (PPT), to increase anti-anxiety- and anti-depressive-like behaviour of OVX rats when these compounds were administered subcutaneously (i.e. the whole brain; 43-45) and directly to the hippocampus (46). In a recent study, a week of once daily administration of DPN, its biologically-active S-enanitomer, or another ERβ agonist, WAY-200070, to OVX rats produced anti-depressant-like effects in the forced swim test (47). Although this report is focused on reviewing the literature in females, it must be noted that there may be sex differences and a role of gonadal status in the ERβ-mediated effect. A previous report demonstrated that DPN reduces anxiety-like responding of gonadectomized rats (43). However, a recent report failed to find such an effect of subcutaneous or oral administration of DPN to gonadally-intact male rats (48). Determining whether the magnitude of ERβ’s action depends upon neuroendocrine context is of interest. Together, these experiments in female mice and rats supported our hypothesis that ERβ in the hippocampus is a target of the beneficial effects of E2 for affective functions. Of further importance is that similar beneficial effects of ERβ SERMs were observed when administered in a regimen that would affect the whole body and brain as well as when administered directly to the brain. Thus, these data lend support to the notion that oestrogens’ effects through ERβ in the hippocampus may be dissociable from effects of oestrogens in peripheral tissues.
Mechanisms of E2’s effects- Potential non-traditional actions
Rapid effects of E2
In addition to having traditional actions at intracellular ERs, E2 may also have some functional effects involving rapid actions at the membrane, alone, or to potentiate subsequent intracellular ER actions. Traditional effects of E2 typically have latencies greater than 10-15 minutes, which is the time necessary to initiate gene transcription and protein synthesis. However, more rapid behavioural effects of E2 have been described, suggesting that E2 may have actions at the plasma membrane (30,49). For example, in the study described above investigating the effects of direct administration of E2 and SERMs to the hippocampus, compounds were administered 10 minutes before behavioral testing (46). Notably, these behavioral effects were similar as was observed in rats administered E2 or SERMs 44-48 hours before behavioral testing (45). Thus, there can be rapid actions of oestrogens.
Membrane targets for E2
Oestrogens’ rapid actions may occur at the membrane. Restricting E2’s actions to the membrane, by administering E2 conjugated to a large molecule (BSA), similarly increases performance in the inhibitory avoidance task as does free E2 which readily crossing into the cell (30,50). There are various potential membrane targets for E2 for its non-traditional effects. The first possibility is that ERs may be associated with the membrane. There is some evidence that ERs, in particular ERα, can associate with caveolae, and interact with a G-protein-coupled receptor (GPCR; 51). In vitro studies have demonstrated that E2 may spur ERβ to translocate to the cell membrane rapidly and transiently (52). Moreover, other membrane receptors as targets of E2 action have been suggested, such as ER-X (53), GPR30 (54), or Gq-mER (55,56). The functional significance of E2 acting at these receptors is not entirely understood, but recent studies have pursued this question. As an example, a compound that is selective for the Gq-mER, but does not bind the ER, STX, was developed and its functional effects are being characterised. STX reduces weight gain in female guinea pigs, which is typically observed following OVX in a dose-related manner (55,56). Thus, there may be functional effects of oestrogens at a membrane-associated ER.
Additionally, E2’s actions may involve interactions with other GPCR or ion-gated neurotransmitter receptors. Receptors of particular interest are serotonin, glutamate, GABA, acetylcholine and dopamine (57) because of their clear relationship to mood and cognition. Several elegant studies have been done investigating the interactions between ERβ, the serotonin system, and the hypothalamic-pituitary-adrenal axis, as related to affective responding. These are briefly reviewed as follows. In support, a high percentage of serotonin cells in the dorsal raphe nucleus co-express ERβ (58). DPN, administered once daily for eight days, subcutaneously or to the dorsal raphe nucleus, increased expression of tryptophan hydroxylase mRNA in the dorsal raphe nucleus, as well as reducing depression-like responses of OVX rats (59). Furthermore, ERβ knockout mice have reductions in serotonin levels and expression of tryptophan hydroxylase mRNA in the dorsal raphe nucleus (37, 60). In addition to these actions involving the serotoninergic system, ERβ activation may reduce HPA responding. Unlike administration of E2 and PPT, DPN decreases corticosterone levels when administered centrally or systemically (43,47). There is high expression of ERβ in the paraventricualr nucleus of female rats compared to ERα expression (61). As well, E2 and PPT, but not DPN, increase expression of the immediate early gene c-fos in the paraventricular nucleus following a stressor (29, 47). Thus, these studies demonstrate the role of oestrogens at neurotransmitter and neuromodulator targets.
It may also be that E2’s functional effects occur downstream of its actions to alter neurosteroidogenesis. E2 can enhance activity of steroid metabolism enzymes, produce de novo P synthesis in astrocytes, and increase 3α,5α-THP concentrations in the hippocampus and other brain regions relevant for affect and cognition (62-64). Finally, it may be that there is a potentiation of intracellular ER signalling following activation of a membrane process. Lordosis behaviour is a well-characterised bioassay for steroid actions, and is a clear example of a behaviour modulated by the traditional actions of E2 at ERs (65). However, more recent studies have demonstrated that signalling of E2 at the membrane enhances subsequent effects in the hypothalamus to facilitate lordosis of OVX rats (66). We are currently investigating the potential interactions between these membrane targets and ERβ for affective and cognitive processes and trophic effects in the body.
Targets downstream of the membrane for E2
Effects of E2 at the membrane interact with several molecular pathways, and the multitude effects of E2 through these membrane targets, described above, have been extensively reviewed (30, 51, 56, 66, 67). In support, E2 can have interactions with other transcription factors (e.g. pCREB, STATs, Elk-1-Srf, ATF-2-Jun), growth factors, as well as initiate major signalling pathways (e.g. phospholipase C, phosphatidylinositol 3 kinase, and mitogen-activated protein kinase-MAPK; 51, 68, 69). We have found that pharmacologically blocking MAPK in the hippocampus of OVX rats attenuates E2’s effects to improve inhibitory avoidance performance (30). Of interest are the other functional effects of E2’s interactions with these targets, and their role in our model investigating E2’s mechanisms in the central nervous system and in peripheral E2-sensitive tissues.
What other actions in addition to ERβ- potential role of cyclin D1 and/or histones in E2-sensitive tissues
SERMs’ effects in the hippocampus vs. uterus vs. mammary tumours
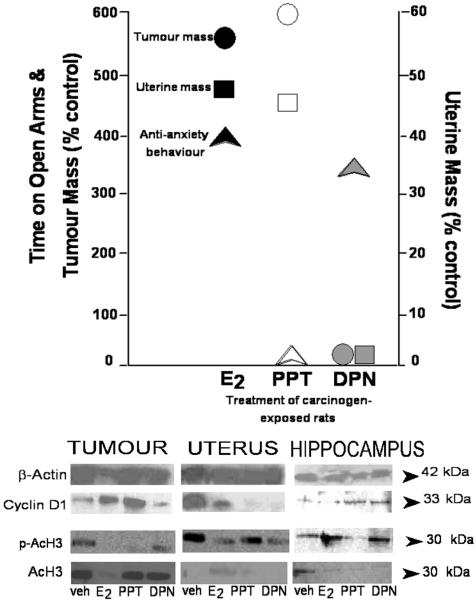
The downstream mechanisms of ERα and ERβ for effects on proliferation in the body and possible trophic effects in the brain are of interest. To this end, we replicated studies investigating the behavioral effects of subcutaneously administered SERMs for behavior and extended these studies to investigate the peripheral effects of SERMs. As described above, rats were OVX and administered the chemical carcinogen (DMBA) or not. Rats were administered E2, PPT, or DPN once a week for 13 weeks. Behavioral analyses occurred weekly, and brains, tumours (if present), and uteri were collected at the end of the study. As depicted in Figure 4, we found that the ERα-SERM, PPT, increased tumour incidence and uterine weight, similar to that observed with E2. The ERβ-SERM, DPN, did not have these effects for tumourigenesis or uterine proliferation; yet, E2 and DPN similarly increased anti-anxiety behaviour (Figure 4). Thus, a possibility is that ERβ may not be associated with proliferative effects in the body to the same extent that ERα is. This study also demonstrates that behavioral and peripheral effects of oestrogens can be dissociated. Further investigation of these mechanisms is ongoing. Recent investigations have focused on changes in the cell cycle that may underlie these ERα- and ERβ-specific actions in the hippocampus, uterus, and mammary tumours.
Figure 4. Dissociable effects of oestrogen receptor (ER) ligands for tumoigenesis, uterine growth, and affective behaviour and protein changes in peripheral and central nervous system tissue.
In ovariectomised rats exposed to a chemical carcinogen (DMBA): 1) Administration of estradiol (E2) increases tumourigenesis, uterine growth, and anti-anxiety behaviour. 2) Administration of an ERα-SERM (propyl pyrazole triol - PPT) increases tumourigenesis, uterine growth, but not anti-anxiety behaviour. 3) Administration of an ERβ-SERM (p diarylpropionitrile - DPN) increases anti-anxiety behaviour, but not tumourigenesis or uterine growth. Pictures below are representative depictions from western blots of changes in protein expression in tumours, uterus, and hippocampus (i.e. β-actin as a loading control, cyclin D1, and phosphoacetylated histone 3-pACH3, and acetylated histone 3-AcH3) of rats in these conditions.
Role of Cyclin D1
We have begun investigating whether some of the functional effects of E2 and SERMs that we have observed involve changes in the cell cycle. As such, we have investigated the expression of cyclin D1 in the uterus, tumours, and hippocampus. Cyclin D1 is important for governing DNA synthesis and is considered a molecular mechanism driving tumourigenesis. Indeed, the cyclin D1 gene is overexpressed in 30-50% of breast cancer, despite not seeming to alter prognosis of the disease in women with ERα+ tumours (69). Cyclin D1 is a positive regulator of ERα transcription, further implicating it in the proliferative effects of E2 in the body. A recent in vitro study suggests that overexpression of cyclin D1 can reduce the efficacy of newly-developed SERMs to act as antagonists in breast cancer models (70). We have demonstrated differences in cyclin D1 expression in the body and brain in our animal model. As expected given its role in tumorigenesis, cyclin D1 expression was particularly increased in the tumours of rats administered E2 and PPT, compared to its expression in the uterus or hippocampus following vehicle or DPN administration (Figure 4). Thus, these data suggest that differences in cyclin D1 may be one target for further investigation of the trophic effects of oestrogens in the brain versus body.
Role of Histone 3
Histone modifications (acetylation, methylation, phosphorylation, etc), downstream of ERs, may underlie divergent trophic effects in the body and brain. Recent studies have demonstrated a clear role of epigenetics in the hormonal control of organization of the brain, and, therefore, behavior (71-73). In adulthood, histone modifications have also been shown to be important for hippocampal plasticity as related to fear and other learning processes (74, 74). Histone modifications are also of interest to us given their role in tumor biology. In support, histone deacetylases (HDACs) modulate chromatin structure and transcriptional activity in the cell by altering acetylation of histones, in particular histone 3 and 4, in the nucleus. HDACs are also involved in regulating transcriptional pathways, by acting as repressors, important for differentiation and growth of cells. Because HDAC recruitment is typically associated with repression, HDAC inhibitors are candidates for cancer treatments owing to their ability to attenuate repression, and, therefore, promote expression of genes involved in cell differentiation and death, and cell cycle arrest. We have begun analyzing changes in histone 3 acetylation and phospho-acetylation in tissues from rats in our model. These experiments have suggested that there are differences in histone 3 acetylation and phosphor-acetylation in the uterus, tumours, and hippocampus of rats administered E2, PPT, DPN or placebo, and DMBA (Figure 4). In the hippocampus, phospho-acetylated form of histone 3 was reduced by E2, PPT, and DPN similarly; however, acetylated histone 3 was increased by E2 and DPN, and decreased by PPT, compared to vehicle. In tumors, both acetylated and phospho-acetylated histone 3 was increased by E2 and PPT in particular. In the uterus, phospho-acetylated, but not acetylated, were increased by PPT more so than E2 or DPN. These findings substantiate further investigation of the effects of E2 on histone modifications to underlie differential trophic effects on reproductive and neural tissue. How beneficial effects are related to their capacity to alter histones and/or actions involving ERβ needs to be investigated further.
Summary and Conclusions
In a whole animal model, E2 regimen that exert trophic effects to increase tumourigenesis and uterotrophic effects can enhance affective behaviour. E2, CEE supplement, or ERβ-SERM enhance affective behaviour, but ERβ-SERMs have no trophic effects. E2 and/or ERβ SERMs to WT, but not βERKO, mice enhance affective behaviour of males and females, and investigation of trophic effects is ongoing. E2 and ERβ SERMs to hippocampus enhance affective behaviour without peripheral trophic effects. Some of these trophic effects in the periphery may be related to changes in cell cycle/division (cyclin D1) and/or histone modifications. Experiments, such as these, are timely because more information is needed in basic science studies to inform clinically-relevant questions, such as oestrogens’ effects for anxiety, mood, cognition, and proliferative processes that may confer increased risk for reproductive cancers. Thus, it may be possible to differentiate the beneficial effects of oestrogens through ERβ, particularly in the CNS, from negative proliferative effects on peripheral tissues. Studies are ongoing to investigate downstream targets of E2 at ERβ for its beneficial effects in the body and brain.
Acknowledgements
The research described in this report has been supported in part by a trainee grant from the USAMRMC Dept. of Defense Breast Cancer Research Program (BC051001), as well as funding to the author’s mentor, Dr. Cheryl A. Frye, from the National Science Foundation (IBN98-96263; IBN03-16083; DBI 00-97342), National Institute of Mental Health (MH0676980), Whitehall Foundation (096-010), and Ronald McNair Research Program to support minority undergraduates. The author wishes to acknowledge the assistance and oversight provided by Dr. Frye in completing these studies, and in writing this manuscript. The author wishes to also thank her mentor, Dr. Jamie Rusconi, for her assistance with western blotting. Technical assistance provided by Dr. Madeline Rhodes, Daniel Da Costa, Amy Kohtz, Carolyn Koonce, Danielle Llaneza, Danielle Osborne, and Jason Paris, is greatly appreciated.
Reference list
- 1.Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45:S116–24. doi: 10.1067/mjd.2001.117432. [DOI] [PubMed] [Google Scholar]
- 2.Nelson RJ. An Introduction to Behavioral Endocrinology. 3rd edition. Sinauer Associates; Sunderland, MA: 2005. [Google Scholar]
- 3.Lewinsohn PM, Rohde P, Seeley JR. Major depressive disorder in older adolescents: prevalence, risk factors, and clinical implications. Clin Psychol Rev. 1998;18:765–94. doi: 10.1016/s0272-7358(98)00010-5. [DOI] [PubMed] [Google Scholar]
- 4.Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J Affect Disord. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- 5.Farage MA, Osborn TW, MacLean AB. Cognitive, sensory, and emotional changes associated with the menstrual cycle: a review. Arch Gynecol Obstet. 2008;278:299–307. doi: 10.1007/s00404-008-0708-2. [DOI] [PubMed] [Google Scholar]
- 6.Hampson E. Variations in sex-related cognitive abilities across the menstrual cycle. Brain Cogn. 1990;14:26–43. doi: 10.1016/0278-2626(90)90058-v. [DOI] [PubMed] [Google Scholar]
- 7.Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44:234–46. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- 8.Rubinow DR, Schmidt PJ. Gonadal steroid regulation of mood: the lessons of premenstrual syndrome. Front Neuroendocrinol. 2006;27:210–6. doi: 10.1016/j.yfrne.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SA, Pettinger M, Liu J, McNeeley SG, Lopez AM, Women’s Health Initiative Investigators Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1739–48. doi: 10.1001/jama.290.13.1739. [DOI] [PubMed] [Google Scholar]
- 10.Prentice RL, Manson JE, Langer RD, Anderson GL, Pettinger M, Jackson RD, Johnson KC, Kuller LH, Lane DS, Wactawski-Wende J, Brzyski R, Allison M, Ockene J, Sarto G, Rossouw JE. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol. 2009;170:12–23. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–95. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- 12.Nilsen J. Estradiol and neurodegenerative oxidative stress. Front Neuroendocrinol. 2008;29:463–75. doi: 10.1016/j.yfrne.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30:201–11. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol. 2007;19:77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- 16.Long JA, Evans HM. The estrous cycle in the rat and its associated phenomena. Memories of University of California. 1992;6:1–148. [Google Scholar]
- 17.Freeman ME. The ovarian cycle of the rat. In: Knobil E, Neil J, editors. Physiology of reproduction. Raven Press Ltd.; New York: 1988. pp. 1893–1928. [Google Scholar]
- 18.Chakraborty TR, Gore AC. Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Exp Biol Med. 2004;229:977–87. doi: 10.1177/153537020422901001. [DOI] [PubMed] [Google Scholar]
- 19.Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- 20.Nass TE, LaPolt PS, Judd HL, Lu JK. Alterations in ovarian steroid and gonadotrophin secretion preceding the cessation of regular oestrous cycles in ageing female rats. J Endocrinol. 1984;100:43–50. doi: 10.1677/joe.0.1000043. [DOI] [PubMed] [Google Scholar]
- 21.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walf AA, Frye CA. Effects of two estradiol regimens on anxiety and depressive behaviors and trophic effects in peripheral tissues in a rodent model. Gender Medicine. 2009;6:300–11. doi: 10.1016/j.genm.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walf AA, Frye CA. Estradiol enhances sociosexual behavior and augments carcinogen-induced tumorigenesis in ovariectomized rats. AGE. 2009;31:221–9. doi: 10.1007/s11357-008-9079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gustafsson JA. What pharmacologists can learn from recent advances in estrogen signaling. Trends Pharmacol Sci. 2003;24:479–85. doi: 10.1016/S0165-6147(03)00229-3. [DOI] [PubMed] [Google Scholar]
- 25.Jensen EV, Jacobson HI, Walf AA, Frye CA. Estrogen action: a historic perspective on the implications of considering alternative approaches. Physiol Behav. 2009 doi: 10.1016/j.physbeh.2009.08.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugiura H, Toyama T, Hara Y, Zhang Z, Kobayashi S, Fujii Y, Iwase H, Yamashita H. Expression of estrogen receptor β wild-type and its variant ERβcx/β2 is correlated with better prognosis in breast cancer. Jpn J Clin Oncol. 2007;37:820–8. doi: 10.1093/jjco/hym114. [DOI] [PubMed] [Google Scholar]
- 27.Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson JA. International Union of Pharmacology. LXIV. Estrogen Receptors. Pharmacol Rev. 2006;58:773–781. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- 28.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Lund TD, Hinds LR, Handa RJ. The androgen 5α-dihydrotestosterone and its metabolite 5α-androstan-3β, 17β-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor β-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–56. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walf AA, Frye CA. Rapid and estrogen receptor β mediated actions in the hippocampus mediate some functional effects of estrogen. Steroids. 2008;73:997–1007. doi: 10.1016/j.steroids.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor β knockout, mice. Behav Neurosci. 2008;122:974–81. doi: 10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor β knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res. 2009;196:254–60. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor β-deficient mice. Proc Natl Acad Sci U S A. 2001;98:12278–82. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW. Involvement of estrogen receptor α, β and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice. Genes Brain Behav. 2006;5:528–39. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- 35.Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor α: specificity for the type of activity. Endocrinology. 2003;144:230–9. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-α gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–81. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 37.Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor β influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84:157–63. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17β-estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-β knockout (BERKO) mice. Psychopharmacology. 2005;179:637–43. doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- 39.Tomihara K, Soga T, Nomura M, Korach KS, Gustafsson JA, Pfaff DW, Ogawa S. Effect of ER-β gene disruption on estrogenic regulation of anxiety in female mice. Physiol Behav. 2009;9:300–6. doi: 10.1016/j.physbeh.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao L, Brinton RD. Select estrogens within the complex formulation of conjugated equine estrogens (Premarin) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer’s disease. BMC Neurosci. 2006;7:24. doi: 10.1186/1471-2202-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walf AA, Frye CA. Conjugated equine estrogen enhances rats’ cognitive, anxiety, and social behavior. Neuroreport. 2008;19:789–92. doi: 10.1097/WNR.0b013e3282fe209c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walf AA, Ciriza I, Garcia-Segura LM, Frye CA. Antisense oligodeoxynucleotides for estrogen receptor-β and α attenuate estradiol’s modulation of affective and sexual behavior, respectively. Neuropsychopharmacology. 2008;33:431–40. doi: 10.1038/sj.npp.1301416. [DOI] [PubMed] [Google Scholar]
- 43.Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 44.Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERβ-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78:523–9. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 45.Walf AA, Frye CA. ERβ-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- 46.Walf AA, Frye CA. Administration of estrogen receptor β-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 2007;86:407–14. doi: 10.1016/j.pbb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-β agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150:1817–25. doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patisaul HB, Burke KT, Hinkle RE, Adewale HB, Shea D. Systemic administration of diarylpropionitrile (DPN) or phytoestrogens does not affect anxiety-related behaviors in gonadally intact male rats. Horm Behav. 2009;55:319–28. doi: 10.1016/j.yhbeh.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cornil CA. Rapid regulation of brain oestrogen synthesis: the behavioural roles of oestrogens and their fates. J Neuroendocrinol. 2009;21:217–26. doi: 10.1111/j.1365-2826.2009.01822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frye CA, Rhodes ME. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Res. 2002;956:285–93. doi: 10.1016/s0006-8993(02)03559-x. [DOI] [PubMed] [Google Scholar]
- 51.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–9. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheldahl LC, Shapiro RA, Bryant DN, Koerner IP, Dorsa DM. Estrogen induces rapid translocation of estrogen receptor β, but not estrogen receptor α, to the neuronal plasma membrane. Neuroscience. 2008;153:751–61. doi: 10.1016/j.neuroscience.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid Biochem Mol Biol. 2008;109:350–3. doi: 10.1016/j.jsbmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–40. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roepke TA. Oestrogen modulates hypothalamic control of energy homeostasis through multiple mechanisms. J Neuroendocrinol. 2009;21:141–50. doi: 10.1111/j.1365-2826.2008.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nadal A, Díaz M, Valverde MA. The estrogen trinity: membrane, cytosolic, and nuclear effects. News Physiol Sci. 2001;16:251–5. doi: 10.1152/physiologyonline.2001.16.6.251. [DOI] [PubMed] [Google Scholar]
- 58.Gundlah C, Lu NZ, Mirkes SJ, Bethea CL. Estrogen receptor β (ERβ) mRNA and protein in serotonin neurons of macaques. Brain Res Mol Brain Res. 2001;91:14–22. doi: 10.1016/s0169-328x(01)00108-5. [DOI] [PubMed] [Google Scholar]
- 59.Donner N, Handa RJ. Estrogen receptor β regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163:705–18. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nomura M, Akama KT, Alves SE, Korach KS, Gustafsson JA, Pfaff DW, Ogawa S. Differential distribution of estrogen receptor (ER)-α and ER-β in the midbrain raphe nuclei and periaqueductal gray in male mouse: Predominant role of ER-β in midbrain serotonergic systems. Neuroscience. 2005;130:445–56. doi: 10.1016/j.neuroscience.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki S, Handa RJ. Estrogen receptor-β, but not estrogen receptor-α, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol. 2005;484:28–42. doi: 10.1002/cne.20457. [DOI] [PubMed] [Google Scholar]
- 62.Cheng YJ, Karavolas HJ. Conversion of progesterone to 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one by rat medical basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology. 1973;93:1157–62. doi: 10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- 63.Frye CA, Rhodes ME. Estrogen-priming can enhance progesterone’s anti-seizure effects in part by increasing hippocampal levels of allopregnanolone. Pharmacol Biochem Behav. 2005;81:907–16. doi: 10.1016/j.pbb.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 64.Micevych PE, Chaban V, Ogi J, Dewing P, Lu JK, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology. 2007;148:782–9. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- 65.Pfaff DW. Drive: Neurobiological and Molecular Mechanisms of Sexual Motivation. The MIT Press; Cambridge, MA: 1999. [Google Scholar]
- 66.Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- 67.Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–42. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 68.Garcia-Segura LM, Diz-Chaves Y, Perez-Martin M, Darnaudéry M. Estradiol, insulin-like growth factor-I and brain aging. Psychoneuroendocrinology. 2007;32:S57–61. doi: 10.1016/j.psyneuen.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Roy PG, Thompson AM. Cyclin D1 and breast cancer. Breast. 2006;15:718–27. doi: 10.1016/j.breast.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 70.Zwart W, Rondaij M, Jalink K, Sharp ZD, Mancini MA, Neefjes J, Michalides R. Resistance to anti-estrogen arzoxifene is mediated by overexpression of cyclin D1. Mol Endocrinol. 2009;23:1335–45. doi: 10.1210/me.2008-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–23. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murray EK, Hien A, de Vries GJ, Forger NG. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology. 2009;150:4241–7. doi: 10.1210/en.2009-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsai HW, Grant PA, Rissman EF. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics. 2009;4:47, 53. doi: 10.4161/epi.4.1.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang Y, Langley B, Lubin FD, Renthal W, Wood MA, Yasui DH, Kumar A, Nestler EJ, Akbarian S, Beckel-Mitchener AC. Epigenetics in the nervous system. J Neurosci. 2008;28:11753–9. doi: 10.1523/JNEUROSCI.3797-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levenson JM, Sweatt JD. Epigenetic mechanisms: a common theme in vertebrate and invertebrate memory formation. Cell Mol Life Sci. 2006;63:1009–16. doi: 10.1007/s00018-006-6026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]