Abstract
Repression by E(spl)M8 during inhibitory Notch (N) signaling (lateral inhibition) is regulated, in part, by protein kinase CK2, but the involvement of a phosphatase has been unclear. The studies we report here employ Tik, a unique dominant-negative (DN) mutation in the catalytic subunit of CK2, in a Gal4-UAS based assay for impaired lateral inhibition. Specifically, overexpression of Tik elicits ectopic bristles in N+ flies and suppresses the retinal defects of the gain-of-function allele Nspl. Functional dissection of the two substitutions in Tik (M161K and E165D), suggests that both mutations contribute to its DN effects. While the former replacement compromises CK2 activity by impairing ATP-binding, the latter affects a conserved motif implicated in binding the phosphatase PP2A. Accordingly, overexpression of microtubule star (mts), the PP2A catalytic subunit closely mimics the phenotypic effects of loss of CK2 functions in N+ or Nspl flies, and elicits notched wings, a characteristic of N mutations. Our findings suggest antagonistic roles for CK2 and PP2A during inhibitory N signaling.
Introduction
Lateral inhibition is critical for the patterning of sensory organs such as the eye and bristles (reviewed in (Calleja et al., 2002; Frankfort and Mardon, 2002; Simpson et al., 1999)). The process of neurogenesis initiates with the expression of proneural transcription factors encoded by the Achaete Scute Complex (ASC) o0r atonal (ato), whose activities are essential for formation of groups of equipotent cells called the proneural clusters (PNC’s). Subsequently, from each PNC a single cell is selected, and this cell goes on to become an R8 photoreceptor in the eye or the sensory organ precursor (SOP) in the bristle. This selection process involves inhibitory Notch signaling between the future R8/SOP and other cells of a PNC, and requires the activities of the Enhancer of split (E(spl)) repressors that antagonize Ato/ASC.
Over the last few years it has been found that the antagonism of Ato/ASC by E(spl) is regulated at least in part by phosphorylation. Specifically, phosphorylation of E(spl)M8 by protein kinase CK2 augments repression in vivo (Karandikar et al., 2004), and this modification is conserved in the mammalian homolog, Hes6 (Gratton et al., 2003). Consistent with a role for CK2 in repression by E(spl)M8, a reduction in CK2 activity compromises lateral inhibition and elicits the specification of supernumerary bristle SOP’s and R8 cells, which manifest in the adult as ectopic bristles and rough eyes, respectively (Bose et al., 2006). These latter studies involved the expression of UAS-CK2α-RNAi constructs or UAS-Tik, a construct that encodes a DN variant of the catalytic CK2α subunit.
CK2 is a highly conserved protein kinase that is composed of catalytic (α) and regulatory (β) subunits whose association generates the α2β2 holoenzyme (Glover et al., 1983). In addition to lateral inhibition (see above), this enzyme regulates the activities of proteins such as Hairy, Antennapedia, Ultrabithorax and Odd-skipped during development (Goldstein et al., 2005; Jaffe et al., 1997; Kahali et al., 2008; Taghli-Lamallem et al., 2008). To date, two alleles of CK2α have been identified in a screen for modifiers of the circadian clock. These are Timekeeper (Tik), a unique dominant allele, and its partial phenotypic revertant called TikR (Lin et al., 2002). While Tik/+ flies displayed lengthened periods, Tik is homozygous lethal. Since recombinant Tik lacks detectable kinase activity (in vitro), its effects on the clock appear to be dominant-negative (DN). Tik harbors two missense mutations, M161K and E165D. Of these, Met161 is located in the ATP-binding pocket and is invariant in all CK2α subunits from yeast to humans (Fig. 1b), and its replacement with Lys is proposed to impede ATP-binding, and attenuate catalysis (Lin et al., 2002). However, the contribution of the E165D substitution to the dominant behavior of Tik is unclear. In contrast, TikR displays a key genetic characteristic of a revertant; that is TikR/+ flies do not display the severe clock defects of Tik/+ flies (Lin et al., 2002). Molecular analysis shows that TikR harbors an internal deletion of seven amino acids and the substitution of Arg242 with Glu, in addition to the two original mutations in Tik (M161K+E165D, Fig. 1b). Consequently, recombinant TikR does not display any kinase activity (in vitro) and is also homozygous lethal. Its revertant behavior likely reflects misfolding due to the internal deletion, which neutralizes the DN activity (of Tik) by impairing association with CK2β and formation of the holoenzyme, a possibility that has not been formally tested.
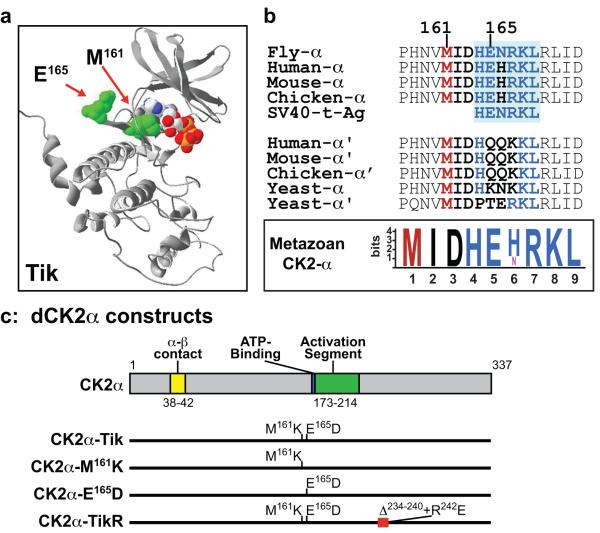
Figure-1. Modeling of Tik and constructs.
(a) Molecular model of Drosophila Tik based on coordinates of human CK2α. The location of the two substitutions and ATP are shown in space-filling mode. (b) Alignment of the α and α’ subunits of CK2. The green box is the region of human CK2α proposed to mediate interaction with PP2A (Heriche et al., 1997). Inset (boxed) shows a Motif-Logo of residues from Met161-Leu169 in CK2α subunits. (c) Drosophila CK2α constructs. The locations of the substitutions or deletions are shown relative to key motifs of dCK2α.
Unlike the clock, Tik/+ flies do not display neural (eye/bristle) patterning defects (Bose et al., 2006), suggesting that CK2 activity in this background is still sufficient for inhibitory N signaling. However, the ability of ectopically (Gal4-UAS mediated) expressed Tik to impair lateral inhibition (see above) provided a means to functionally dissect the substitutions in Tik and TikR. We thus generated variants of CK2α that harbored these substitutions/deletions individually. Following biochemical analysis, we tested for their impact on bristle and eye development in N+ and Nspl flies. These studies suggest that TikR is structurally compromised and likely to be a true ‘null’ allele. In the case of Tik, however, our studies suggest that both M161K and E165D contribute to its DN-behavior. As is the case for Tik, overexpression of CK2α-M161K or CK2α-E165D elicits ectopic bristles in N+ flies and both variants suppresses the retinal defects of Nspl, a gain of function allele. Expression of wild type CK2α does not alter bristle patterning in N+ or suppress the retinal defects of Nspl, consistent with our findings that levels of (endogenous) CK2 are not rate limiting for N signaling ((Bose et al., 2006) and Kahali et al, Genesis, In Press). The ability of a CK2 variant that harbors only the E165D substitution to mimic the DN-effects of Tik during Drosophila neurogenesis is of interest, because this residue is located in a motif that is similar to that in human CK2α, and one previously reported to bind the phosphatase PP2A (Heriche et al., 1997). A potential role for PP2A is now supported by our findings that increased dosage of microtubule star (mts), the catalytic subunit of this phosphatase, elicits neural phenotypes in N+ and in Nspl backgrounds that closely mimic the effects of ectopic Tik, CK2α-M161K or CK2α-E165D. Our observations that loss-of-CK2 phenotypes mimic a gain-of-PP2A function suggest that coordinated activities of this kinase and phosphatase regulate inhibitory N signaling in an antagonistic manner.
Results
Molecular modeling of Tik
To better understand the potential impact of the M161K and E165D substitutions, we first modeled the Tik protein using the atomic coordinates of human CK2 (Niefind et al., 2001), which is highly similar to the fly protein. Both substitutions in Tik are located in the vicinity of the active site (Fig. 1a). Met161 is located in a hydrophobic pocket at the base of the ATP-binding site, and its replacement with Lys has been suggested to impede nucleotide binding and attenuate catalysis (Lin et al., 2002; Rasmussen et al., 2005). In contrast, Glu165 is located in a hinge region (Fig. 1a) that connects the (β-strand rich) N- and (α-helix rich) C-terminal subdomains, typical of most Ser/Thr protein kinases. In the crystal structure of human CK2 (Niefind et al., 2001), this Glu residue does not contact other regions of CK2α in its monomeric state, or participate in the CK2α-CK2β interaction (Fig. 1c) that is required for formation of the α2β2 holoenzyme (Chantalat et al., 1999; Niefind et al., 2001).
Given the location and solvent accessibility of Glu165, one might a priori predict that the Asp replacement should not alter electrostatic potential or hinge bending, and have minimal effects on catalytic functions per se. However, sequence alignments indicate that Glu165 is invariant in metazoan CK2α, but not CK2α’, subunits (Fig. 1b). With the exception of the single CK2α isoform in Drosophila (Saxena et al., 1987), metazoan organisms contain two subunits (α and α’) that are encoded by non-redundant genes (Lou et al., 2008; Xu et al., 1999). The invariant nature of Glu165 and its flanking residues in metazoan CK2α subunits (Fig. 1b) suggested that this conservation might be critical for proper function. Glu165 resides in a motif, HE(N/H)RKL (Fig. 1b), that is also conserved in SV40 t-antigen, and mediates binding of mammalian CK2 with the Ser/Thr phosphatase PP2A (Heriche et al., 1997).
Since overexpression of mts also elicits clock defects (Sathyanarayanan et al., 2004), the possibility arose that Tik is, perhaps, a fortuitous ‘double hit’; M161K attenuates kinase activity, whereas E165D perturbs binding to PP2A. To parse the contributions of these substitutions to the dominant defects of ectopic Tik during neurogenesis (see Introduction), we generated two variants of Tik, which we call CK2α-M161K and CK2α-E165D, and a construct that recapitulates the molecular lesions in TikR (Fig. 1c).
Characterization of Tik and its variants
Prior to in vivo studies, we biochemically characterized these variants. We first tested for interaction with the regulatory CK2β subunit, given its requirement for formation of the α2β2 holoenzyme. Using the interaction trap, we find that CK2α-WT, Tik, CK2α-M161K and CK2α-E165D interact robustly and equivalently with CK2β (Fig. 2a). This was not the case for TikR (Fig. 2a), even though it is expressed at levels comparable to CK2α-WT, Tik, CK2α-M161K or CK2α-E165D (Fig. 2b). The immunoreactive bands are specific to Drosophila CK2α, as this antibody does not cross-react with extracts of non-transformed cells (Fig. 2b). We also assessed for activity using a yeast complementation assay (Kuntamalla et al., 2008) in which the lethality of yeast lacking endogenous CK2 is fully rescued by Drosophila CK2α (Fig. 2c). Using this assay, we find that CK2α-E165D rescued akin to CK2α-WT, whereas CK2α-M161K elicited weak rescue (slow growth) in a temperature-sensitive manner (Fig. 2c). In contrast, Tik did not rescue lethality of yeast at 29oC (or at a lower temperature of 25oC), suggesting that the two mutations have additive effects (data not shown). Similarly, TikR was found to be nonfunctional for rescue (data not shown). These studies suggest that CK2α-M161K is impaired for activity, while CK2α-E165D more closely resembles CK2α-WT (in yeast).
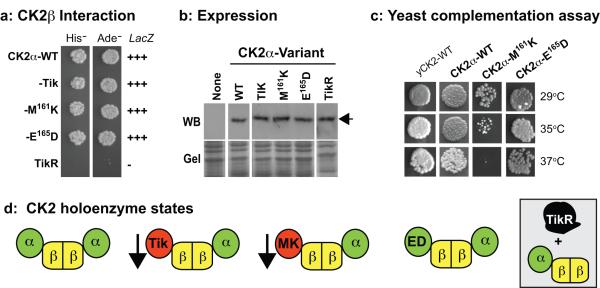
Figure-2. Biochemical analysis of CK2α variants in yeast.
(a) Interaction trap analysis. Cells expressing CK2α-variants plus CK2β were tested for induction of the reporters on media lacking His or Ade, and for LacZ, which is reported as strong (+++) or none (−). (b) Expression of CK2α variants. Arrow denotes dCK2α protein. (c) Complementation of the lethality of yeast lacking endogenous CK2. Yeast cells were rescued by a plasmid encoding yeast-CK2 (yCK2-WT), or were rescued by plasmids containing the indicated Drosophila CK2α-variants. Growth was assessed at the indicated temperatures. (d) Tik and CK2α-M161K are deficient for kinase activity (arrow), but interact normally with CK2β. Both CK2α-E165D and CK2α-WT retain activity and CK2β-binding. TikR is non-functional for structure and activity.
Our biochemical analysis suggests that the DN-effects of ectopic Tik on inhibitory N signaling (see Introduction) might reflect its ability to interact with CK2β and ‘poison’ the holoenzyme (Fig. 2d). If attenuated CK2 activity underlies these effects, CK2α-M161K is likely to also behave as a DN-construct. On the other hand, CK2α-E165D should behave akin to CK2α-WT (Fig. 2d), but only if the E165D substitution is silent in Drosophila. The revertant behavior of TikR likely reflects aberrant folding that impairs interaction with CK2β and incorporation into the holoenzyme (inset in Fig. 2d). No in vivo analysis was conducted with TikR as it appears nonfunctional for activity or interaction with CK2β.
CK2α-M161K and CK2α-E165D elicit dominant bristle defects in N+ flies
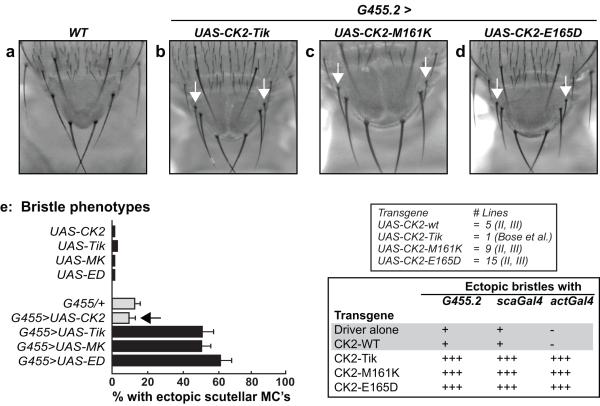
To compare the activities of CK2α-M161K and CK2α-E165D, we used the ectopic bristle defects of overexpressed Tik (see Introduction). To eliminate position effects, multiple independent lines harboring UAS-constructs were generated (inset in Fig. 3e), and expression was driven in a localized (G455.2 and scaGal4) or ubiquitous (actGal4) manner. Expression with G455.2 is restricted to the scutellar PNC’s, whereas that with scaGal4 encompasses the scutellar and thoracic PNC’s (Giebel and Campos-Ortega, 1997). Expression of Tik with G455.2 elicited ectopic scutellar bristles (Fig. 3b), and a similar phenotype was observed upon expression of CK2α-M161K as well as CK2α-E165D (Fig. 3c, d). These ectopic bristles were observed upon expression of all of the UAS-lines encoding CK2α-M161K or CK2α-E165D (data not shown), but were not seen upon expression of wild type CK2α(see Fig. 3e, 5 lines tested). Moreover, none of these bristle defects are intrinsic to any of the UAS-lines, by themselves (see below), indicating that expression of the variants was required. Similar results were obtained with scaGal4 or actGal4 (inset in Fig. 3e).
Figure-3. Ectopic bristle phenotypes of CK2α variants.
(a-d) The indicated CK2α-variants were expressed with G455.2. The arrows denote ectopic scutellar bristles. (e) Quantitation of the bristle defects. Inset shows number of lines of UAS-constructs and chromosomal locations. Summary of bristle defects upon expression of CK2α/variants with G455.2, scaGal4 and actGal4. Note that ‘Driver alone’ or expression of CK2-WT elicits baseline bristle defects with similar (+) penetrance. In contrast, expression of Tik, CK2α-M161K or CK2α-E165D elicits ectopic bristles with enhanced (+++) severity.
We next quantified the ectopic bristle phenotype. In the case of G455.2/+ flies, ~15% display ectopic bristles, and this number was used as the ‘baseline’ (Fig. 3e). When expressed with G455.2, ectopic bristles were found in 50-60% of flies upon overexpression of Tik, CK2α-M161K or CK2α-E165D (Fig. 3e), a 3- to 4-fold increase over the baseline (G455.2/+). Quantitatively similar results were obtained upon expression of 9 UAS-CK2α-M161K and 15 UAS-CK2α-E165D lines (data not shown). The close correspondence between Tik and CK2α-M161K suggests that M161K significantly attenuates CK2 activity on its own, a finding supported by the yeast bioassay (see above). The similar penetrance of ectopic bristle in flies overexpressing CK2α-E165D was unexpected, because overexpression of wild type CK2α was without effect, i.e., it elicited ectopic bristles in ~10% flies, a number similar to the baseline (G455.2/+ flies, see Fig. 3e). The lack of any effect of wild type CK2α is not due to a non-expressed (or defective) transgene, because expression of this UAS-construct (line) fully suppresses the eye/R8 defects of a UAS-CK2α-RNAi construct (Bose et al., 2006). The presence of ectopic bristles in the relevant UAS lines, by themselves, was typically ≤5% (Fig. 3e), indicating that expression was necessary. Quantitatively similar results were obtained upon expression of all three variants with scaGal4 or actGal4 (Fig. 3e, and data not shown). The ectopic bristles of CK2α-E165D, with different drivers and multiple (15 lines) UAS-insertions, suggests that this variant does not behave akin to wild type CK2α in vivo and is likely to be functionally perturbed.
CK2α-M161K and CK2α-E165D suppress the retinal defects of Nspl
To independently assess our finding on CK2α-E165D we next employed the recessive split allele, Nspl. It has been previously shown that this gain-of-function allele renders R8 precursors sensitive to inhibitory N signaling (Li et al., 2003). Consequently, in Nspl flies, both patterning and differentiation of the R8 photoreceptors are impaired. Since differentiated R8′s serve as ‘founding’ photoreceptors and are necessary for recruitment of all other retinal cell types (reviewed in (Frankfort and Mardon, 2002; Hsiung and Moses, 2002)), this manifests in the adult as a uniformly rough and reduced eye (Fig. 4a). It has been previously shown that the rough and reduced eye of Nspl is suppressed by Df(3R)BX22 (Shepard et al., 1989), a deficiency that uncovers m5, m7, m8 and groucho. Since m8, a CK2 target, is expressed during R8 patterning (Ligoxygakis et al., 1999), and that m5/7 are not expressed in the developing eye (Cooper et al., 2000), we have tested and found that reduced CK2 activity (UAS-Tik or UAS-CK2α-RNAi) also suppresses the retinal defects of Nspl with a potency similar to that of Df(3R)BX22 (Kahali et al, Genesis, In Press). The possibility thus arose that decreased CK2 activity elicits hypo-phosphorylation of M8, which would then attenuate inhibitory N signaling in the sensitized R8′s (in Nspl). Thus Nspl appears to provide a background that is highly sensitive to lowered CK2 activity.
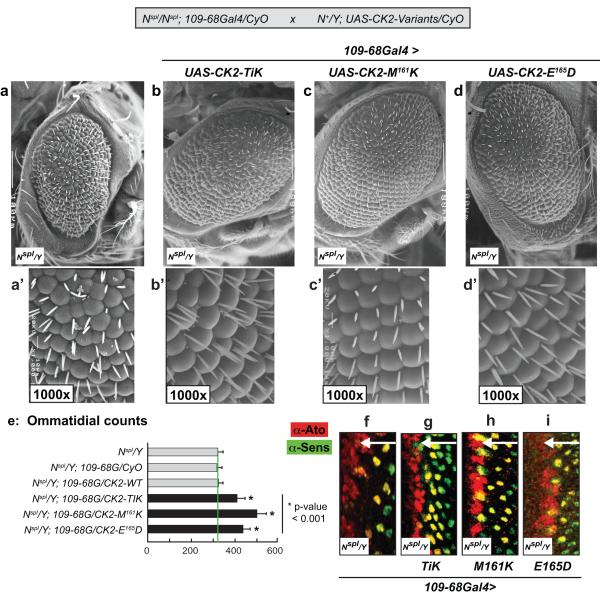
Figure-4. Suppression of the retinal defects of Nspl by CK2α-variants.
Crosses were conducted as shown (inset, grey box), and eye phenotypes were assessed in (Nspl/Y) males. (a-d) UAS-constructs were expressed using 109-68Gal4. Magnifications are 200x and anterior is to the right. (e) Ommatidial (facet) counts were determined in ≥15 flies of the indicated genotypes; 109-68G denotes 109-68Gal4. (f-i) Eye discs were stained with α-Ato (Red) and α-Sens (Green) to assess R8 patterning and differentiation, respectively. Arrows denote direction of MF progression.
To assess for suppression of the retinal defects of Nspl, we expressed the CK2α-variants in Nspl males using 109-68Gal4. This driver elicits expression in the morphogenetic furrow (MF) and in R8 precursors (Powell et al., 2004), and does not, by itself, modulate the retinal defects of Nspl (see Figs. 4a, 5e). Expression of Tik or CK2α-M161K with 109-68Gal4 suppressed the rough eye of Nspl, and appeared to restore ommatidial phasing; this effect was more pronounced in the ventral half of the eye (Fig. 4b, b’ and c, c’). Furthermore, suppression by CK2α-M161K was stronger (compare Fig. 4c’ and b’), and this variant also restored patterning of the interommatidial bristles (IOB’s) at alternating positions in the ommatidial lattice, akin to that in wild type flies. While the lower potency of Tik might reflect weaker expressivity of the UAS-construct (expression levels), this variant nevertheless enhanced the IOB defects of Nspl (compare Fig. 4b’ and a’). Expression of CK2α-E165D also suppressed the rough and reduced eye (Fig. 4d, d’), but did not suppress the IOB defects; in this case multiple IOB’s were often found, as with Tik (compare Fig. 4d’ and b’). Expression of CK2α-WT was without effect, in agreement with our previous findings (Kahali et al, Genesis, In Press, and see below). As controls, we tested and found that Nspl/Y; UAS-CK2-variants/CyO flies (absence of 109-68Gal4) displayed the rough/reduced eye and IOB defects of Nspl (data not shown). Expression of the CK2-variants was therefore necessary for suppression of the ommatidial and/or IOB phenotypes of Nspl.
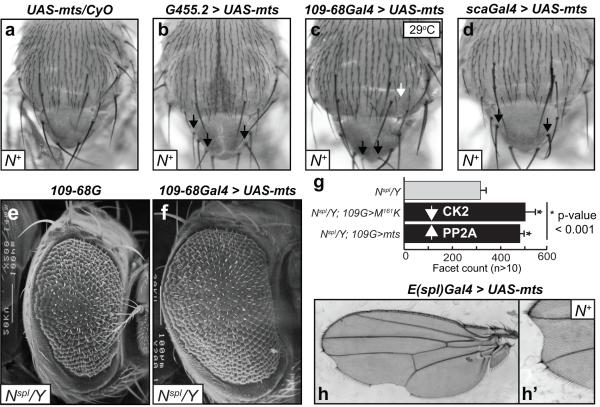
Figure-5. Increased dosage of mts elicits bristle, eye, and wing phenotypes akin to N loss of function.
(a-d) Ectopic bristle phenotypes upon overexpression of mts in N+ flies. Crosses were at 24°C, except for 109-68Gal4 (29°C). The arrows denote ectopic bristles. (e-f) UAS-mts was expressed with 109-68Gal4 in Nspl males. Magnifications are 200x and anterior is to the right. (g) Ommatidial (facet) counts were determined in ≥15 flies of the indicated genotypes; 109-68G denotes 109-68Gal4. (h) Notched wing defects upon expression of mts with E(spl)Gal4. The relevant areas of two wings are shown.
To quantify the suppression (rescue) of Nspl, we determined the ommatidial (facet) number in ≥15 flies of each of the relevant genotypes as described (Jones et al., 2006). Typically, Nspl males display ~323±10 facets, and this number was used as the ‘baseline’ (Fig. 4e). Virtually identical numbers were found in the control Nspl/Y; 109-68Gal4/+ flies (Fig. 4e), results consistent with adult eye phenotypes (see Figs. 4a, 5e). As previously found (Kahali et al, Genesis, In Press), overexpression of CK2α-WT did not enhance/suppress facet numbers, which were indistinguishable from those in the relevant controls (Fig. 4e). In contrast, expression of Tik, CK2α-M161K or CK2α-E165D resulted in facet numbers significantly higher than in Nspl/Y or in Nspl/Y; 109-68Gal4/+ flies (Fig. 4e). Our observation that CK2α-E165D mimics the in vivo effect of Tik (in N+ and Nspl) further supports the possibility that the E165D substitution is not silent and negatively impacts CK2 functions in vivo (see Discussion).
We next assessed whether Tik and its variants restored R8 patterning and differentiation, by staining eye discs for Ato and Senseless (Sens), respectively. Ato expression is inconsistent in the MF of Nspl/Y or Nspl/Y; 109-68Gal4/+ eye discs (Fig. 4f and data not shown), and these lowered Ato levels impair R8 differentiation (note spacing defects in Ato- and Sens-positive cells in Fig. 4f). Consistent with the adult eye phenotypes, expression of Tik, CK2α-M161K or CK2α-E165D appeared to restore R8 precursor specification (Ato-positive cells at the anterior margin of the MF, see Fig. 4g, h, i) and these cells also differentiated as R8 photoreceptors (Sens-positive cells in Fig. 4g, h, i). Expression of wild type CK2α did not elicit these effects (data not shown), consistent with its inability to suppress the rough/reduced eye of Nspl (Fig. 4e).
Increased PP2A dosage mimics the effects of lowered CK2, and elicits bristle, eye and wing phenotypes akin to N loss of function
Based on the in vivo effects of CK2α-E165D, we next tested whether increasing PP2A dosage would mimic phenotypes seen with lowered CK2 activity or altered functions. PP2A is a ubiquitously expressed heterotrimeric enzyme, composed of single catalytic and scaffolding subunits, and multiple regulatory subunits (Mumby, 2007; Xu et al., 2006). As information on expression/function of the diverse regulatory subunits during eye development is incompletely understood, we focused our efforts on mts, which encodes the unique catalytic subunit. To enable a comparison to our results with the CK2 variants, we tested the effects of mts overexpression in N+ and in Nspl backgrounds.
Expression of a UAS-mts construct with G455.2, 109-68Gal4, or scaGal4 elicited ectopic bristles (Fig. 5b-d) and in all three cases the percent flies displaying these bristle defects was ~50-60%, a number approximately 3-fold higher than the Gal4 drivers, by themselves (data not shown, and see Fig. 3e). In the case of 109-68Gal4, however, expression at 29oC was required consistent with observations (by others and us) that expressivity of this driver is temperature-dependent (Kahali et al., 2009; White and Jarman, 2000). Importantly, these bristle defects were not intrinsic to the UAS-mts insertion (Fig. 5a), indicating that overexpression was required. Thus increased mts dosage elicits a N loss of function phenotype, and mimics the effects of decreased CK2 activity (Tik, CK2α-M161K, Fig. 4), or to expression of a variant (CK2α-E165D, Fig. 4) that is altered for the putative PP2A binding site.
Based on these findings, we tested whether ectopic mts would suppress the rough and reduced eye of Nspl. Indeed, overexpression of mts by 109-68Gal4 suppressed the rough and reduced eye of Nspl (Fig. 5e, f). This suppression manifests as restored ommatidial phasing and is more pronounced in the ventral half of the eye (Fig. 5f), akin to the effects of CK2α-M161K or CK2α-E165D (see Fig. 4). Moreover, overexpression of mts increased facet numbers to levels similar to those with Tik, CK2α-M161K or CK2α-E165D (Figs. 5g, 4e). Thus the effects of decreased CK2 functions in N+ or Nspl backgrounds are also recapitulated by increased PP2A dosage. Given the suppressive effects of mts overexpression, we tested and found that overexpression of an mts-DN construct (Sathyanarayanan et al., 2004) with 109-68Gal4 did enhance the retinal defects of Nspl, i.e., it elicited a severely reduced eye (data not shown). However, these results were deemed to not be informative, because loss of PP2A activity elicited cell lethality and hypoproliferation of the eye imaginal discs, consistent with a requirement of this phosphatase for cell viability (reviewed in (Eichhorn et al., 2009)).
Expression of mts with E(spl)Gal4 elicited a notched wing (Fig. 5h, h’). Moreover, the regions of wing nicking were also devoid of the mechanosensory (stout) bristles at the anterior wing margin or the wing hairs at the posterior wing margin (Fig. 5h, h’). These wing nicking and wing margin bristle phenotypes have been described with loss of N pathway components (Go and Artavanis-Tsakonas, 1998; Verheyen et al., 1996). It appears that increased dosage of mts also reduces N signaling activity during wing development. While expression of Tik, CK2α-M161K or CK2α-E165D does not elicit such effects (data not shown), a notched wing phenotype is, in fact, elicited, in an allele-specific manner, between Tik and alleles of N (Kahali and Bidwai, In preparation). In summary, our studies demonstrate that decreased CK2 functions or increased PP2A dosage attenuate N signaling and elicit phenotypes characteristic of loss of N functions such as ectopic bristles, suppression of Nspl, and notched wings. We conclude that CK2 and PP2A exert antagonistic effects on inhibitory N signaling.
Discussion
Inhibitory N signaling is vital for stereotyped patterning of sense organs such as the eye and the bristles. This signaling pathway is required for proper SOP/R8 selection and involves cell-cell communications. Specifically, the future SOP/R8 cell expresses the highest levels of the N ligand, Delta, which activates N in all cells of the PNC, but the future SOP/R8 (Baonza and Freeman, 2001; Simpson, 1990; Simpson et al., 1992). This, in turn, elicits expression of the E(spl) repressors, a family of homologous basic-helix-loop-helix (bHLH) proteins (Delidakis and Artavanis-Tsakonas, 1991; Jennings et al., 1994; Klambt et al., 1989). These bHLH proteins, along with the co-repressor Groucho, then antagonize ASC/Ato (reviewed in (Bray, 2006)). As a result, cells that receive N signaling are redirected from adopting the default (SOP/R8) neural fate. This model reflects the findings that loss of inhibitory N signaling leads to excess SOP and R8 specification, which manifest as ectopic bristles and rough eyes, respectively. It is, therefore, important to fully define the mechanisms that regulate this critical step in neural patterning.
Earlier studies suggested that transcription of E(spl) and the ensuing rise in protein levels was, perhaps, sufficient for restriction of the R8/SOP fate. Accumulating evidence, however, suggests that phosphorylation of E(spl) proteins is important for repression. Evidence has so far been obtained for M8 and its structurally related repressor Hairy, and in either case phosphorylation by CK2 augments repression in the eye and/or the bristle (Kahali et al., 2008; Karandikar et al., 2004). It has, however, remained unclear whether protein phosphatases act to oppose CK2 functions. The characterization of such a regulation would open the possibility that phosphorylation and repression by E(spl) (inhibitory N signaling) is dynamically controlled in vivo. A role for PP2A has been implicated in studies showing ectopic bristle defects upon increased dosage of the regulatory subunits widerborst (wdb) or twins (tws) (Abdelilah-Seyfried et al., 2000; Shiomi et al., 1994) and in screens for modifiers of N (Muller et al., 2005). However, interactions between PP2A and alleles of N, such as Nspl have not yet been described. The studies we describe provide new insights into the genetic behaviors of Tik and its revertant allele TikR, and implicate a tripartite regulatory nexus, involving CK2, PP2A and inhibitory N signaling.
As stated above (see Introduction), both Tik and TikR lack CK2 kinase activity (in vitro). The severe clock defect of Tik/+ flies is, however, not observed in TikR/+ animals (Lin et al., 2002), and in this sense TikR meets the criteria of a revertant allele. Our studies suggest that the TikR protein is not only devoid of kinase activity, but more importantly is deficient for binding CK2β, a prerequisite for CK2-holoenzyme formation and for proper functions in vivo (Jauch et al., 2006). The most parsimonious interpretation is that misfolding of TikR prevents its incorporation into the holoenzyme. It seems reasonable to, therefore, suggest that the ability of Tik to incorporate into and ‘poison’ the endogenous holoenzyme (by binding CK2β) underlies its strong DN effects in vivo. However, it has been generally thought that these effects of Tik primarily reflect the M161K, but not the E165D, substitution. Our studies on site-specific variants, suggest that these substitutions have additive effects on activity and N signaling, and Tik is likely to therefore be a ‘double hit’.
The studies we present in N+ and Nspl backgrounds provide evidence that both substitutions in Tik affect proper CK2 functions. How might one interpret the effects on Nspl? Unlike the bristle, where N signaling occurs only after the specification of the bristle PNC’s (reviewed in (Gibert and Simpson, 2003; Modolell, 1997; Simpson et al., 1999)), the development of patterned founding R8 photoreceptors requires N signaling in a biphasic manner in the MF of the developing third instar eye disc (reviewed in (Baker, 2002; Baker et al., 1996)). At the anterior margin of the MF, N elicits ato expression (for R8 specification), whereas in the MF it drives expression of E(spl) enabling refinement of a single R8 cell from the PNC’s (Ligoxygakis et al., 1998). Nspl only perturbs the latter. Specifically, Nspl renders R8 precursors hypersensitive to inhibitory N signaling, and consequently impairs R8 differentiation (Li et al., 2003). These impaired R8′s are defective in the presentation of signals such as Hedgehog and Decapentaplegic, whose activities are necessary for ato expression at the anterior margin of the MF. As a result, the reduced ato expression in the MF of Nspl perpetuates throughout retinal histogenesis, and elicits the rough and reduced eye of Nspl. Consistent with the notion that this allele renders R8′s sensitive to inhibitory N signaling, the retinal defect of Nspl are strongly suppressed by conditions that attenuate E(spl) activity, such as halved dosage of Delta or E(spl) (Brand and Campos-Ortega, 1990; Shepard et al., 1989), or by reduced CK2 activity (Kahali et al, In Press, and this report).
The dominant-negative effects of CK2α-M161K and CK2α-E165D in N+ and in Nspl animals are likely to involve the ability of either variant to robustly interact with CK2β and efficiently incorporate into the endogenous holoenzyme, in a manner akin to wild type CK2α (see Fig. 2d). We suggest that incorporation of the former variant attenuates endogenous CK2 activity. In contrast, the dominant-negative effects of the E165D substitution might not involve impaired CK2 kinase activity, but instead reflect its ability to perturb the interaction of endogenous CK2 with PP2A, an interaction that is increasingly suspect in the regulation of this protein phosphatase. These possibilities are addressed below.
The effects of CK2α-M161K in N+ or in Nspl are easier to reconcile given its position in the ATP-binding site. This substitution substantially impairs kinase activity (Fig. 2), and consequently ectopic CK2α-M161K mimics the neural defects of knockdown of this enzyme by RNAi (Bose et al., 2006). It would therefore seem to be the case that ectopic CK2α-M161K binds CK2β, efficiently incorporates into the endogenous CK2-holoenzyme and attenuates activity, and this lowered activity impairs phosphorylation of, and repression by, endogenous E(spl). If so, this will reduce the ‘strength’ of inhibitory N signaling and elicit ectopic bristles in N+, and suppress the eye/R8 defects of Nspl. The effects of CK2α-M161K in these three developmental contexts (Fig. 3, 4) are consistent with this model.
However, the behavior of the E165D substitution was unexpected. Our suggestion that this substitution exerts a negative impact on CK2 functions is supported by multiple findings, in addition to the extraordinary conservation of Glu165 in metazoan CK2α subunits (Fig. 1). First, CK2α-E165D elicits ectopic bristles in N+ and suppresses the retinal defects of Nspl (Figs. 3, 4), and these effects are observed with multiple independent insertions and with multiple drivers. Second, CK2α-E165D restores eye size and the hexagonal phasing of the facets in Nspl, akin to Tik or CK2α-M161K. Third, CK2α-E165D appears to restore Ato expression anterior to the MF and increases the number of Sens-positive R8 cells at its posterior margin (Fig. 4). Therefore, its effects closely correlate, in time and space, to R8 cell specification, which is defective in Nspl (see above). Together, these results suggest that the E165D substitution impairs CK2 functions. These functions, however, might not involve perturbed kinase activity per se, but may instead be related to the interaction of this enzyme with PP2A (see below).
Our studies with mts overexpression are of interest, because to our knowledge this is the first demonstration that increased dosage of the PP2A catalytic subunit elicits developmental defects that are hallmarks of loss of N functions (Fig. 5). Specifically, mts overexpression elicits ectopic bristles and notched wings in N+ flies, and suppresses the retinal defects of Nspl. Furthermore, its effects on restored ommatidial phasing and eye size (facet numbers) are comparable to those seen with Tik, CK2α-M161K or CK2α-E165D. These studies lead us to suggest that interaction of PP2A with CK2 down-regulates phosphatase activity, perhaps by competing with the regulatory subunit such as Wdb, which is essential for target recognition and dephosphorylation (reviewed in (Eichhorn et al., 2009)). Such a mechanism would reflect the mutually exclusive binding of the catalytic (Mts) subunit of PP2A with Wdb or SV40 t-antigen (Guergnon et al., 2006; Mateer et al., 1998). If so, ectopic Mts would override the binding capacity of endogenous CK2, and upon recruiting Wdb attenuate repression by E(spl) through dephosphorylation.
This model could account for the dominant-negative effects of CK2α-E165D (see above). In this case, ectopic CK2α-E165D would bind CK2β, incorporate into the endogenous CK2-holoenzyme, and impair PP2A binding and downregulation. Its effects should therefore mimic Mts overexpression, a proposal that is consistent with our findings. If so, overexpression of CK2-E165D probably leads to enhanced PP2A activity. In contrast, the effects of ectopic CK2α-M161K more likely reflect a negative influence on CK2 activity itself, and suggest that this variant may represent a more precise dominant-negative construct of CK2.
The possibility arises that a precise regulation of repression by E(spl) proteins involves a balance between the opposing activities of CK2 and PP2A, perhaps involving direct interactions. Indeed, direct interactions between CK2 and PP2A have been identified by proteomic analysis in the mouse model (Arrigoni et al., 2008) and in cultured cells (Heriche et al., 1997). While consensus sequences for kinases are easier to identify computationally and biochemically, similar analysis with phosphatases has been less forthcoming (reviewed in (Eichhorn et al., 2009)). For example, in the case of Period (Per), the central clock protein, coordinated activities of CK2, CK1, and PP2A are required for proper function (Bae and Edery, 2006; Kim and Edery, 2006; Sathyanarayanan et al., 2004). While Per is phosphorylated by CK2 and CK1 in vitro and in vivo, evidence for its dephosphorylation by PP2A is lacking especially as it relates to its site preference(s). In the future it will be important to determine whether E(spl) proteins are direct targets of PP2A, and if so how a balance between PP2A and CK2 activities regulates repression. PP2A may play a similar role in the regulation of mammalian Hes6 (the homolog of fly M8), given its phosphorylation by CK2 (Gratton et al., 2003). A reversible switch could be important in neural patterning to confer a rapid and precise temporal control over the onset of repression, or prevent a protracted block to the neural fate once resolution of the PNC has occurred and the SOP’s and R8′s have been selected.
Methods
Construction of CK2 variants
Variants of Tik (CK2α-M161K, and CK2α-E165D) were generated by PCR based mutagenesis of wild type CK2α, and contained BamH1 and Xho1 sites 5′ and 3′ to the open reading frame, respectively. TikR was PCR amplified using DNA from TikR/Tm3, Sb1, Ser1 flies. The PCR product was enriched for TikR by digestion with Mfe1, which only cleaves wild type CK2α. The TikR PCR product is also flanked with BamH1 and Xho1 sites 5′ and 3′ to the open reading frame, respectively. All constructs were verified by sequencing.
Biochemical analysis
Interactions of CK2α with CK2β were assessed using a mating two-hybrid system (James et al., 1996). CK2α-variants were expressed as activation-domain fusions in the vector pOAD, and CK2β was expressed as a DNA-binding domain-fusion of Gal4 in the vector pOBD. Interactions were assessed in yeast strains PJ69-4a and PJ69-4α, and induction of HIS3, ADE2 and LacZ was as described (James et al., 1996). Expression levels were assessed by Western blot using rabbit anti-Drosophila embryo CK2 (1:1000 (Karandikar et al., 2004)) and goat-anti-rabbit IgG coupled to alkaline phosphatase (1:3000, Bio-Rad).
Rescue of yeast lacking endogenous CK2 subunits were conducted in yeast strain YDH8. Strain YDH8 harbors a double disruption of the yeast CKA1+2 genes and is rescued by a LEU2-marked plasmid containing a temperature-sensitive allele of yeast CKA2, pcka2-8 (Hanna et al., 1995). CK2α variants were cloned into pESC-URA, a GAL1/10-inducible vector (Stratagene). The resulting plasmids were used to transform yeast strain YDH8 (Hanna et al., 1995). The pcka2-8 plasmid was evicted as described (Kuntamalla et al., 2008), and cells rescued by dCK2α-variants were confirmed by leucine auxotrophy.
Molecular modeling
Swiss-pdb viewer was used to generate the molecular models used in this study. The molecular model of Tik was generated using the coordinates of human CK2α (PDB file 1jwh (Niefind et al., 2001)).
Fly stocks, crosses and phenotypes
Flies harboring the UAS-constructs encoding CK2α-wt and Tik have been previously described (Bose et al., 2006). For in vivo expression, cDNA’s encoding CK2α-M161K and CK2α-E165D were cloned into the plasmid pUAST (Brand and Perrimon, 1993). Aside from the mutations, all 4 constructs are identical in length and contain the same 5′ and 3′ ends relative to the open reading frame. Germ line transformants were generated using a commercial embryo injection facility (BestGene, Inc.) as described (Rubin, 1983). w+ progeny were identified and the location of insertions was determined via crosses to lines harboring chromosomes carrying dominant visible markers. Between 5-15 independent insertions of each construct have been used in these studies.
Flies were raised at 24oC on standard Yeast-Glucose medium. The Gal4 drivers used in these studies are G455.2 (Giebel and Campos-Ortega, 1997), scaGal4 (Nakao and Campos-Ortega, 1996), actGal4, and 109-68Gal4 (Jarman and Ahmed, 1998). Fly heads were dehydrated by sequential passes through a graded alcohol series (25-50-75-absolute) and finally through Hexamethyldisalizane. Heads were mounted on EM stubs, dried for 24 hours, sputter coated with gold, and examined with a JEOL-6400 scanning electron microscope at an accelerating voltage of 20 kV. For bristle phenotypes, newly eclosed adults were photographed. For quantitative analysis of the bristle phenotypes, multiple crosses were established (≥triplicates), and adults were scored for bristle artifacts. In every case multiple independent insertions of UAS-constructs were used.
Late third instar larval imaginal discs were isolated and processed as described (Bose et al., 2006). The following antibodies were used in this study: rabbit anti-Ato (1:1000, gift of Yuh Nun Jan) and guinea pig anti-Sens (1:800, gift of Hugo Bellen). Secondary antibodies (Molecular Probes) were goat-anti rabbit-IgG coupled to Alexa Fluor 594 (1:1000) and donkey anti-guinea pig-IgG coupled to Alexa Flour 488 (1:1000). Discs were mounted in Vectashield. An Olympus FluoView (FV1000) was used for confocal imaging. Images were acquired every 1 μm along the apicobasal axis of the discs and then compressed as a Z-stack. No layers were omitted.
Acknowledgments
We thank Sophia Zhang for technical assistance. We are grateful to Ravi Allada and Amita Sehgal for flies, Yuh Nung Jan for anti-Ato antibodies, and Hugo Bellen for anti-Sens antibodies. We thank Diane Schwegler-Berry (NIOSH, CDC) for assistance with electron microscopy. This work was supported by a National Institutes of Health Grant EY015718 to A.P.B.
References
- Abdelilah-Seyfried S, Chan YM, Zeng C, Justice NJ, Younger-Shepherd S, Sharp LE, Barbel S, Meadows SA, Jan LY, Jan YN. A gain-of-function screen for genes that affect the development of the Drosophila adult external sensory organ. Genetics. 2000;155:733–752. doi: 10.1093/genetics/155.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni G, Pagano MA, Sarno S, Cesaro L, James P, Pinna LA. Mass spectrometry analysis of a protein kinase CK2beta subunit interactome isolated from mouse brain by affinity chromatography. J Proteome Res. 2008;7:990–1000. doi: 10.1021/pr070500s. [DOI] [PubMed] [Google Scholar]
- Bae K, Edery I. Regulating a circadian clock’s period, phase and amplitude by phosphorylation: insights from Drosophila. J Biochem. 2006;140:609–617. doi: 10.1093/jb/mvj198. [DOI] [PubMed] [Google Scholar]
- Baker NE. Notch and the patterning of ommatidial founder cells in the developing Drosophila eye. In: Moses K, editor. Drosophila eye development. Springer-Verlag; Heidelberg: 2002. pp. 35–58. [DOI] [PubMed] [Google Scholar]
- Baker NE, Yu S, Han D. Evolution of proneural atonal expression during distinct regulatory phases in the developing Drosophila eye. Curr Biol. 1996;6:1290–1301. doi: 10.1016/s0960-9822(02)70715-x. [DOI] [PubMed] [Google Scholar]
- Baonza A, Freeman M. Notch signalling and the initiation of neural development in the Drosophila eye. Develop. 2001;128:3889–3898. doi: 10.1242/dev.128.20.3889. [DOI] [PubMed] [Google Scholar]
- Bose A, Kahali B, Zhang S, Lin J-M, Allada R, Karandikar U, Bidwai A. Drosophila CK2 regulates lateral-inhibition during eye and bristle development. Mech Dev. 2006;123:649–664. doi: 10.1016/j.mod.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Develop. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brand M, Campos-Ortega JA. Second-site modifiers of the split mutation of Notch defines genes involved in neurogenesis in Drosophila melanogaster. Dev Genes Evol. 1990;198:275–285. doi: 10.1007/BF00377394. [DOI] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Calleja M, Renaud O, Usui K, Pistillo D, Morata G, Simpson P. How to pattern an epithelium: lessons from achaete-scute regulation on the notum of Drosophila. Gene. 2002;292:1–12. doi: 10.1016/s0378-1119(02)00628-5. [DOI] [PubMed] [Google Scholar]
- Chantalat L, Leroy D, Filhol O, Nueda A, Benitez MJ, Chambaz EM, Cochet C, Dideberg O. Crystal structure of the human protein kinase CK2 regulatory subunit reveals its zinc finger-mediated dimerization. EMBO J. 1999;18:2930–2940. doi: 10.1093/emboj/18.11.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MTD, Tyler DM, Furriols M, Chalkiadaki A, Delidakis C, Bray SJ. Spatially Restricted Factors Cooperate with Notch in the Regulation of Enhancer of split Genes. Dev Biol. 2000;221:390–403. doi: 10.1006/dbio.2000.9691. [DOI] [PubMed] [Google Scholar]
- Delidakis C, Artavanis-Tsakonas S. The enhancer of split [E(spl)] locus of Drosophila encodes seven independant helix-loop-helix proteins. Proc Natl Acad Sci U S A. 1991;89:8731–8735. doi: 10.1073/pnas.89.18.8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2009;1795:1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Frankfort BJ, Mardon G. R8 development in the Drosophila eye: a paradigm for neural selection and differentiation. Develop. 2002;129:1295–1306. doi: 10.1242/dev.129.6.1295. [DOI] [PubMed] [Google Scholar]
- Gibert JM, Simpson P. Evolution of cis-regulation of the proneural genes. Int J Dev Biol. 2003;47:643–651. [PubMed] [Google Scholar]
- Giebel B, Campos-Ortega JA. Functional dissection of the Drosophila enhancer of split protein, a suppressor of neurogenesis. Proc Natl Acad Sci U S A. 1997;94:6250–6254. doi: 10.1073/pnas.94.12.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover CVC, Shelton ER, Brutlag DL. Purification and characterization of a type II casein kinase from Drosophila melanogaster. J Biol Chem. 1983;258:3258–3265. [PubMed] [Google Scholar]
- Go MJ, Artavanis-Tsakonas S. A genetic screen for novel components of the Notch signaling pathway during Drosophila bristle development. Genetics. 1998;150:211–220. doi: 10.1093/genetics/150.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RE, Cook O, Dinur T, Pisante A, Karandikar UC, Bidwai A, Paroush Z. An eh1-like motif in odd-skipped mediates recruitment of groucho and repression in vivo. Mol Cell Biol. 2005;25:10711–10720. doi: 10.1128/MCB.25.24.10711-10720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton M-O, Torban E, Jasmin SB, Theriault FM, German MS, Stifani S. Hes6 Promotes Cortical Neurogenesis and Inhibits Hes1 Transcription Repression Activity by Multiple Mechanisms. Mol Cell Biol. 2003;23:6922–6935. doi: 10.1128/MCB.23.19.6922-6935.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guergnon J, Dessauge F, Dominguez V, Viallet J, Bonnefoy S, Yuste VJ, Mercereau-Puijalon O, Cayla X, Rebollo A, Susin SA, Bost PE, Garcia A. Use of penetrating peptides interacting with PP1/PP2A proteins as a general approach for a drug phosphatase technology. Mol Pharmacol. 2006;69:1115–1124. doi: 10.1124/mol.105.019364. [DOI] [PubMed] [Google Scholar]
- Hanna DE, Rethinaswamy A, Glover CVC. Casein kinase II is required for cell cycle progression during G1 and G2/M in Saccharomyces cerevisiae. J Biol Chem. 1995;270:25905–25914. doi: 10.1074/jbc.270.43.25905. [DOI] [PubMed] [Google Scholar]
- Heriche JK, Lebrin F, Rabilloud T, Leroy D, Chambaz EM, Goldberg Y. Regulation of protein phosphatase 2A by direct interaction with casein kinase 2alpha. Science. 1997;276:952–955. doi: 10.1126/science.276.5314.952. [DOI] [PubMed] [Google Scholar]
- Hsiung F, Moses K. Retinal development in Drosophila: specifying the first neuron. Hum Mol Genet. 2002;11:1207–1214. doi: 10.1093/hmg/11.10.1207. [DOI] [PubMed] [Google Scholar]
- Jaffe L, Ryoo H-D, Mann RS. A role for phosphorylation by casein kinase II in modulating Antennapedia activity in Drosophila. Genes & Dev. 1997;11:1327–1340. doi: 10.1101/gad.11.10.1327. [DOI] [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA. Genomic libraries and a host strain designed for efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman AP, Ahmed I. The specificity of proneural genes in determining Drosophila sense organ identity. Mech Dev. 1998;76:117–125. doi: 10.1016/s0925-4773(98)00116-6. [DOI] [PubMed] [Google Scholar]
- Jauch E, Wecklein H, Stark F, Jauch M, Raabe T. The Drosophila melanogaster DmCK2beta transcription unit encodes for functionally non-redundant protein isoforms. Gene. 2006;374:142–152. doi: 10.1016/j.gene.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Jennings B, Preiss A, Delidakis C, Bray SJ. The Notch signaling pathway is required for Enhancer of split bHLH protein expression during neurogenesis in Drosophila. Develop. 1994;120:3537–3548. doi: 10.1242/dev.120.12.3537. [DOI] [PubMed] [Google Scholar]
- Jones C, Reifegerste R, Moses K. Characterization of Drosophila mini-me, a Gene Required for Cell Proliferation and Survival. Genetics. 2006;173:793–808. doi: 10.1534/genetics.106.056762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahali B, Bose A, Karandikar U, Bishop CP, Bidwai A. On the mechanism underlying the divergent retinal and bristle defects of M8* (E(spl)D) in Drosophila. Genesis. 2009 doi: 10.1002/dvg.20521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahali B, Trott R, Paroush Z, Allada R, Bishop CP, Bidwai AP. Drosophila CK2 phosphorylates Hairy and regulates its activity in vivo. Biochem Biophys Res Commun. 2008;373:637–642. doi: 10.1016/j.bbrc.2008.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karandikar U, Trott RL, Yin J, Bishop CP, Bidwai AP. Drosophila CK2 regulates eye morphogenesis via phosphorylation of E(spl)M8. Mech Dev. 2004;121:273–286. doi: 10.1016/j.mod.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Kim EY, Edery I. Balance between DBT/CKIepsilon kinase and protein phosphatase activities regulate phosphorylation and stability of Drosophila CLOCK protein. Proc Natl Acad Sci U S A. 2006;103:6178–6183. doi: 10.1073/pnas.0511215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klambt C, Knust E, Tietze K, Campos-Ortega JA. Closely related transcripts encoded by the neurogenic gene complex Enhancer of split of Drosophila melanogaster. EMBO J. 1989;8:203–210. doi: 10.1002/j.1460-2075.1989.tb03365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntamalla P, Kunttas E, Karandikar U, Bishop C, Bidwai A. Drosophila protein kinase CK2 is rendered temperature-sensitive by mutations of highly conserved residues flanking the activation. Mol Cell Biochem. 2008;323:49–60. doi: 10.1007/s11010-008-9963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lei L, Irvine KD, Baker NE. Notch activity in neural cells triggered by a mutant allele with altered glycosylation. Develop. 2003;130:2829–2840. doi: 10.1242/dev.00498. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Bray SJ, Apidianakis Y, Delidakis C. Ectopic expression of individual E(spl) genes has differential effects on different cell fate decisions and underscores the biphasic requirement for notch activity in wing margin establishment in Drosophila. Develop. 1999;126:2205–2214. doi: 10.1242/dev.126.10.2205. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Yu SY, Delidakis C, Baker NE. A subset of Notch functions during Drosophila eye development require Su(H) and E(spl) gene complex. Develop. 1998;125:2893–2900. doi: 10.1242/dev.125.15.2893. [DOI] [PubMed] [Google Scholar]
- Lin JM, Kilman VL, Keegan K, Paddock B, Emery-Le M, Rosbash M, Allada R. A role for casein kinase 2alpha in the Drosophila circadian clock. Nature. 2002;420:816–820. doi: 10.1038/nature01235. [DOI] [PubMed] [Google Scholar]
- Lou DY, Dominguez I, Toselli P, Landesman-Bollag E, O’Brien C, Seldin DC. The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol Cell Biol. 2008;28:131–139. doi: 10.1128/MCB.01119-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateer SC, Fedorov SA, Mumby MC. Identification of structural elements involved in the interaction of simian virus 40 small tumor antigen with protein phosphatase 2A. J Biol Chem. 1998;273:35339–35346. doi: 10.1074/jbc.273.52.35339. [DOI] [PubMed] [Google Scholar]
- Modolell J. Patterning of the adult peripheral nervous system of Drosophila. Perspect Dev Neurobiol. 1997;4:285–296. [PubMed] [Google Scholar]
- Muller D, Kugler SJ, Preiss A, Maier D, Nagel AC. Genetic modifier screens on Hairless gain-of-function phenotypes reveal genes involved in cell differentiation, cell growth and apoptosis in Drosophila melanogaster. Genetics. 2005;171:1137–1152. doi: 10.1534/genetics.105.044453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby M. The 3D structure of protein phosphatase 2A: new insights into a ubiquitous regulator of cell signaling. ACS Chem Biol. 2007;2:99–103. doi: 10.1021/cb700021z. [DOI] [PubMed] [Google Scholar]
- Nakao K, Campos-Ortega JA. Persistent expression of genes of the Enhancer of Split Complex suppress neural development in Drosophila. Neuron. 1996;16:275–286. doi: 10.1016/s0896-6273(00)80046-x. [DOI] [PubMed] [Google Scholar]
- Niefind K, Guerra B, Ermakowa I, Issinger OG. Crystal structure of human protein kinase CK2: insights into basic properties of the CK2 holoenzyme. EMBO J. 2001;20:5320–5331. doi: 10.1093/emboj/20.19.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LM, Zur Lage PI, Prentice DR, Senthinathan B, Jarman AP. The proneural proteins Atonal and Scute regulate neural target genes through different E-box binding sites. Mol Cell Biol. 2004;24:9517–9526. doi: 10.1128/MCB.24.21.9517-9526.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Skjøth IHE, Jensen HH, Niefind K, Boldyreff B, Issinger OG. Biochemical characterization of the recombinant human Drosophila homologues Timekeeper and Andante involved in the Drosophila circadian oscillator. Mol Cell Biochem. 2005;274:151–161. doi: 10.1007/s11010-005-2944-0. [DOI] [PubMed] [Google Scholar]
- Rubin G. Vectors for P element-mediated gene transfer in Drosophila. Nuc Acids Res. 1983;11:6341. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayanan S, Zheng X, Xiao R, Sehgal A. Posttranslational regulation of Drosophila PERIOD protein by protein phosphatase 2A. Cell. 2004;116:603–615. doi: 10.1016/s0092-8674(04)00128-x. [DOI] [PubMed] [Google Scholar]
- Saxena A, Padmanabha R, Glover CVC. Isolation and sequencing of cDNA clones encoding a and b subunits of Drosophila melanogaster casein kinase II. Mol Cell Biol. 1987;7:3409–3417. doi: 10.1128/mcb.7.10.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard SB, Broverman SA, Muskavitch MA. A tripartite interaction among alleles of Notch, Delta, and Enhancer of split during imaginal development of Drosophila melanogaster. Genetics. 1989;122:429–438. doi: 10.1093/genetics/122.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi K, Takeichi M, Nishida Y, Nishi Y, Uemura T. Alternative cell fate choice induced by low-level expression of a regulator of protein phosphatase 2A in the Drosophila peripheral nervous system. Develop. 1994;120:1591–1599. doi: 10.1242/dev.120.6.1591. [DOI] [PubMed] [Google Scholar]
- Simpson P. Lateral inhibition and the development of the sensory bristles of the adult peripheral nervous system of Drosophila. Develop. 1990;109:509–519. doi: 10.1242/dev.109.3.509. [DOI] [PubMed] [Google Scholar]
- Simpson P, Bourouis M, Heitzler P, Ruel L, Haenlin M, Ramain P. Delta, notch, and shaggy: elements of a lateral signaling pathway in Drosophila. Cold Spring Harb Symp Quant Biol. 1992;57:391–400. doi: 10.1101/sqb.1992.057.01.044. [DOI] [PubMed] [Google Scholar]
- Simpson P, Woehl R, Usui K. The development and evolution of bristle patterns in Diptera. Develop. 1999;126:1349–1364. doi: 10.1242/dev.126.7.1349. [DOI] [PubMed] [Google Scholar]
- Taghli-Lamallem O, Hsia C, Ronshaugen M, McGinnis W. Context-dependent regulation of Hox protein functions by CK2 phosphorylation sites. Dev Genes Evol. 2008;218:321–332. doi: 10.1007/s00427-008-0224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyen EM, Purcell KJ, Fortini ME, Artavanis-Tsakonas S. Analysis of dominant enhancers and suppressors of activated Notch in Drosophila. Genetics. 1996;144:1127–1141. doi: 10.1093/genetics/144.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N, Jarman A. Drosophila atonal controls photoreceptor R8-specific properties and modulates both receptor tyrosine kinase and Hedgehog signalling. Develop. 2000;127:1681–1689. doi: 10.1242/dev.127.8.1681. [DOI] [PubMed] [Google Scholar]
- Xu X, Toselli PA, Russell LD, Seldin DC. Globozoospermia in mice lacking the casein kinase II alpha’ catalytic subunit. Nat Genet. 1999;23:118–121. doi: 10.1038/12729. [DOI] [PubMed] [Google Scholar]
- Xu Y, Xing Y, Chen Y, Chao Y, Lin Z, Fan E, Yu JW, Strack S, Jeffrey PD, Shi Y. Structure of the protein phosphatase 2A holoenzyme. Cell. 2006;127:1239–1251. doi: 10.1016/j.cell.2006.11.033. [DOI] [PubMed] [Google Scholar]