Abstract
The anticancer agent 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) and its methyl ester (CDDO-Me) typically induce a broad spectrum of growth-inhibitory, proapoptotic, and antiangiogenic responses. Treatment of Panc1, Panc28, and L3.6pL pancreatic cancer cells with low micromolar concentrations of CDDO or CDDO-Me resulted in growth inhibition, induction of apoptosis, and down-regulation of cyclin D1, survivin, vascular endothelial growth factor (VEGF), and its receptor (VEGFR2). RNA interference studies indicate that these repressed genes are regulated by specificity protein (Sp) transcription factors Sp1, Sp3, and Sp4, and Western blot analysis of lysates from pancreatic cancer cells treated with CDDO and CDDO-Me shows for the first time that both compounds decreased the expression of Sp1, Sp3, and Sp4. Moreover, CDDO-Me (7.5 mg/kg/day) also inhibited pancreatic human L3.6pL tumor growth and down-regulated Sp1, Sp3, and Sp4 in tumors using an orthotopic pancreatic cancer model. CDDO-Me also induced reactive oxygen species (ROS) and decreased mitochondrial membrane potential (MMP) in Panc1 and L3.6pL cells, and cotreatment with antioxidants (glutathione and dithiothreitol) blocked the formation of ROS, reversed the loss of MMP, and inhibited down-regulation of Sp1, Sp3, and Sp4. Repression of Sp and Sp-dependent genes by CDDO-Me was due to the down-regulation of microRNA-27a and induction of zinc finger and BTB domain containing 10 (ZBTB10), an Sp repressor, and these responses were also reversed by antioxidants. Thus, the anticancer activity of CDDO-Me is due, in part, to activation of ROS, which in turn targets the microRNA-27a:ZBTB10-Sp transcription factor axis. This results in decreased expression of Sp-regulated genes, growth inhibition, induction of apoptosis, and antiangiogenic responses.
Extracts of plants and microorganisms and individual natural products have been extensively used as traditional medicines for the treatment of several diseases, including cancer. Individual natural products including aspirin, morphine, quinine, statins, penicillins, taxanes, and many other compounds are widely used pharmaceutical agents and serve as templates for the synthesis of more potent analogs (Koehn and Carter, 2005). Triterpenoids are derived from cyclization of oxidosqualene, and different cyclization pathways coupled with postcyclization modifications can give several thousand possible analogs, including oleanolic acid, which contains a pentacyclic oleanane skeleton and a C28 carboxyl group. Oleanolic acid has been used by Sporn, Honda, and their collaborators as a template for extensive structure-activity studies to identify anti-inflammatory drugs, and the most active compounds identified were 2-cyano-3,12-dioxoleana-1,9-dien-28-oic acid (CDDO) and its corresponding methyl (CDDO-Me) and imidazole (CDDO-Im) esters (Honda et al., 1998, 2000; Liby et al., 2007).
The anticancer activities of CDDO and related compounds have been extensively investigated in several different cancer cell lines and in vivo, and their remarkable potency is due to modulation of several important pathways (Liby et al., 2007). Initial studies showed that CDDO was a peroxisome proliferator-activated receptor γ (PPARγ) agonist (Wang et al., 2000); however, most subsequent studies indicate that the anticancer activities of CDDO and related compounds were PPARγ-independent (Melichar et al., 2004; Ray et al., 2006). The effects of CDDO, CDDO-Me, and CDDO-Im vary among different cell lines and are dependent on the specific parameters measured; however, treatment with these compounds invariably resulted in growth inhibition, antiangiogenic activity, and induction of apoptosis (Liby et al., 2007). Induction of these responses is associated with the modulation of several pathways and genes, including activation of endoplasmic reticulum stress, microtubule disruption, direct binding to specific thiol groups in proteins, inhibition of nuclear factor-κB signaling, and mitochondriotoxicity, resulting in decreased mitochondrial membrane potential (MMP) (Ikeda et al., 2003, 2004; Samudio et al., 2005, 2006; Yore et al., 2006; Yue et al., 2006; Liby et al., 2007). For example, in pancreatic cancer cells, CDDO-Im inhibits cell growth and induces apoptosis, and this is associated with decreased MMP and mitochondrial glutathione (GSH) and induction of reactive oxygen species (ROS) (Samudio et al., 2005).
Studies in this laboratory have characterized the anticancer activity of 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oic acid (CDODA) and its methyl ester (CDODA-Me), which are structurally similar to CDDO and CDDO-Me but are derived from the triterpenoid glycyrrhetinic acid, a bioactive component of licorice (Chintharlapalli et al., 2007a, 2009; Chadalapaka et al., 2008b; Papineni et al., 2008; Jutooru et al., 2009). A recent study reported that one of the underlying mechanisms of action of CDODA-Me in colon cancer cells was due to the down-regulation of specificity protein (Sp) transcription factors Sp1, Sp3, and Sp4 and Sp-dependent genes (Chintharlapalli et al., 2009). In this study, we demonstrate for the first time that CDDO and CDDO-Me also decrease the expression of Sp1, Sp3, and Sp4 and Sp-dependent gene products (VEGF, cyclin D1, surviving, and VEGFR2) in pancreatic cancer cells and tumors in an orthotopic mouse model. The mitochondriotoxicity of CDDO-Me results in decreased MMP, induction of ROS, ROS-dependent down-regulation of microRNA-27a, and induction of ZBTB10 (an Sp repressor protein), which in turn down-regulates Sp transcription factors and Sp-dependent genes. Thus, CDDO-Me-dependent repression of Sp1, Sp3, and Sp4 contributes to the potent anticancer activity of CDDO and related compounds.
Materials and Methods
Cell Lines.
Panc28 cell line was a generous gift from Dr. Paul Chiao, and L3.6pL cells were kindly provided by Dr. Isaiah Fidler (University of Texas M. D. Anderson Cancer Center, Houston, TX), and Panc1 cells were obtained from the American Type Culture Collection (Manassas, VA). The HPDE immortalized pancreatic cell line was kindly provided by. Dr. M-T. Tsao (Ontario Cancer Institute, Toronto, ON, Canada).
Antibodies and Reagents.
All three pancreatic cancer cell lines were maintained in DMEM/Ham's F-12 medium supplemented with 5% FBS, 0.22% sodium bicarbonate, and 10 ml/l of 100× antibiotic/antimycotic cocktail solution (Sigma-Aldrich, St. Louis, MO). Cells were grown in 150-cm2 culture plates in an air/CO2 (95:5) atmosphere at 37°C. Cyclin D1, Sp3, Sp4, VEGF, and VEGFR2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Cleaved PARP antibody was purchased from Cell Signaling Technology (Danvers, MA), and Sp1 antibody was purchased from Millipore (Billerica, MA). Survivin antibody was purchased from R&D systems (Minneapolis, MN). Monoclonal β-actin antibody was purchased from Sigma-Aldrich. Horseradish peroxidase substrate for Western blot analysis was obtained from Millipore. Dithiothreitol and glutathione (GSH) were obtained from Sigma-Aldrich. Superscript II, LipofectAMINE, and LipofectAMINE 2000 were purchased from Invitrogen (Carlsbad, CA). Reporter lysis buffer and luciferase reagent were purchased from Promega (Madison, WI). β-Galactosidase reagent was obtained from Tropix (Bedford, MA). Primers for TBP and ZBTB10 were purchased from Integrated DNA Technologies (Coralville, IA). Primers for Sp3 and Sp4 were obtained from QIAGEN (Valencia, CA); ZBTB10 expression vector and empty vector (pCMV6-XL4) were from Origene (Rockville, MD). miRNA mirvaRNA extraction kits and the reverse transcription and real-time polymerase chain reaction (PCR) amplification kits were purchased from Applied Biosystems (Foster City, CA). The VEGF and survivin promoter constructs were provided by Drs. Gerhard Siemeister and Gunter Finkenzeller (Institute of Molecular Medicine, Tumor Biology Center, Freiburg, Germany) and Dr. M. Zhou (Emory University, Atlanta, GA). Sp1 and Sp3 promoter constructs were kindly provided by Drs. Carlos Cuidad and Veronique Noe (University of Barcelona, Barcelona, Spain).
Cell Proliferation Assay.
Pancreatic cancer cells (3 × 104/well) were plated in 12-well plates and allowed to attach for 24 h. The medium was then changed to DMEM/Ham's F-12 medium containing 2.5% charcoal-stripped FBS, and either vehicle (DMSO) or different doses of CDDO or CDDO-Me were added. Cells were trypsinized and counted every 48 h using a Coulter Z1 particle counter for 6 days (Beckman Coulter, Fullerton, CA). Fresh medium and test compounds were added every 48 h. Each experiment was done in triplicate, and results are expressed as means ± S.E. for each treatment group.
Transfection and Luciferase Assay.
Pancreatic cancer cells (105/well) were plated in 12-well plates in DMEM/Ham's F-12 medium supplemented with 2.5% charcoal-stripped FBS. After 24 h, various amounts of DNA [i.e., 0.4 μg of PGL2-Luc, 0.4 μg of PGL3-Luc, 0.04 μg of β-galactosidase, and 0.4 μg of pSp1 (4)-Luc or 0.4 μg of pSp3-Luc or 0.4 μg of VEGF (2068)-Luc or 0.4 μg of pSurvivin (269)-Luc] were transfected using Lipofectamine reagent according to the manufacturer's protocol. Five hours after transfection, the transfection mix was replaced with complete medium containing either vehicle (DMSO) or the indicated compound in DMSO. After 22 h, cells were then lysed with 100 μl of 1X reporter lysis buffer, and cell extracts (30 ml) were used for luciferase and β-galactosidase assays. A Lumicount luminometer was used to quantitate luciferase and β-galactosidase activities, and the luciferase activities were normalized to β-galactosidase activity.
Transfection With Antisense microRNA-27a, Small Inhibitory RNAs against Sp1, Sp3, and Sp4, and ZBTB10 Expression Vector.
Pancreatic cancer cells (105/well) were plated in 12-well plates in DMEM/Ham's F-12 medium supplemented with 2.5% charcoal-stripped FBS. After 24 h, cells were transfected with empty vector (pCMV6-XL4) or 4 μg/well ZBTB10 expression plasmid pCMV6-XL4 vector using Lipofectamine 2000 reagent according to the manufacturer's protocol. After transfection for 5 h, the transfection mix was replaced with complete medium and incubated for 48 h. A similar approach was used in studies with antisense microRNA-27a (as-miR-27a) to determine its effect on cell proliferation and apoptosis. Small inhibitory RNAs for Sp1 (iSp1), Sp3 (iSp3), Sp4 (iSp4), and their combination (iSp1/3/4) were also transfected into Panc1 and L3.6pL cells, and inhibition of cell proliferation and induction of PARP cleavage were determined after 72 h. The oligonucleotides used for this study have been described previously (Chadalapaka et al., 2008a, 2010).
Western Blots.
Pancreatic cancer cells (3 × 105/well) were seeded in six-well plates in DMEM/Ham's F-12 medium containing 2.5% charcoal-stripped FBS, and after 24 h, cells were treated with either vehicle (DMSO) or the indicated compounds. Cells were collected using high-salt buffer (50 mM HEPES, 0.5 mol/l NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, and 1% Triton-X-100) and 10 μl/ml Protease Inhibitor Cocktail (Sigma-Aldrich). Protein lysates were incubated for 3 min at 100°C before electrophoresis and then separated on 10% SDS-polyacrylamide gel electrophoresis 120 V for 3 to 4 h. Proteins were transferred onto polyvinylidene difluoride membranes by wet electroblotting in a buffer containing 25 mM Tris, 192 mM glycine, and 20% methanol for 1.5 h at 180 mA. Membranes were blocked for 30 min with 5% TBST-Blotto (10 mM Tris-HCl, 150 mM NaCl, pH 8.0, 0.05% Triton X-100, and 5% nonfat dry milk) and incubated in fresh 5% TBST-Blotto with 1:500 primary antibody overnight with gentle shaking at 4°C. After washing with TBST for 10 min, the polyvinylidene difluoride membrane was incubated with secondary antibody (1:5000) in 5% TBST-Blotto for 2 h by gentle shaking. The membrane was washed with TBST for 10 min, incubated with 6 ml of chemiluminescence substrate for 1 min, and exposed to Kodak image station 4000 mm Pro (Carestream Health, Rochester, NY).
Animals and Orthotopic Implantation of Tumor Cells.
Male athymic nude mice (NCI-nu) were purchased from the Animal Production Area of the National Cancer Institute Frederick Cancer Research and Development Center (Frederick, MD). Mice were housed and maintained under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the United States Department of Agriculture, United States Department of Health and Human Services, and the National Institutes of Health. Mice of 8 to 12 weeks of age were used for the current study. L3.6pL cells were harvested from subconfluent cultures by a brief exposure to 0.25% trypsin and 0.02% EDTA. Trypsinization was stopped with medium containing 10% fetal bovine serum, and the cells were washed once in serum-free medium and resuspended in Hanks' balanced salt solution. Only suspensions consisting of single cells with >90% viability were used for the injections. Injection of cells into the pancreas was performed as described previously (Abdelrahim et al., 2006). One week after the injection of the cells, four to five mice were randomly selected and sacrificed, and the pancreas was isolated from all the animals and tested under microscope to confirm the initiation of tumor growth. The remaining mice were divided into two groups (at least five animals per group) and treated (orally) with vehicle (control) or 7.5 mg/kg CDDO-Me daily for 4 weeks. Animals were sacrificed, and the primary pancreatic tumors were isolated. All of the tumors were measured, and their weights were recorded. The tumor tissues were properly isolated into three portions for preparing 1) protein extracts (snap-frozen in liquid nitrogen and stored at −80°C); 2) RNA [treated with RNA stabilization solution (RNAlater) and then stored at −80°C]; and 3) paraffin sections for immunohistochemistry (fixed in formaldehyde).
ROS Estimation.
Cellular ROS levels were evaluated with the cell-permeable probe 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) from Invitrogen. After treatment for 20 to 24 h, cells plated on 96-well cell culture plates were loaded with 10 μM CM-H2DCFDA for 30 min, washed once with serum-free medium, and analyzed for ROS levels using the BioTek Synergy 4 plate reader (Winooski, VT) set at 480 and 525 nm excitation and emission wavelengths, respectively. After reading of ROS, cultures were then treated with Janus green, and cell counts were determined with the plate reader set to an absorbance of 610 nm, and ROS intensities were then corrected accordingly. Each experiment was done in triplicate, and results are expressed as means ± S.E. for each treatment group.
Measurement of MMP.
MMP was measured with the Mitochondrial Membrane Potential Detection Kit (Stratagene Cedar Creek, TX) according to manufacturer's protocol using JC-1 dye. Pancreatic cancer cells were plated on two-well Lab-Tex Coverglass slides (NUNC A/S, Roskilde, Denmark), and after 24 h, cells were treated with DMSO or CDDO-Me alone or with GSH for 16 h. Cells were then incubated with 1× JC-1 dye at 37°C for 15 min and washed twice with assay buffer according to manufacturer's protocol, and then cells were subjected to microscopic analysis using Zeiss Stallion Dual Detector Imaging System (Carl Zeiss Microimaging Inc., Thornwood, NY) using a C-Apochromat 63×, 1.2 numerical aperture water immersion lens. J-aggregates are detected as red fluorescence, and J-monomers are detected as green fluorescence. The ratio of red fluorescence to green fluorescence was measured using ImageJ Software (http://rsbweb.nih.gov/ij/). Cells were examined in more than 10 fields per slide on multiple slides. Data represent the average of all the fields.
Quantitative Real-Time PCR of mRNA and miRNA.
cDNA was prepared from Panc1 and L3.6pL cell lines using Superscript II reverse transcriptase (Invitrogen) according to manufacturer's protocol. Each PCR was carried out in triplicate in a 20-μl volume using SYBR GreenER (Invitrogen) for of 95°C for 10 min, then 40 cycles of 95°C for 15 s, and 60°C for 1 min in the Applied Biosystems 7500 Fast Real-time PCR System. The following primers were used: TBP (forward), 5′-TGCACAGGAGCCAAGAGTGAA-3′; TBP (reverse), 5′-CACATCACAGCTCCCCACCA-3′; ZBTB10 (forward), 5′-GCTGGATAGTAGTTATGTTGC-3′; and ZBTB10 (reverse), 5′-CTGAGTGGTTTGATGGACAGA-3′.
mirVana miRNA extraction kit (Applied Biosystems) was used for the extraction of miRNA according to manufacturer's protocol. Quantification of miRNA (RNU6B and miRNA-27a) was done using the Taqman miRNA kit (Applied Biosystems) according to the manufacturer's protocol with real-time PCR. U6 small nuclear RNA was used as a control to determine relative miRNA expression.
Statistical Analysis.
Statistical significance of differences between the treatment groups was determined by an analysis of variance and/or Student's t test, and levels of probability were noted. IC50 values were calculated using linear regression analysis and are expressed in micromolar concentrations at 95% confidence intervals.
Results
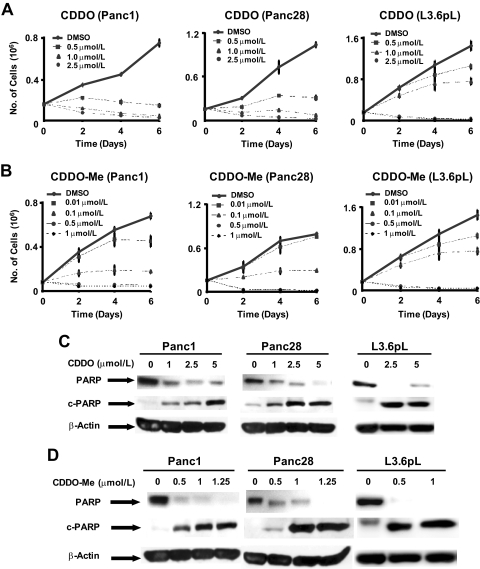
The synthetic oleanolic acid derivatives CDDO, CDDO-Me, and CDDO-Im are cytotoxic to several different cancer cell lines, and the latter derivative was a potent inhibitor of pancreatic cancer cell proliferation (Samudio et al., 2005). In this study, we initially compared the effects of CDDO and CDDO-Me on the proliferation of Panc1, Panc28, and L3.6pL pancreatic cancer cell lines for up to 144 h (Fig. 1, A and B). IC50 values for growth inhibition by CDDO-Me and CDDO after treatment for 48 h were 5.2 and 0.37 (Panc1), 5.3 and 0.37 (Panc28), and 3.0 and 0.4 μM (L3.6pL), respectively. CDDO-Me was more active than CDDO in all three pancreatic cancer cell lines, and there were minimal differences between cell lines to the cytotoxic effects of both compounds. Supplemental Fig. 1 shows that CDDO-Me also inhibits the growth of nontransformed HPDE pancreatic cells; however, this cell line was more resistant to the effects of CDDO-Me than pancreatic cancer cell lines. After prolonged treatment (144 h) with CDDO-Me and CDDO, IC50 values were 0.25 and 1.8 (Panc1), 0.30 and 2.3 (Panc28), and 0.28 and 1.4 μM (L3.6pL), respectively, and the relative potencies of both compounds and cellular responsiveness were similar to that observed after 48 h. Inhibition of Panc1, Panc28, and L3.6pL cell growth by CDDO and CDDO-Me was also accompanied by apoptosis, and Fig. 1, C and D, shows that both compounds induced caspase-dependent PARP cleavage in the pancreatic cancer cell lines.
Fig. 1.
CDDO and CDDO-Me inhibit cell growth and induce apoptosis in pancreatic cancer cell lines. Inhibition of Panc1, Panc28, and L3.6pL cell growth by CDDO (A) and CDDO-Me (B). Cells were treated with DMSO (solvent control), CDDO (0.5, 1.0, or 2.5 μM), or CDDO-Me (0.01, 0.1, 0.5, or 1.0 μM), and effects on cell growth were determined over a period of 6 days as described under Materials and Methods. Induction of PARP cleavage by CDDO (C) and CDDO-Me (D). Panc1, Panc28, and L3.6pL cells were treated with DMSO, CDDO (1.0, 2.5, or 5.0 μM), or CDDO-Me (0.5, 1.0, or 1.25 μM) for 24 h, and whole-cell lysates were analyzed by Western blot analysis as described under Materials and Methods. β-Actin served as a loading control.
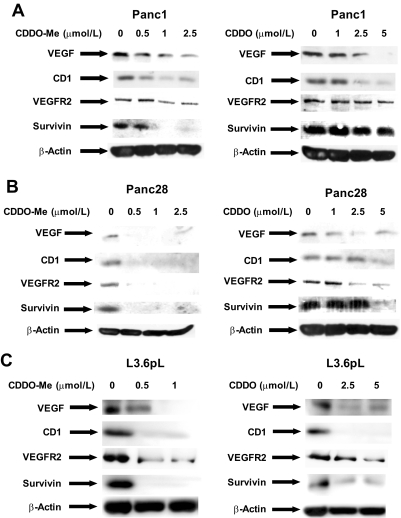
CDDO and related compounds inhibit growth, induce apoptosis, and exhibit antiangiogenic activity in cancer cells derived from multiple tumor types (Liby et al., 2007), and the effects of CDDO-Me and CDDO on the expression of prototypical gene products representing these activities were investigated in the three pancreatic cancer cell lines. Results in Fig. 2A show that 0 to 1.25 μM CDDO-Me and 0 to 5 μM CDDO decrease the expression of cyclin D1, survivin, VEGF, and VEGFR2 proteins in Panc1 cells. Moreover, similar results were observed in Panc28 (Fig. 2B) and L3.6pL (Fig. 2C) cells, and results in Figs. 1 and 2 are consistent with previous studies on CDDO and related compounds in cancer cells (Honda et al., 1998, 2000; Wang et al., 2000; Ikeda et al., 2003, 2004; Melichar et al., 2004; Samudio et al., 2005, 2006; Ray et al., 2006; Yore et al., 2006; Yue et al., 2006; Liby et al., 2007).
Fig. 2.
CDDO and CDDO-Me decrease the expression of VEGF, VEGFR2, cyclin D1 (CD1), and survivin proteins in Panc1 (A), Panc28 (B) and L3.6pL (C) pancreatic cancer cell lines. Cells were treated with DMSO, CDDO (1.0, 2.5, or 5.0 μM), or CDDO-Me (0.5, 1.0, or 1.25 μM) for 24 h, and whole-cell lysates were analyzed by Western blot analysis as described under Materials and Methods. β-Actin served as a loading control. The gels were typical of results of at least two replicate determinations per treatment group.
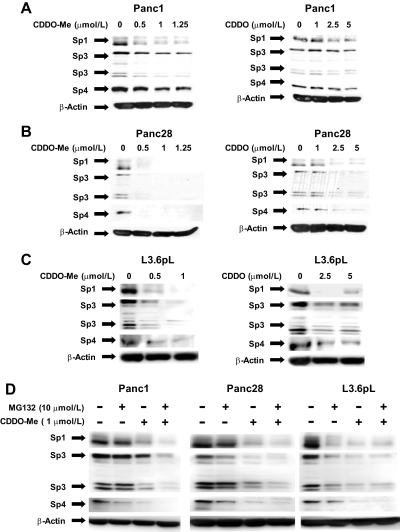
Knockdown of Sp1, Sp3, and Sp4 transcription factors by RNA interference in pancreatic cancer cells showed that VEGF, VEGFR1, and VEGFR2 expression were regulated by Sp1, Sp3, and Sp4 (Abdelrahim et al., 2004, 2006; Higgins et al., 2006), suggesting that an underlying mechanism of action of CDDO and CDDO-Me may involve the down-regulation of Sp proteins as described previously for other bioactive triterpenoids in cancer cells (Chintharlapalli et al., 2007b, 2009). Results in Fig. 3A demonstrate that 0 to 1.25 μM CDDO-Me and 0 to 5.0 μM CDDO decreased the expression of Sp1, Sp3, and Sp4 proteins in Panc1 cells, and similar results were observed in Panc28 (Fig. 3B) and L3.6pL (Fig. 3C) cells. The observed CDDO-/CDDO-Me-dependent down-regulation of Sp transcription factors (Fig. 3, A–C) is consistent with their parallel decrease in Sp-dependent genes, as illustrated in Fig. 2. Previous studies show that the nonsteroidal anti-inflammatory drug tolfenamic acid induced proteasome-dependent down-regulation of Sp1, Sp3, and Sp4 in Panc1 cells that was blocked by the proteasome inhibitor N-benzoyloxycarbonyl (Z)-Leu-Leu-leucinal (MG132) (Abdelrahim et al., 2006); however, results in Fig. 3D using CDDO-Me indicate that down-regulation of Sp1, Sp3, and Sp4 by this compound in pancreatic cancer cells was not blocked by the proteasome inhibitor MG132, indicating a proteasome-independent pathway. The proximal regions of the Sp1, Sp3, VEGF, and survivin promoters are GC-rich, and the effects of CDDO-Me and CDDO on promoter activity were investigated in Panc1 cells transfected with pSp1For4, pSp3For5, pVEGF, and pSurvivn constructs, which contain −751 to −20 (Sp1), −417 to −38 (Sp3), − 2018 to +50 (VEGF), and −269 to +49 (survivin) promoter inserts, respectively. CDDO and CDDO-Me significantly decreased luciferase (reporter gene) activity in Panc1 cells transfected with these constructs (Supplemental Fig. 2), indicating that CDDO and CDDO-Me act at the transcriptional level, and this is consistent with the observed proteasome-independent down-regulation results shown in Fig. 3D. We also observed that CDDO-Me decreased Sp1 mRNA levels (Supplemental Fig. 3), which is consistent with the inhibitory effects of this compound on transcription.
Fig. 3.
CDDO-Me down-regulates Sp proteins in a proteosome-independent manner. CDDO-Me decreases Sp protein expression in Panc1 (A), Panc28 (B), and L3.6pL (C). Cells were with DMSO, CDDO (1.0, 2.5, or 5.0 μM), or CDDO-Me (0.5, 1.0, or 1.25 μM) for 24 h, and whole-cell lysates were analyzed for Sp1, Sp3, and Sp4 by Western blot analysis as described under Materials and Methods. D, proteosome-independent down-regulation of Sp proteins by CDDO-Me. Cells were treated with DMSO and CDDO-Me (1.0 μM) in the presence or absence of proteasome inhibitor MG132 (10 μM), and the effects on Sp protein degradation were determined after treatment for 24 h by Western blot as described under Materials and Methods. β-Actin served as a loading control.
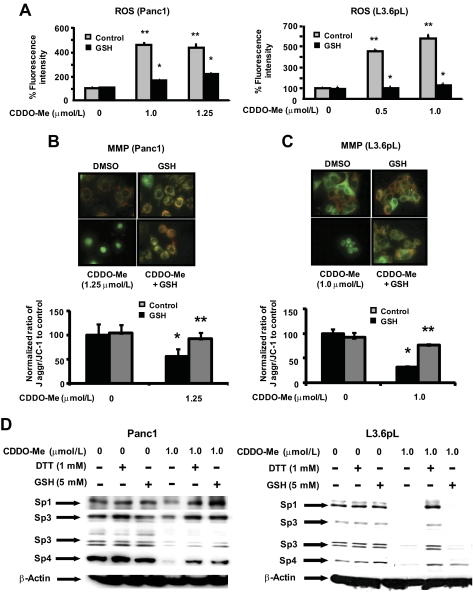
Previous reports showed that the imidazole derivative of CDDO was mitochondriotoxic in pancreatic cancer cells, and this was characterized by decreased MMP and accompanied by induction of ROS (Samudio et al., 2005). Treatment of Panc1 or L3.6pL cells with CDDO-Me also increased total ROS levels as determined by CM-H2DCFDA fluorescent dye, and this response was also attenuated after cotreatment with the antioxidant glutathione (Fig. 4A). Panc1 and L3.6pL cells were incubated with the fluorescent dye JC-1, and after treatment with DMSO or GSH, the typical orange/red fluorescent staining of mitochondria was observed (Figs. 4A). However, in Panc1 or L3.6pL cells treated with 1.25 or 1.0 μM CDDO-Me, respectively, there was a significant decrease in orange/red staining and an increase in green fluorescence indicative of decreased MMP, and this response was attenuated after cotreatment with GSH in both cell lines (Fig. 4B). These results demonstrate that CDDO-Me was mitochondriotoxic in pancreatic cancer cells, and we therefore investigated the possible connection between CDDO-Me-induced ROS and mitochondrial effects and CDDO-Me-dependent down-regulation of Sp1, Sp3, and Sp4 proteins. Results in Fig. 4C demonstrate that Sp1, Sp3, and Sp4 are down-regulated in Panc1 and L3.6pL cells after treatment with CDDO-Me, and decreased expression of Sp1, Sp3, and Sp4 proteins was inhibited after cotreatment with thiol antioxidants (DTT and/or GSH) and similar results were observed in Panc28 cells (data not shown). Hydrogen peroxide or t-butyl hydroperoxide was used as prototypical model ROS, and both compounds decreased the expression of Sp1, Sp3, and Sp4 proteins (data not shown), confirming that induction of ROS in pancreatic cancer cells is a critical upstream response in targeting the repression of Sp transcription factors, and comparable results have been observed in other cancer cell lines.
Fig. 4.
Role of oxidative stress and MMP in mediating the effects of CDDO-Me on Sp proteins in pancreatic cancer cells. Effect of CDDO-Me on ROS (A) and MMP (B and C). Panc1 and L3.6pL cells were treated with DMSO and CDDO-Me (0.5, 1.0, or 1.25 μM) for 24 h in the presence or absence of antioxidant GSH. ROS was measured using BioTek Synergy 4 plate reader using CM-H2DCFDA (10 μM) dye as described under Materials and Methods, and normalized fluorescence intensity against control is plotted as a bar diagram. MMP was determined using JC-1 dye, and quantitation of the ratio of red to green fluorescence was measured using ImageJ Software as described under Materials and Methods. D, reversal of CDDO-Me-mediated down-regulation of Sp proteins by thiol antioxidants. Cells were treated with DMSO or CDDO-Me (1.0 μM) in the presence or absence of DTT or GSH for 24 h, and whole-cell lysates were analyzed by Western blots as described under Materials and Methods. β-Actin served as a loading control. Results in A and C are expressed as means ± S.E. for three replicate determinations for each treatment group, and significant (P < 0.05) CDDO-Me-mediated decreases (*) or increases (**) after cotreatment with antioxidants compared with the solvent (DMSO) control are indicated.
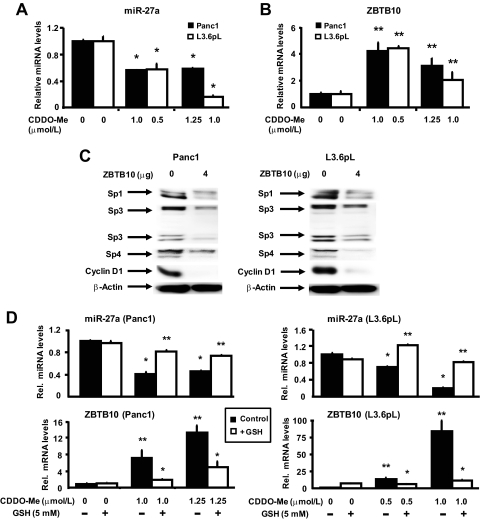
CDODA-Me, a triterpenoid structurally related to CDDO-Me, decreased Sp proteins in colon cancer cells through down-regulation of microRNA-27a (miR-27a) and induction of ZBTB10, a zinc finger Sp-repressor protein (Chintharlapalli et al., 2009). After treatment of Panc1 and L3.6pL cells with CDDO-Me, there was a decrease in expression of miR-27a (Fig. 5A), and this was accompanied by increased ZBTB10 mRNA levels (Fig. 5B). Because ZBTB10 suppresses the expression of Sp-regulated genes through competitive interactions with GC-rich promoter elements (Tillotson, 1999; Mertens-Talcott et al., 2007; Chintharlapalli et al., 2009), we investigated the effects of ZBTB10 overexpression in Panc1 and L3.6pL cells (Fig. 5C). Overexpression of ZBTB10 in pancreatic cancer cells decreased the expression of Sp1, Sp3, and Sp4 proteins, and similar results have been observed previously in breast and colon cancer cells (Mertens-Talcott et al., 2007; Chintharlapalli et al., 2009). CDDO-Me-dependent induction of ROS and down-regulation of Sp proteins is inhibited after cotreatment with antioxidants (Fig. 4), and therefore, we investigated the role of ROS induction and the effects of antioxidants on the expression of ZBTB10 and miR-27a. CDDO-Me decreased miR-27a and induced ZBTB10 in Panc1 and L3.6pL cells, and in cells cotreated with CDDO-Me plus glutathione, these responses were significantly reversed (Fig. 5D). This demonstrates that CDDO-Me-induced ROS is a common upstream factor regulating disruption of miR-27a:ZBTB10–Sp axis.
Fig. 5.
Effect of CDDO-Me on the expression miR-27a and ZBTB10 mRNA and role of ZBTB10 overexpression on Sp proteins in pancreatic cancer cells. CDDO-Me decreases miR-27a (A) and induces ZBTB10 mRNA (B). Panc1 and L3.6pL cells were treated with the indicated doses of CDDO-Me for 24 h, and miR-27a and ZBTB10 levels were analyzed by real-time PCR as described under Materials and Methods. C, effect of ZBTB10 overexpression on Sp proteins and Sp-dependent genes. Panc1 and L3.6pL cells were transfected with empty vector (pCMV6-XL4) or 4 μg/well ZBTB10 expression plasmid pCMV6-XL4 vector, and whole-cell lysates were analyzed by Western blots as described under Materials and Methods. D, effect of GSH on CDDO-Me-mediated miR-27a and ZBTB10 mRNA expression. Panc1 and L3.6pL cells were treated with the indicated doses of CDDO-Me in the presence or absence of GSH for 24 h, and miR-27a and ZBTB10 mRNA levels were analyzed by real-time PCR as described under Materials and Methods. Results in A, B, and D are expressed as means ± S.E. for three replicate determinations for each treatment group, and significant (P < 0.05) inhibition (*) or induction (**) of responses are indicated.
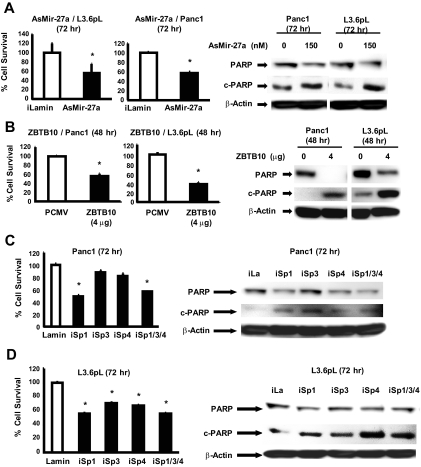
We also investigated the comparative effects of as-miR-27a, ZBTB10 overexpression, and knockdown of Sp1, Sp3, and Sp4 alone or in combination as described previously (Chadalapaka et al., 2008a, 2010; Chintharlapalli et al., 2009) on growth inhibition and induction of PARP cleavage in Panc1 and L3.6pL cells (Fig. 6). Transfection of cells with as-miR-27a inhibited the growth of Panc1 and L3.6pL cell growth (Fig. 6B), and the results were similar to that observed for as-miR-27a; however, overexpression of ZBTB10 was more potent than as-miR-27a as an inducer of PARP cleavage. Because both as-miR-27a and ZBTB10 are involved in the down-regulation of Sp1, Sp3, and Sp4 (Fig. 5C) (Mertens-Talcott et al., 2007), the effects of individual Sp knockdown (iSp1, iSp3, and iSp4) or combined knockdown (iSp1/3/4) by RNA interference on cell growth and PARP cleavage were investigated (Fig. 6, C and D). Panc1 and L3.6pL cell growth was decreased in cells transfected with iSp1/3/4, and the effects of individual Sp knockdown were variable and cell context-dependent; however, loss of Sp1 resulted in the most pronounced growth inhibition in both cell lines. In Panc1 cells, iSp1, iSp3, iSp4, and iSp1/3/4 induced PARP cleavage; however, iSp3 was the most effective (Fig. 6C), whereas iSp4 and iSp1/3/4 induced PARP cleavage in L3.6pL cells (Fig. 6D). These results demonstrate that the growth inhibitory and proapoptotic effects of CDDO-Me are also observed in pancreatic cancer cells transfected with as-miR-27a or ZBTB10 overexpression or after knockdown of Sp transcription factors.
Fig. 6.
Growth inhibition and induction of apoptosis by as-miR-27a, ZBTB10, and Sp knockdown. Panc1 and L3.6pL cells were transfected with as-miR-27a (A), ZBTB10 expression plasmid (B), and small inhibitory RNAs against Sp transcription factors (C and D), and their effects on cell proliferation and induction of PARP cleavage were determined as described under Materials and Methods. Western blots are typical of at least two replicate determinations. Cell numbers are given as means ± S.E. of three replicate determinations. Significant (P < 0.05) growth inhibition is indicated (*). RNA interference decreased expression of individual Sp proteins approximately 60 to 80% as described previously (Chadalapaka et al., 2008a, 2010).
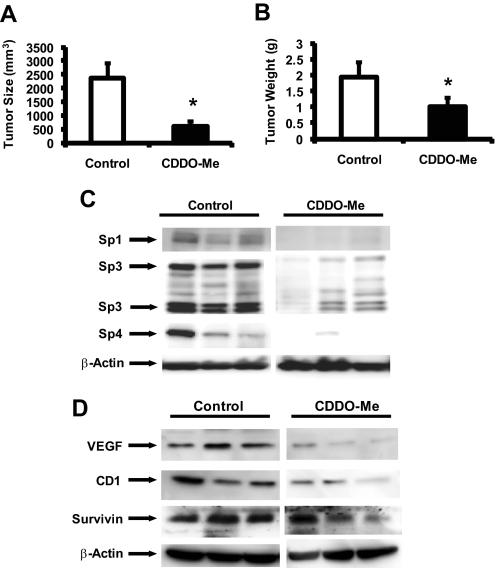
The in vivo anticancer activity of CDDO-Me was also investigated in an orthotopic model of pancreatic cancer in which L3.6pL cells were injected directly into the pancreas of 8- to 12-week-old male thymic nude mice (Baker et al., 2002; Abdelrahim et al., 2006). Treatment with CDDO-Me (7.5 mg/kg daily by oral administration) was initiated 7 days after injection of the cells and continued for an additional 28 days. Treatment with CDDO-Me significantly decreased pancreatic tumor volume and weight (Fig. 7, A and B) compared with the vehicle control group. In addition, lysate from tumors treated with the vehicle or CDDO-Me (from three different animals per group) were also analyzed by Western blots, and there was a marked decrease in expression of Sp1, Sp3, and Sp4 proteins in tumors from mice treated with CDDO-Me compared with the control group (Fig. 7C). Moreover, we also observed decreased expression of VEGF, cyclin D1, and survivin in tumors from CDDO-Me-treated mice compared with animals receiving vehicle control (Fig. 7D). Body and organ weight changes and evidence for toxicity were not observed (data not shown), and this is consistent with the applications of CDDO compounds in chemoprevention studies (Liby et al., 2007). These in vivo results are consistent with cell culture studies (Figs. 2 and 3) demonstrating for the first time that CDDO-Me represses the expression of Sp1, Sp3, and Sp4 transcription factors and Sp-dependent gene products, suggesting that this hitherto unrecognized pathway also contributes to the anticancer activity of CDDO-Me and related compounds.
Fig. 7.
CDDO-Me inhibits pancreatic tumor growth and down-regulates Sp proteins and Sp dependent genes. Tumor weights (A) and volume (B). Male athymic nude mice bearing orthotopic pancreatic (L3.6pL) tumors were treated with corn oil or CDDO-Me (7.5 mg/kg) for 4 weeks, and tumor weights and tumor volumes (in cubic millimeters) were determined as described under Materials and Methods. Significant (P < 0.05) inhibition (*) is indicated in results as means ± S.E. for five animals per treatment. Western blot analysis of tumor lysates for Sp proteins (C) and Sp-dependent proteins (D). Lysates from three mice in the treated and control groups were analyzed by Western blots as described under Materials and Methods. β-Actin served as loading control.
Discussion
Sp1 was the first transcription factor identified and is a member of the Sp/Krüppel-like family of zinc finger transcription factors that exhibit a broad range of tissue-specific and overlapping functions (Bouwman and Philipsen, 2002; Safe and Abdelrahim, 2005). Although Sp transcription factors are important during embryonic development, there is evidence that in humans and laboratory animals of a marked decrease in Sp1 with aging (Ammendola et al., 1992; Oh et al., 2007), and studies in this laboratory show that Sp1, Sp3, and Sp4 levels in nontumor tissue of mice are minimal to nondetectable (Abdelrahim et al., 2006, 2007; Chintharlapalli et al., 2007b). Several reports show that Sp1 protein is overexpressed in different human tumor types, including gastric, colorectal, pancreatic, epidermal, thyroid, and breast cancers (Zannetti et al., 2000; Shi et al., 2001; Chiefari et al., 2002; Wang et al., 2003; Hosoi et al., 2004; Yao et al., 2004; Mertens-Talcott et al., 2007; Jiang et al., 2008). In patients with gastric cancer, there was an association between Sp1 expression with advanced stage of the tumor, VEGF expression, and poor survival (Yao et al., 2004). Although the mechanism of Sp overexpression has not been determined, Lou et al. (2005) have shown that malignant transformation of human fibroblasts resulted in an 8- to 18-fold increase in Sp1 expression and the transformed cells formed tumors in athymic nude mouse xenografts. In contrast, these transformed cells were not tumorigenic after knockdown of Sp1. Sp1 is overexpressed in pancreatic cancer cells (Shi et al., 2001), and there is a correlation between the expression of Sp1 and the angiogenic factor VEGF. Moreover, it was recently reported that Sp1 was a biomarker that identifies patients with a highly aggressive subtype of pancreatic ductal adenocarcinomas (Jiang et al., 2008). Studies in this laboratory show that Sp1, Sp3, and Sp4 are overexpressed in pancreatic and other cancer lines and knockdown of Sp1, Sp3, and Sp4 by RNA interference indicates that expression of several growth-promoting, prosurvival, and angiogenic genes/responses are regulated by these transcription factors (Abdelrahim et al., 2004, 2006; Chintharlapalli et al., 2007b; Mertens-Talcott et al., 2007; Chadalapaka et al., 2008a).
The high expression of Sp1, Sp3, and Sp4 proteins in cancer cells and tumors coupled with their regulation of several critical pro-oncogenic genes suggests that these transcription factors are potentially important drug targets. The nonsteroidal anti-inflammatory drug tolfenamic acid, the triterpenoid betulinic acid, curcumin, and CDODA-Me have been characterized previously as agents that inhibit cancer cell and tumor growth in rodent models and induce down-regulation of Sp1, Sp3, and Sp4 through proteasome-dependent and -independent pathways (Abdelrahim et al., 2006; Chintharlapalli et al., 2007b, 2009; Chadalapaka et al., 2008a). CDDO/CDDO-Me and CDODA/CDODA-Me activate PPARγ and exhibit some common and different activities in colon and pancreatic cancer cells (Wang et al., 2000; Chintharlapalli et al., 2007a). A recent study in colon cancer cells showed that CDODA-Me down-regulated the expression of Sp1, Sp3, and Sp4 and Sp-regulated gene products (Chintharlapalli et al., 2009). Results in Figs. 2 and 3 show that CDDO and CDDO-Me also decreased the expression of Sp1, Sp3, and Sp4 and Sp-regulated genes such as cyclin D1, VEGF, VEGFR2, and survivin in Panc1, Panc28, and L3.6pL pancreatic cancer cells. Moreover, repression of these gene products by CDDO and CDDO-Me were observed at concentrations that were comparable with those required for inhibition of Panc1, Panc28, and L3.6pL cell growth and induction of apoptosis (Fig. 1). In contrast to previous reports with tolfenamic acid in pancreatic cancer cells (Abdelrahim et al., 2006, 2007), CDDO-Me induced proteasome-independent down-regulation of Sp1, Sp3, and Sp4 in pancreatic cancer cells (Fig. 3D). CDDO-Me also decreased luciferase activity in cells transfected with constructs containing Sp1, Sp4, VEGF, and survivin gene promoter inserts and decreased Sp1 mRNA expression (Supplemental Figs. 2 and 3), which is consistent with the effects of CDDO-Me on transcription. These in vitro cell culture studies confirm the potency of CDDO-Me as an inhibitor of pancreatic cancer cell growth and demonstrate for the first time that the anticancer activity is due, in part, to targeting of Sp transcription factors and Sp-regulated genes. Further evidence for the role of this pathway in the activity of CDDO-Me was observed in an orthotopic model for pancreatic cancer, in which CDDO-Me (7.5 mg/kg/day) not only inhibited tumor growth but also decreased the expression of Sp1, Sp3, and Sp4 proteins in pancreatic tumors (Fig. 7). These results suggest that CDDO-Me activates comparable pathways both in vitro and in vivo, and this has been observed previously for other agents that target Sp transcription factors (Abdelrahim et al., 2006, 2007; Chadalapaka et al., 2008a; Chintharlapalli et al., 2009).
CDDO-Im-dependent anticarcinogenic activity in pancreatic cancer cells has been linked to its mitochondriotoxic activity (Samudio et al., 2005), which was characterized by decreased MMP and GSH and induction of ROS, and these responses were blocked in cells cotreated with thiol antioxidants. Not surprisingly, CDDO-Me also induced a similar pattern of mitochondriotoxic responses in pancreatic cancer cells, which were inhibited after cotreatment with thiol antioxidants (Fig. 4). A recent study in prostate cancer cells also linked the proapoptotic activity of CDDO-Me to the induction of ROS (Deeb et al., 2010) Because induction of ROS and ROS-dependent responses play an important role in the anticancer activity of mitochondriotoxic anticancer drugs such as arsenic trioxide (Miller et al., 2002), we hypothesized that repression of Sp transcription factors may be an ROS-dependent response. Results in Fig. 4D show that CDDO-Me-dependent down-regulation of Sp1, Sp3, and Sp4 proteins is significantly inhibited in Panc1 and L3.6pL cells after cotreatment with GSH and DTT. Moreover, using hydrogen peroxide and t-butyl hydroperoxide as prototypical ROS, both oxidants also decreased the expression of Sp1, Sp3, and Sp proteins in pancreatic cancer cells (data not shown). These results demonstrate that the effects of CDDO-Me on mitochondria are linked to ROS-dependent down-regulation of Sp1, Sp3, and Sp4, and we have also observed similar effects with arsenic trioxide in pancreatic cancer cells (Miller et al., 2002).
The role of miR-27a and its suppression of the zinc finger protein ZBTB10 in regulating the expression of Sp1, Sp3, and Sp4 has been characterized previously in breast and colon cancer cells (Mertens-Talcott et al., 2007; Chintharlapalli et al., 2009). Both antisense miR-27a and ZBTB10 overexpression decrease Sp and Sp-dependent genes in these cell lines (Abdelrahim et al., 2006; Mertens-Talcott et al., 2007), and this is related to the effects of ZBTB10, which binds GC-rich sites and acts as a transcriptional repressor (Tillotson, 1999). Treatment of Panc1 and L3.6pL cells with CDDO-Me decreased the expression of miR-27a (Fig. 5A), and this was accompanied by the induction of ZBTB10 (Fig. 5B). These results are comparable with those observed for the structurally related triterpenoid CDODA-Me in colon cancer cells (Mertens-Talcott et al., 2007; Chintharlapalli et al., 2009). We also confirmed that ZBTB10 overexpression in Panc1 and L3.6pL cells decreased levels Sp1, Sp3, and Sp4 proteins (Fig. 5C) as observed in colon and breast cancer cells (Mertens-Talcott et al., 2007; Chintharlapalli et al., 2009). These studies also demonstrate for the first time that CDDO-Me-dependent induction of ROS is critical for downstream events because antioxidants inhibit the down-regulation of miR-27a, induction of ZBTB10 (Fig. 5D), and repression of Sp1, Sp3, and Sp4 (Fig. 4D) in Panc1 and L3.6pL cells treated with CDDO-Me. Because the downstream effects of CDDO-Me are associated with decreased expression of as-miR-27a, which results in the induction of ZBTB10 and down-regulation of Sp1, Sp3, and Sp4, we also investigated the growth inhibitory/proapoptotic effects of individual steps in this pathway. Transfection of Panc1 and L3.6pL cells with as-miR-27a or ZBTB10 expression plasmid or a cocktail of small inhibitory RNAs against Sp1, Sp3, and Sp4 (iSp1/3/4) inhibited cell growth and induced PARP cleavage. There were some cell context-dependent differences in the effects of individual Sp knockdown; however, these results indicate that CDDO-Me-dependent perturbation of the miR-27a:ZBTB10–Sp1/3/4 axis contributes to the growth inhibitory and proapoptotic effects of CDDO-Me.
In summary, we have shown that CDDO-Me is highly cytotoxic to pancreatic cancer cells and tumors, and this is consistent with previous reports on the potent anticancer activity of CDDO and related compounds (Honda et al., 1998, 2000; Wang et al., 2000; Ikeda et al., 2003, 2004; Melichar et al., 2004; Samudio et al., 2005, 2006; Ray et al., 2006; Yore et al., 2006; Yue et al., 2006; Liby et al., 2007). Our results demonstrate for the first time that CDDO and CDDO-Me induce the down-regulation of Sp1, Sp3, and Sp4 transcription factors and several Sp-dependent genes that are associated with cancer cell survival, growth, and angiogenesis. These observations suggest that this pathway contributes to the potent antitumorigenic activity of CDDO and related compounds and may also explain, in part, the broad spectrum of activities of these drugs in cancer cell lines. CDDO-Me, like its imidazole derivative (Samudio et al., 2005), also induces ROS and decreases MMP in pancreatic cancer cells. We show that the induction of ROS leads to a cascade of events in which miR-27a is decreased, and this is accompanied by the induction of ZBTB10, a suppressor of Sp transcription factors and Sp-dependent genes (Tillotson, 1999; Mertens-Talcott et al., 2007; Chintharlapalli et al., 2009). Activation of the ROS-miR-27a:ZBTB10–Sp axis by CDDO-Me represents a novel and highly practical route for targeting several Sp-regulated genes that play an important role in carcinogenesis. Current research is focused on improving the efficacy of drugs that target Sp transcription factors and on determining the specific ROS-induced factors and other pathways that lead to down-regulation of miR-27a and other miRs that regulate potential Sp-repressor genes.
Supplementary Material
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This study was supported by the National Institutes of Health National Cancer Institute [Grants R01-CA108718, P20-CA10193, and R01-CA136571]; and Texas A&M AgriLife Research.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.064451.
- CDDO
- 2-cyano-3,12-dioxoleana-1,9-dien-28-oic acid
- CDDO-IM
- imidazole ester of 2-cyano-3,12-dioxoleana-1,9-dien-28-oic acid
- CDDO-Me
- methyl ester of 2-cyano-3,12-dioxoleana-1,9-dien-28-oic acid
- CDODA
- 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oic acid
- CDODA-Me
- methyl ester of 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oic acid
- GSH
- glutathione
- MMP
- mitochondrial membrane potential
- PPARγ
- peroxisome proliferator-activated receptor γ
- ROS
- reactive oxygen species
- Sp
- specificity protein
- VEGF
- vascular endothelial growth factor
- DMEM
- Dulbecco’s modified Eagle’s medium
- FBS
- fetal bovine serum
- PARP
- poly(ADP-ribose) polymerase
- TBP
- TATA binding protein
- ZBTB10
- zinc finger and BTB domain containing 10
- PCR
- polymerase chain reaction
- DMSO
- dimethyl sulfoxide
- TBST
- Tris-buffered saline/Tween 20
- MG132
- N-benzoyloxycarbonyl (Z)-Leu-Leu-leucinal
- DTT
- dithiothreitol
- as-miR-27a
- antisense microRNA-27a
- CM-H2DCFDA
- 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester.
References
- Abdelrahim M, Baker CH, Abbruzzese JL, Safe S. (2006) Tolfenamic acid and pancreatic cancer growth, angiogenesis, and Sp protein degradation. J Natl Cancer Inst 98:855–868 [DOI] [PubMed] [Google Scholar]
- Abdelrahim M, Baker CH, Abbruzzese JL, Sheikh-Hamad D, Liu S, Cho SD, Yoon K, Safe S. (2007) Regulation of vascular endothelial growth factor receptor-1 expression by specificity proteins 1, 3, and 4 in pancreatic cancer cells. Cancer Res 67:3286–3294 [DOI] [PubMed] [Google Scholar]
- Abdelrahim M, Smith R, 3rd, Burghardt R, Safe S. (2004) Role of Sp proteins in regulation of vascular endothelial growth factor expression and proliferation of pancreatic cancer cells. Cancer Res 64:6740–6749 [DOI] [PubMed] [Google Scholar]
- Ammendola R, Mesuraca M, Russo T, Cimino F. (1992) Sp1 DNA binding efficiency is highly reduced in nuclear extracts from aged rat tissues. J Biol Chem 267:17944–17948 [PubMed] [Google Scholar]
- Baker CH, Solorzano CC, Fidler IJ. (2002) Blockade of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling for therapy of metastatic human pancreatic cancer. Cancer Res 62:1996–2003 [PubMed] [Google Scholar]
- Bouwman P, Philipsen S. (2002) Regulation of the activity of Sp1-related transcription factors. Mol Cell Endocrinol 195:27–38 [DOI] [PubMed] [Google Scholar]
- Chadalapaka G, Jutooru I, Burghardt R, Safe S. (2010) Drugs that target specificity proteins downregulate epidermal growth factor receptor in bladder cancer cells. Mol Cancer Res 8:739–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadalapaka G, Jutooru I, Chintharlapalli S, Papineni S, Smith R, 3rd, Li X, Safe S. (2008a) Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res 68:5345–5354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadalapaka G, Jutooru I, McAlees A, Stefanac T, Safe S. (2008b) Structure-dependent inhibition of bladder and pancreatic cancer cell growth by 2-substituted glycyrrhetinic and ursolic acid derivatives. Bioorg Med Chem Lett 18:2633–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiefari E, Brunetti A, Arturi F, Bidart JM, Russo D, Schlumberger M, Filetti S. (2002) Increased expression of AP2 and Sp1 transcription factors in human thyroid tumors: a role in NIS expression regulation? BMC Cancer 2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, Abdelrahim M, Abudayyeh A, Jutooru I, Chadalapaka G, Wu F, Mertens-Talcott S, Vanderlaag K, Cho SD, et al. (2009) Oncogenic microRNA-27a is a target for anticancer agent methyl 2-cyano-3,11-dioxo-18beta-olean-1,12-dien-30-oate in colon cancer cells. Int J Cancer 125:1965–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, Jutooru I, McAlees A, Safe S. (2007a) Structure-dependent activity of glycyrrhetinic acid derivatives as peroxisome proliferator-activated receptor {gamma} agonists in colon cancer cells. Mol Cancer Ther 6:1588–1598 [DOI] [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, Ramaiah SK, Safe S. (2007b) Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res 67:2816–2823 [DOI] [PubMed] [Google Scholar]
- Deeb D, Gao X, Jiang H, Janic B, Arbab AS, Rojanasakul Y, Dulchavsky SA, Gautam SC. (2010) Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells through a ROS-dependent mechanism. Biochem Pharmacol 79:350–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins KJ, Abdelrahim M, Liu S, Yoon K, Safe S. (2006) Regulation of vascular endothelial growth factor receptor-2 expression in pancreatic cancer cells by Sp proteins. Biochem Biophys Res Commun 345:292–301 [DOI] [PubMed] [Google Scholar]
- Honda T, Rounds BV, Bore L, Finlay HJ, Favaloro FG, Jr, Suh N, Wang Y, Sporn MB, Gribble GW. (2000) Synthetic oleanane and ursane triterpenoids with modified rings A and C: a series of highly active inhibitors of nitric oxide production in mouse macrophages. J Med Chem 43:4233–4246 [DOI] [PubMed] [Google Scholar]
- Honda T, Rounds BV, Gribble GW, Suh N, Wang Y, Sporn MB. (1998) Design and synthesis of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, a novel and highly active inhibitor of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett 8:2711–2714 [DOI] [PubMed] [Google Scholar]
- Hosoi Y, Watanabe T, Nakagawa K, Matsumoto Y, Enomoto A, Morita A, Nagawa H, Suzuki N. (2004) Up-regulation of DNA-dependent protein kinase activity and Sp1 in colorectal cancer. Int J Oncol 25:461–468 [PubMed] [Google Scholar]
- Ikeda T, Nakata Y, Kimura F, Sato K, Anderson K, Motoyoshi K, Sporn M, Kufe D. (2004) Induction of redox imbalance and apoptosis in multiple myeloma cells by the novel triterpenoid 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid. Mol Cancer Ther 3:39–45 [PubMed] [Google Scholar]
- Ikeda T, Sporn M, Honda T, Gribble GW, Kufe D. (2003) The novel triterpenoid CDDO and its derivatives induce apoptosis by disruption of intracellular redox balance. Cancer Res 63:5551–5558 [PubMed] [Google Scholar]
- Jiang NY, Woda BA, Banner BF, Whalen GF, Dresser KA, Lu D. (2008) Sp1, a new biomarker that identifies a subset of aggressive pancreatic ductal adenocarcinoma. Cancer Epidemiol Biomarkers Prev 17:1648–1652 [DOI] [PubMed] [Google Scholar]
- Jutooru I, Chadalapaka G, Chintharlapalli S, Papineni S, Safe S. (2009) Induction of apoptosis and nonsteroidal anti-inflammatory drug-activated gene 1 in pancreatic cancer cells by a glycyrrhetinic acid derivative. Mol Carcinog 48:692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehn FE, Carter GT. (2005) The evolving role of natural products in drug discovery. Nat Rev Drug Discov 4:206–220 [DOI] [PubMed] [Google Scholar]
- Liby KT, Yore MM, Sporn MB. (2007) Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer 7:357–369 [DOI] [PubMed] [Google Scholar]
- Lou Z, O'Reilly S, Liang H, Maher VM, Sleight SD, McCormick JJ. (2005) Down-regulation of overexpressed sp1 protein in human fibrosarcoma cell lines inhibits tumor formation. Cancer Res 65:1007–1017 [PubMed] [Google Scholar]
- Melichar B, Konopleva M, Hu W, Melicharova K, Andreeff M, Freedman RS. (2004) Growth-inhibitory effect of a novel synthetic triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, on ovarian carcinoma cell lines not dependent on peroxisome proliferator-activated receptor-γ expression. Gynecol Oncol 93:149–154 [DOI] [PubMed] [Google Scholar]
- Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. (2007) The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res 67:11001–11011 [DOI] [PubMed] [Google Scholar]
- Miller WH, Jr, Schipper HM, Lee JS, Singer J, Waxman S. (2002) Mechanisms of action of arsenic trioxide. Cancer Res 62:3893–3903 [PubMed] [Google Scholar]
- Oh JE, Han JA, Hwang ES. (2007) Downregulation of transcription factor, Sp1, during cellular senescence. Biochem Biophys Res Commun 353:86–91 [DOI] [PubMed] [Google Scholar]
- Papineni S, Chintharlapalli S, Safe S. (2008) Methyl 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oate is a peroxisome proliferator-activated receptor γ agonist that induces receptor-independent apoptosis in LNCaP prostate cancer cells. Mol Pharmacol 73:553–565 [DOI] [PubMed] [Google Scholar]
- Ray DM, Morse KM, Hilchey SP, Garcia TM, Felgar RE, Maggirwar SB, Phipps RP, Bernstein SH. (2006) The novel triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) induces apoptosis of human diffuse large B-cell lymphoma cells through a peroxisome proliferator-activated receptor γ-independent pathway. Exp Hematol 34:1202–1211 [DOI] [PubMed] [Google Scholar]
- Safe S, Abdelrahim M. (2005) Sp transcription factor family and its role in cancer. Eur J Cancer 41:2438–2448 [DOI] [PubMed] [Google Scholar]
- Samudio I, Konopleva M, Hail N, Jr, Shi YX, McQueen T, Hsu T, Evans R, Honda T, Gribble GW, Sporn M, et al. (2005) 2-Cyano-3,12-dioxooleana-1,9-dien-28-imidazolide (CDDO-Im) directly targets mitochondrial glutathione to induce apoptosis in pancreatic cancer. J Biol Chem 280:36273–36282 [DOI] [PubMed] [Google Scholar]
- Samudio I, Konopleva M, Pelicano H, Huang P, Frolova O, Bornmann W, Ying Y, Evans R, Contractor R, Andreeff M. (2006) A novel mechanism of action of methyl-2-cyano-3,12 dioxoolean-1,9 diene-28-oate: direct permeabilization of the inner mitochondrial membrane to inhibit electron transport and induce apoptosis. Mol Pharmacol 69:1182–1193 [DOI] [PubMed] [Google Scholar]
- Shi Q, Le X, Abbruzzese JL, Peng Z, Qian CN, Tang H, Xiong Q, Wang B, Li XC, Xie K. (2001) Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res 61:4143–4154 [PubMed] [Google Scholar]
- Tillotson LG. (1999) RIN ZF, a novel zinc finger gene, encodes proteins that bind to the CACC element of the gastrin promoter. J Biol Chem 274:8123–8128 [DOI] [PubMed] [Google Scholar]
- Wang L, Wei D, Huang S, Peng Z, Le X, Wu TT, Yao J, Ajani J, Xie K. (2003) Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res 9:6371–6380 [PubMed] [Google Scholar]
- Wang Y, Porter WW, Suh N, Honda T, Gribble GW, Leesnitzer LM, Plunket KD, Mangelsdorf DJ, Blanchard SG, Willson TM, et al. (2000) A synthetic triterpenoid, 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO), is a ligand for the peroxisome proliferator-activated receptor gamma. Mol Endocrinol 14:1550–1556 [DOI] [PubMed] [Google Scholar]
- Yao JC, Wang L, Wei D, Gong W, Hassan M, Wu TT, Mansfield P, Ajani J, Xie K. (2004) Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clin Cancer Res 10:4109–4117 [DOI] [PubMed] [Google Scholar]
- Yore MM, Liby KT, Honda T, Gribble GW, Sporn MB. (2006) The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-κB activation through direct inhibition of IκB kinase β. Mol Cancer Ther 5:3232–3239 [DOI] [PubMed] [Google Scholar]
- Yue P, Zhou Z, Khuri FR, Sun SY. (2006) Depletion of intracellular glutathione contributes to JNK-mediated death receptor 5 up-regulation and apoptosis induction by the novel synthetic triterpenoid methyl-2-cyano-3,12-dioxooleana-1,9-dien-28-oate (CDDO-Me). Cancer Biol Ther 5:492–497 [DOI] [PubMed] [Google Scholar]
- Zannetti A, Del Vecchio S, Carriero MV, Fonti R, Franco P, Botti G, D'Aiuto G, Stoppelli MP, Salvatore M. (2000) Coordinate up-regulation of Sp1 DNA-binding activity and urokinase receptor expression in breast carcinoma. Cancer Res 60:1546–1551 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.