Abstract
Pax2 is essential for the development of the urogenital system, neural tube, otic vesicle, optic cup and optic tract. Within the visual system, a loss-of-function leads to lack of choroid fissure closure (known as a coloboma), a loss of optic nerve astrocytes, and anomalous axonal pathfinding at the optic chiasm. This study is directed at determining the effects of ectopic Pax2 expression in the chick ventral optic cup past the normal developmental period when Pax2 is found. In ovo electroporation of Pax2 into the chick ventral optic cup results in the formation of colobomas, a condition typically associated with a loss of Pax2 expression. While the overexpression of Pax2 appears to phenocopy a loss of Pax2, the mechanism of the failure of choroid fissure closure is associated with a cell fate switch from ventral retina and retinal pigmented epithelium (RPE) to an astrocyte fate. Further, ectopic expression of Pax2 in RPE appears to have non-cell autonomous effects on adjacent RPE, creating an ectopic neural retina in place of the RPE.
Keywords: Retina, Pax2, Chick, Optic Cup, Coloboma, Ectopic retina
Introduction
The morphogenetic events that surround the development of the vertebrate optic vesicle and optic cup are key in forming the inductive interactions that pattern the eye (reviewed in Chow and Lang, 2001). A good example of this type of event is the formation and closure of a transient gap in the ventral eye cup, known as the choroid fissure. The fissure extends through the optic cup and optic stalk and eventually closes as the two edges of the fissure undergo fusion. During its existence, this region is key for the migration of mesenchymal cells into the eye to form the vasculature that supports the lens and retina (Hughes et al., 2000). As development proceeds, the edges of the fissure fuse together to complete the ventral portion of the eye. The region of the fissure where the optic cup transitions to the optic stalk becomes the optic nerve head upon closure of the fissure (Chow and Lang, 2001). This region expresses molecules, such as cadherins, netrins, sonic hedgehog, and slits, all of which combine forces to instruct ganglion cell axons to exit the eye to form the optic nerve (Gerhardt et al., 2000; Wallace and Raff, 1999; Oster et al., 2004).
The morphogenesis of the ventral-most portions of the optic cup requires that cells remain undifferentiated until those that will make up the retinal pigmented epithelium (RPE) and neural retina have moved into place, the basal laminae that separates the two opposing lips of the choroid fissure have broken down, cells in the improper position have undergone cell death, and the two lips have fused together (Hero, 1989, 1990; Hero et al., 1991). Defects in the closure of the choroid fissure are referred to as a coloboma, a Greek word meaning “curtailed” or “mutilated”. The presentation of colobomas alone in humans is a rare condition; however, colobomatous eyes frequently occur as part of syndromes that include other congenital defects, such as microphthalmia, deafness and defects in the formation of the urogenital system (Eccles and Schimmenti, 1999).
While the mechanisms that direct the choroid fissure closure are generally not well understood, a number of genetic mutations have been identified in humans, mice, and zebrafish that can give rise to colobomatous eyes (Gregory-Evans et al., 2004; Azuma et al., 2003; Barbieri et al., 2002). In particular, the loss of two transcription factors, Pax2 and Vax have been associated with colobomas. Pax2, a member of paired homeobox family of transcription factors, has been associated with ocular defects in humans (Gregory-Evans et al., 2004). Pax2 loss-of-function mutations and/or haploinsufficiency have been associated primarily with colobomas, microphthalmia, and optic nerve defects in the eye in addition to kidney, inner ear, and neural tube defects (Benetti et al., 2007; Sanyanusin et al., 1995). Pax2 overexpression has devastating consequences in the kidney, leading to the formation of childhood tumors known as Wilms tumors (Dressler and Douglass, 1992).
Pax2 expression has been shown to be induced by the actions of several secreted factors, including bone morphogenetic protein 7 (BMP7), sonic hedgehog (SHH) and fibroblast growth factors (FGFs) (Macdonald et al., 1995; Morcillo et al., 2006; Nakamura 2001). Both BMP7 and SHH are expressed in the prechordal mesoderm underlying the ventral diencephalon and both are thought to control the identity of diencephalon (Dale et al., 1999). PAX2 is co-expressed throughout the early optic vesicle with other transcription factors such as PAX6 and CHX10 (Baumer et al., 2003). The expression of these factors becomes restricted to specific portions of the optic vesicle as, 1) inductive signals from surrounding tissues interact with the cells of optic vesicle, and 2) the cell type specific transcription factors suppress the expression of factors that induce competing cell types. At this point Pax2 expression is restricted to the part of the optic cup destined to be ventral neural retina, RPE and optic stalk (Bovolenta et al., 1997). Pax2 expression is exquisitely regulated in the optic cup and stalk. Expression remains in the ventral optic cup during the period in which the choroid fissure must be closed, and downregulates within the optic cup at the point when the tissue that forms the ventral retina and RPE begins to differentiate. A few stages later, Pax2 expression becomes restricted to the cells of the optic stalk and cells that line the choroid fissure (Mansouri et al., 1996). It is generally unknown whether PAX2-positive cells at the edge of the choroid fissure undergo cell death as a result of the closure, or downregulate their expression as they take on new cell fates. Regardless, the end result is that PAX2 expressing cells are found exclusively in astrocyte precursor cells and mature astrocytes of the optic stalk/nerve and glial cells of a vascular structure peculiar to avian and reptile species, called the pecten. Here, we seek to understand the reason for the complex spatio-temporal regulation of Pax2 during optic cup development and the consequence of a loss in Pax2 regulation, particularly in ventral optic cup differentiation.
Previous results from our lab have shown that overexpression of the BMP binding protein, noggin, in the chick optic cup results in the loss of dorsal retinal markers and the simultaneous expansion of ventral markers, such as PAX2 (Adler and Belecky-Adams, 2002). We hypothesize that the lack of choroid fissure closure in noggin-overexpressing eyes is due to the ectopic expression of PAX2 in the ventral retina past the time at which it should be present, rather than secondary effects of noggin overexpression. Further, we propose that the mechanism of choroid fissure failure, following the ectopic expression of Pax2 is the result of the abnormal differentiation of cells normally fated to become ventral retina and RPE, to glial cells.
Material and methods
Reagents used were as follows: phenol-chloroform, RNase A, DNase I (Invitrogen; Carlsbad, CA), isopropanol, sucrose, paraformaldehyde, EDTA, formamide, Tris, NaCl, KCl, monobasic sodium phosphate, dibasic sodium phosphate, superfrost plus slides (Fisher Scientific; Hanover Park, IL), Chaps, diethyl pyrocarbonate (Dep-C), sodium citrate-trisodium salt dehydrate, Triton X-100, Trypan-blue, Propidium iodide (Sigma; St. Louis, MO), normal Goat Serum, normal Donkey serum (Chemicon International; Temecula, CA), penicillin-streptomycin (Invitrogen; Grand Island, NY), 5-bromo-3-indolyl-phosphate (BCIP), nitro blue tetrazolium chloride (NBT) (Roche; Indianapolis, IN), blocking buffer for in situ hybridization (Roche, Cat# 1096176), in situ cell death detection kit POD, (Roche, Cat# 1684817), plasmid midi kit (Qiagen, Valencio, CA), OCT embedding compound, (Sakura; Torrance, CA), molds and aqua Polymount (Polysciences; Warrington, PA).
Materials
Eggs
White Leghorn eggs used for electroporation studies were provided by Purdue Poultry Farm (West Lafayette, IN) and the Ohio State Univeristy (Columbus, Ohio).
Electroporation Constructs: Plasmid expressing chick Pax2 was a kind gift from Harukazu Nakamura, Tohoku University, Japan (Okafuji et al., 1999), β-gal expression vector was a kind gift from Ruben Adler (Toy et al., 2002). The coding sequence of GFP (BD Biosciences; Palo Alto, CA) was driven by the cytomegalovirus promoter (CMV).
In situ hybridization probes
cRaldh3 (Suzuki et al., 2000) a gift from Amasaharu Noda (National Inst. Basic Biology, Okazaki Japan), Tbx5 (described as cTbx5) a gift from Katherine Yutzey (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH) and Bmp4 was a kind gift from Thomas M. Jessell (Columbia University, New York).
Antibodies
Mouse anti-visinin (Developmental Studies Hybridoma Bank at the University of Iowa, 1:50); mouse anti-vimentin (H5, Developmental Studies Hybridoma Bank at the University of Iowa, 1:1000); mouse anti-P0 (schwann cell marker) (1E8, Developmental Studies Hybridoma Bank at the University of Iowa, 1:100); rabbit anti-GFAP (DAKO 1 μg/ml); rabbit anti-PAX6 (Covance, 1:300); rabbit anti-phospho-Histone H3 (Upstate Biotechnology, 1:200); rabbit anti-PROX1 (Covance, 1:1000); rabbit anti-PAX2 (Babco, 1:200); sheep anti-CHX10 (exAlpha,1:100), mouse anti-HuC/D (Molecular Probes – Invitrogen, 1:200), mouse anti-MMP115 (Mochii et al., 1988, 1:250) and RPE65 (Chemicon, 1:250) and anti-digoxigenin (DIG) antibody (Roche,1:1000). Secondary antibodies conjugated to Alexa Fluor (Molecular Probes – Invitrogen) were used at a concentration of 1:1000.
Methods
Electroporation
DNA used for electroporation was purified using Qiagen Plasmid Midi Kit. Pax2 and β-gal expression vectors were diluted to a final concentration of 1.5–2.0 μg/μl and control GFP expression vector was diluted to a final concentration of 0.2–0.4 μg/μlin Tris–EDTA buffer (TE). Vectors were then diluted in TE containing 0.05% trypan blue as a tracking dye. Microinjection needles were beveled on micro pipette beveler (Sutter Instruments; Novato, CA). E3 embryos were windowed and the extraembryonic membranes were removed to expose the eye. DNA constructs were injected into the eye with beveled glass needles using a PLI-90 picoliter injector (Sutter Instruments; Novato, CA) for 1–2 ms at 5–10 psi. Following the injections a current of 5, 50 ms pulses at 15–20 mV was delivered by a BTX 830 Electro Square Porator (BTX; San Diego, CA) with a 3 mm platinum anode and tungsten wire cathode. A few drops of 1% solution of penicillin/streptomycin in phosphate buffered saline (PBS) were applied to the embryo. The window was then covered with cellophane packing tape and eggs were placed in an egg incubator until harvest time.
Immunohistochemistry
Tissues to be processed for immunohistochemistry were fixed in 4% paraformaldehyde in PBS, and infiltrated in an ascending series of sucrose diluted in 0.1 M phosphate buffer, pH 7.4 (P-buff) (Barthel and Raymond, 1993) and were frozen in a 3:1 mixture of OCT embedding compound and 20% sucrose diluted in PBS and sectioned at 10–12 μm. Frontal sections were processed as described previously (Wilson et al., 2007).
Mitosis and cell survival analysis and quantification
Frontal sections of β-gal/GFP or Pax2/GFP electroporated eyes were analyzed for TUNEL using in situ cell death detection kit (Roche) according to the manufacturer’s instructions. For analysis of mitotic cells, frontal sections of the β-gal/GFP or Pax2/GFP electroporated eyes were immunolabeled with anti phospho-Histone H-3. The apoptotic and mitotic cells were counted in series of 10 fields of about three hundred cells; in the electroporated and unelectroporated eye of control β-gal/GFP and Pax2/GFP embryos. A minimum of three embryos were counted and six sections per embryo for each experimental condition. The index factor was calculated for each experimental condition which is the ratio between numbers of apoptotic cells on the β-gal/GFP or Pax2/GFP electroporated side divided by unelectroporated side.
In situ hybridization
Tissues to be processed for in situ hybridization were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS), and infiltrated in an ascending series of sucrose diluted in PBS, pH7.4. Following incubation with 20% sucrose, the tissues were frozen in a 3:1 mixture of OCT embedding compound and 20% sucrose diluted in PBS and sectioned at 10–12 μm. The frontal sections were then rinsed with PBS containing active DepC for 10 min at room temp prior to incubating in hybridization buffer, and processed as described previously (Belecky-Adams et al., 1997).
Results
Co-expression of the GFP and Pax2
Pax2 coding sequences were originally cloned into the bi-cistronic vector pIRES upstream of the green fluorescent protein (GFP) to simplify the localization of electroporated retinal cells in vivo and sequenced to verify correct orientation. We were unable to detect both the Pax2 and GFP derived from this construct in electroporated embryos, despite detection of both in transfected chick fibroblasts in vitro (data not shown). Embryos co-electroporated with separate vectors containing GFP and Pax2 at ratio of 1:10 (GFP: Pax2) showed brightly fluorescing cells when viewed under a fluorescent stereomicroscope (Figs. 1A–C). Sections through the embryos co-electroporated at stage 10, also showed excellent co-localization of GFP and Pax2 (Figs. 1D–F). In all subsequent experiments described, localization of electroporated cells was determined by visualizing GFP in intact embryos prior to the subsequent analysis of the optic cups. Embryos in which GFP appeared not to be electroporated into the eye were not used in analyses.
Fig. 1.
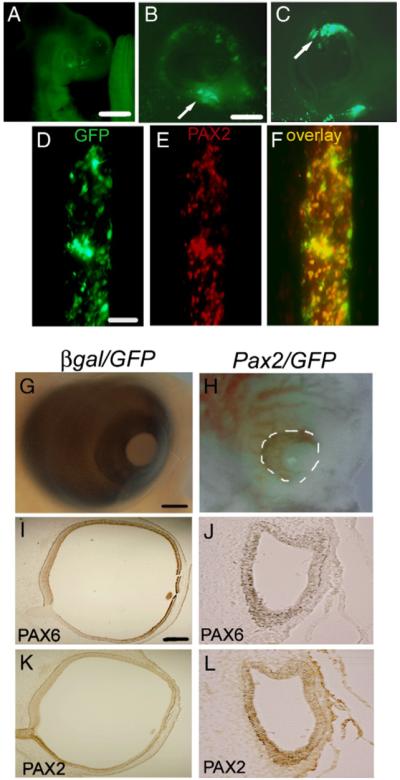
(A–F) Co-expression of GFP with β-gal and Pax2. E3 chick eyes (HH 13–15) were electroporated with β-gal/GFP (10:1) and analyzed at E4. (A, B) GFP is expressed throughout the optic cup, but expression predominates in the ventral portion (arrow). (C) Shows GFP expression in dorsal optic cup (arrow). (D, E) Stage 10 chicks were electroporated with Pax2/GFP (10:1) and sections through forebrain were labeled with GFP (D) and PAX2 (E). (F) Electroporated cells co-express GFP and Pax2. Scale bar (500 μm) in panel A; scale bar (50 μm) in panel B applies to (B, C); scale bar (50 μm) in panel D applies to panels D–F. (G, H) Embryos electroporated at stage 10 were allowed to develop until E6. Embryos electroporated with β-gal/GFP developed normally (G) and showed the typical PAX6 expression patterns throughout the retina (I) and PAX2 expression in the optic nerve (K). In comparison, Pax2/GFP electroporated embryos developed microphthalmia (H; white dashes) in which the optic vesicle-like structure that developed showed very little PAX6 expression (J) and ectopic PAX2 expression (L). Scale bar (50 μm) in panel G applies to panels G, H; scale bar (500 μm) in panel I applies to panels I-L.
Electroporation of Pax2 at stage 10 leads to microphthalmia
Hamburger and Hamilton stage 10 chick embryos were co-electroporated with either β-gal and GFP (β-gal/GFP) or Pax2 and GFP (Pax2/GFP) and allowed to survive until embryonic day 6 (E6) (Hamburger and Hamilton, 1951). Eyes electroporated with the Pax2/GFP expression vectors were consistently much smaller in comparison to the eyes of control β-gal/GFP electroporated embryos (Figs. 1G and H white dashes). Sections through control eyes co-immunolabeled with PAX6 and PAX2 showed the typical bi-layered optic cup at E6, with expression of PAX6 restricted to the developing retina (Fig. 1I) and PAX2 primarily to the optic nerve (Fig. 1K). In comparison to controls, Pax2/GFP co-electroporated eyes appeared to be completely lacking in the formation of a retina and instead had a single-layered neuroepithelium containing pigment granules. Immunolabel for PAX2 and PAX6 showed expression within the vesicle was limited primarily to PAX2, while PAX6 was undetectable. (Figs. 1J, L). Since Pax2/GFP electroporated eyes were clearly aberrant and did not progress beyond what appeared to be the optic vesicle stage, we could not assess the effects of ectopic Pax2 expression on optic fissure closure.
Electroporation of optic cup does not alter the cell type specification of optic cup cells
To address the effects of ectopic Pax2 expression on optic fissure closure, constructs were electroporated between Hamburger–Hamilton stages 13–15 (E3). Introduction of DNA at this stage would circumvent lack of optic cup formation seen in our previous results and would also allow us to electroporate in smaller regions of the developing optic cup. At the start of this study, electroporation had not been used to introduce genes into the developing retina beyond the optic vesicle stage (Stage 10; Hamburger and Hamilton, 1951). We first tested whether we were able to drive and detect expression of the constructs in a region-specific manner within the optic cup. Theoretically, we should be able to drive the expression of Pax2 and GFP in specific regions of the eye, since the method relies upon the electrophoresis of the negatively charged DNA molecules toward the positively charged electrode and the opening of pores in the cell membranes forced by the current passing between electrodes (Itasaki et al., 1999). While we were able to obtain restricted expression in the ventral retina on occasion, we more often obtained expression patterns in both the dorsal and ventral optic cup (not shown).
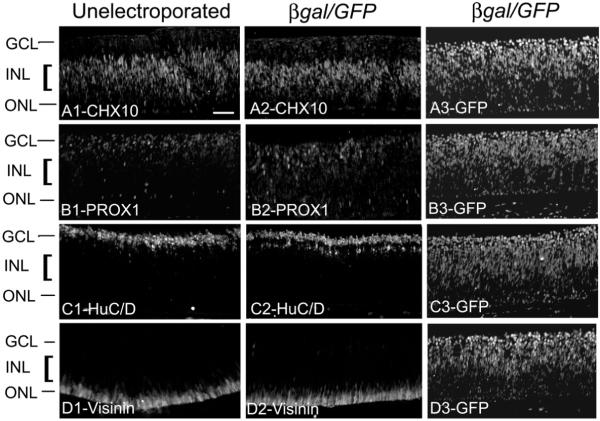
We also sought to determine if there were any changes in differentiation of retina associated with the electroporation performed at stages 13–15. Sections through E8 unelectroporated and β-gal/GFP electroporated optic cups were immunolabeled with antibodies specific to bipolar cells (CHX10; Figs. 2A1 and A2), horizontal cells (PROX1; Figs. 2B1 and B2), amacrine and ganglion cells (HuC/D; Figs. 2C1 and C2) and photoreceptors (visinin; Figs. 2D1 and D2). There was no significant change in the expression of retinal markers in the β-gal/GFP electroporated eye as compared to the unelectroporated eye. Comparisons of cell death and mitosis in unelectroporated and electroporated optic cups are included in a later section of this study.
Fig. 2.
Electroporation has no effect on the normal development of retina. Chick embryos were electroporated with β-gal/GFP at E3 (stage 13–15) and compared with unelectroporated embryos at E8. Immunolabeling was performed on sections through unelectroporated and β-gal/GFP electroporated eyes for CHX10 (Bipolar cells) A1–A2, PROX-1(horizontal cells) B1–B2, HuC/D (amacrine and ganglion cells) C1–C2 and visinin (photoreceptors) D1–D2. A3, B3, C3 and D3 show GFP expression in the respective adjacent sections of the β-gal/GFP electroporated embryos immunolabeled with the retinal markers. ONL; outer nuclear layer, INL; inner nuclear layer, GCL; ganglion cell layer. Scale bar (50 μm) in (A1) applies to (A1–D3).
Overexpression of Pax2 Leads to colobomatous eyes
To address whether Pax2 gain-of-function can lead to a phenocopy of a Pax2 loss-of-function in regards to failure of choroid fissure closure, β-gal/GFP or Pax2/GFP expression vectors were co-electroporated into ventral optic cup at stage 13. Embryos were allowed to survive until E8 (the stage at which the anterior fissure has undergone fusion in normal chick eyes) and embryos were then fixed and scored as to whether the embryo had developed a coloboma, by an investigator masked to the identity of the vectors electroporated into the embryo (see Fig. 3). While 0% of the control embryos developed coloboma, 25% of the embryos electroporated with Pax2/GFP in the ventral eye developed coloboma (n=20) (compare Figs. 3A and B; inset b, arrows). Further, 20% of the embryos that were electroporated in the dorsal optic cup with Pax2/GFP developed an unpigmented region (n=20) (Fig. 3F) while β-gal/GFP electroporated embryos were normal (Fig. 3E). Embryos were subsequently scored as to whether the Pax2/GFP had been electroporated near the choroid fissure. 100% of the embryos that had been electroporated near the choroid fissure developed a coloboma (Fig. 3B).
Fig. 3.
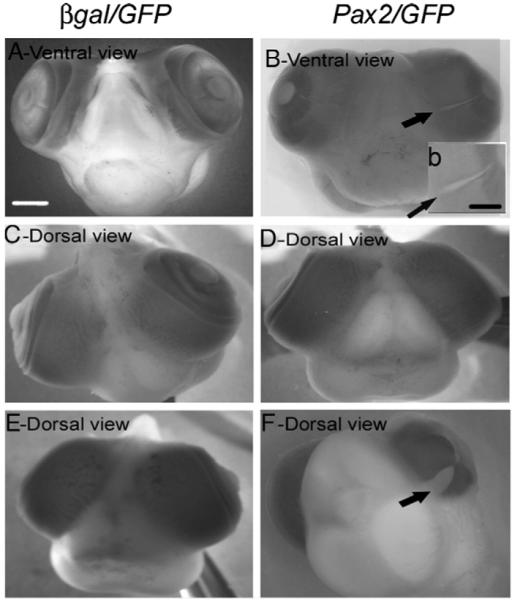
Pax2 overexpression leads to lack of choroid fissure closure and ectopic retina. E3 chick embryos were electroporated with β-gal/GFP (A, C, E) or Pax2/GFP (B, D, F) and analyzed at E8. (A) Choroid fissure of β-gal/GFP electroporated embryos showed normal closure at E8, whereas Pax2/GFP electroporated embryos (B) showed the development of a coloboma (B arrow; inset b). panels C and D showed normal development of dorsal eye structures of same embryos shown in panels A and B respectively. Whereas no abnormalities of eye structures were noted in β-gal/GFP electroporated embryos (A, C and E), some Pax2/GFP electroporated embryos also showed regions where RPE appeared to be missing in dorsal eye (F). Scale bar (500 μm) in panel A applies to panels A–F, scale bar (50 μm) in inset (b).
Pax2 does not stimulate changes in survival or mitosis in optic cup cells
To determine if Pax2 overexpression leads to changes in cell death, embryos were eletroporated at stage 13 with control β-gal/GFP or Pax2/GFP and were allowed to survive for 5 days post electroporation. Control and colobomatous Pax2 electroporated embryos were fixed, sectioned, and analyzed for apoptosis by TUNEL and propidium iodide labeling (see Supplemental figure). Propidium iodide has been used by many studies to highlight condensed nuclei present in apoptotic cells in comparison to the weakly-labeled healthy neighboring cells (Barres et al., 1992a,b; Cook et al., 1995; Nitsch et al., 2000). The number of TUNEL (+) cells were quantitated in sections through Pax2/GFP, β-gal/GFP electroporated and unelectroporated embryos (n=3 embryos per condition) and index factor was calculated as described in materials and methods section. There were no apparent differences in the number of TUNEL (+) cells among the three groups (Fig. 4A). Further, sections labeled with propidium iodide showed no difference between experimental and control groups. There were an average of 129+/−5 labeled cells in β-gal/GFP electroporated, 128+/−6 in unelectroporated side of the same embryos (n=3), and 122+/−12 in Pax2/GFP electroporated and 124+/−9 in unelectroporated eye of Pax2/GFP electroporated embryos (n=3).
Fig. 4.
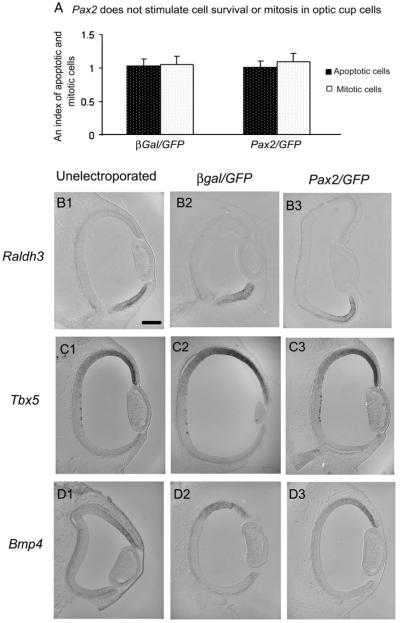
(A) Pax2 does not stimulate cell survival or mitosis in optic cup cells. Quantification of cell death (TUNEL) and mitosis (phospho-Histone H3 immunolabeling), was performed at E8 in β-gal/GFP or Pax2/GFP eyes electroporated at E3 and unelectroporated eyes. The index, with standard deviations (n=6), is the ratio of number of mitotic or apoptotic cells on the β-gal/GFP or Pax2/GFP electroporated side divided by unelectroporated side in each experimental condition. (B1–D3) Pax2 overexpression has no effect on dorso-ventral markers in the eye. E3 unelectroporated (B1, C1, D1), β-gal/GFP electroporated (B2, C2, D2) and Pax2/GFP electroporated (B3, C3, D3) eyes were analyzed at E4 with ventral marker Raldh3 (B1, B2, B3) or dorsal markers Tbx5 (C1, C2, C3) and Bmp4 (D1, D2, D3) by in situ hybridization. No differences in dorsal or ventral markers were noted in unelectroporated and electroporated eyes. Scale bar (50 μm) in (B1) applies to (B1–D3).
To determine if there were changes in mitosis, cryosections from the unelectroporated, β-gal/GFP, and Pax2/GFP electroporated em-bryos were immunolabeled with phospho-Histone-3 (a marker for cells in M-phase). Again the labeled cells were quantitated and the index factor was calculated for each experimental condition. No significant differences were noted between β-gal/GFP or Pax2/GFP electroporated and unelectroporated eyes (Fig. 4A).
Electroporation of Pax2 does not affect expression of other dorso-ventral markers
To determine if Pax2 might be indirectly regulating the failure of choroid fissure closure by affecting dorso-ventral patterning of retina, we electroporated either control β-gal/GFP or Pax2/GFP in the ventral optic cup at stage 13 and allowed embryos to survive for 24 h post-electroporation. The expression of markers present in the dorsal-ventral hemi-retinae were investigated to determine if there were changes in patterns associated with the normally patterned retina. The expression patterns of markers typical of ventral (retinaldehyde dehydrogenase-3 [Raldh3] Figs. 4B1–B3) and dorsal optic cup (T-box 5 [Tbx5] Figs. 4C1–C3) and (bone morphogenetic protein 4 [Bmp4] Figs. 4D1–D3) were compared in unelectroporated wild type, control β-gal/GFP electroporated, and Pax2/GFP electroporated embryos. We were unable to detect any obvious differences in any of the dorsal or ventral markers between β-gal/GFP control and Pax2-electroporated optic cups (Figs. 4B1-D3).
Glial cells differentiate in the choroid fissure of Pax2-expressing cells
To determine if the coloboma formed by Pax2 overexpression involves a cell fate change from retinal or RPE to glial cells, colobomatous eyes were sectioned and double label immunofluorescence performed for glial cell markers (Fig. 5). PAX2 was expressed in the optic nerve head and pecten of control unelectroporated (not shown) and β-gal/GFP electroporated retinae (Fig. 5C, arrow). The choroid fissure was no longer present in either of the controls and no PAX2 (+) cells were present where the choroid fissure had been (not shown). In contrast, Pax2/GFP electroporated embryos with coloboma expressed both PAX2 and vimentin in the cells lining the still present choroid fissure (Fig. 5D, d’, arrow; F, f’) and vimentin positive processes filled the fissure itself (arrow, inset f’). Consistent with the published data, β-gal/GFP electroporated embryos did not express glial fibrillary acidic protein (GFAP) in the optic nerve at this stage of development (Wallace and Raff, 1999) (Fig. 5G). In contrast, GFAP (typically not expressed in the optic nerve until E15; Schuck et al., 2000), was detected in the Pax2/GFP electroporated embryos (Fig. 5H) and the GFAP (+) processes filled the gap between the lips of the unfused retina (see arrow, inset h’).
Fig. 5.
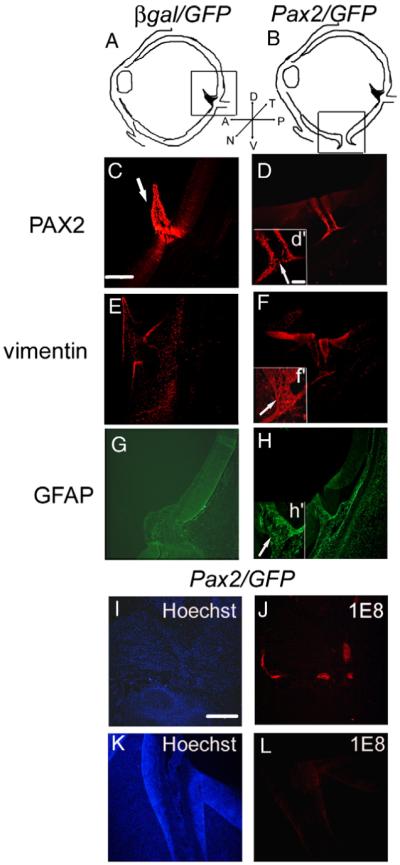
Pax2 expression leads to differentiation of astrocyte processes within the choroid fissure. (A) Shows the schematic of β-gal/GFP electroporated eyes; box represents area shown in panels C, E and G although pecten and optic nerve always do not coincide with each other. (B) shows the schematic of Pax2/GFP electroporated eyes; box represents area shown in panels D, F, H, K and L. (C–H) Pax2 expression leads to differentiation of astrocyte processes within the choroid fissure. PAX2 (C) and vimentin (E) are expressed in the optic nerve, optic nerve head and pecten (arrow in panel C) in control β-gal/GFP electroporated eyes and Pax2/GFP electroporated eyes (not shown). Unlike control E8 eyes, Pax2/GFP electroporated eye showed a persistence of PAX2 (D, d’;arrow) and vimentin (F, f’) expression in cells surrounding the choroid fissure (Hoechst staining of the same region is shown in panel K) and vimentin expression in the processes within the fissure (arrow f’), at E8. Whereas control eyes do not express GFAP in the optic nerve at this stage (G), Pax2/GFP electroporated eye showed GFAP immunolabeled with the cells surrounding the choroid fissure and in the processes of astrocytes within the fissure (H, h’, arrow). Scale bar (50 μm) in panel C applies to panels C–H; scale bar (20 μm) in inset (d’) applies to (d’,f’,h’). (I–L) Cells in the choroid fissure do not express markers characteristic of Schwann cells. Pax2/GFP electroporated embryos with colobomas were immunolabeled for 1E8, a Schwann cell marker. Hoechst label indicates nuclei of cells (I, K). Schwann cells in the peripheral nerves of head can be detected with immunolabel using 1E8 in colobomatous Pax2/GFP electroporated embryos (J), whereas 1E8 antigen cannot be detected in choroid fissure of the same embryo (L). Scale bar (30 μm) in panel I applies to panels I-L.
Previous reports have indicated that ectopic expression of Pax2 in the neural tube leads to the differentiation of glial cells expressing Schwann cell markers (Soukkarieh et al., 2007). To investigate whether glial cells ectopically expressing Pax2 in coloboma region were also expressing Schwann cell markers, sections through colobomatous Pax2/GFP electroporated embryos were immunolabeled with 1E8 antibody, which recognizes Protein zero (P0) found only in Schwann cells (Bhattacharyya et al., 1991; Zhang et al., 1995). While the Schwann cells found in peripheral nerves innervating the head were labeled with 1E8 (Fig. 5J), those in the coloboma region of the same embryo were negative for 1E8 (Fig. 5L).
Ectopic expression of Pax2 leads to ectopic neural retina in the region adjacent to Pax2-electroporated region
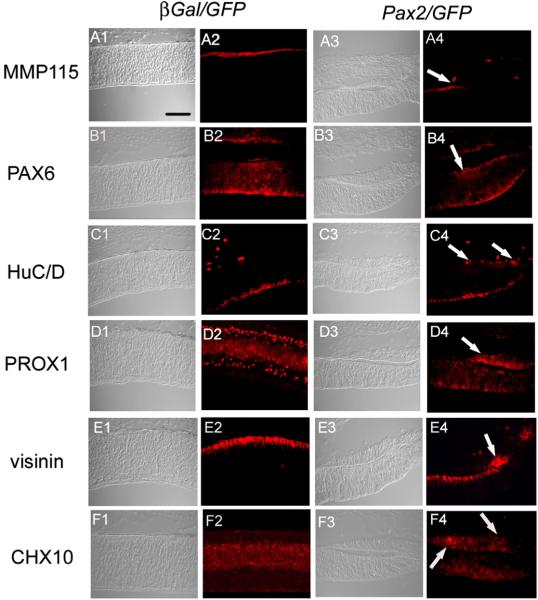
The eyes of embryos electroporated with Pax2/GFP in the dorsal presumptive RPE also had large regions that were devoid of RPE cells. Upon gross morphological examination, these regions appeared to be ectopic choroid fissure or colobomas, however histological examination revealed their true nature. Sections through the unpigmented areas of E8 Pax2/GFP electroporated retina showed what appeared to be the formation of an ectopic neural retina in place of the RPE. While control β-gal/GFP electroporated eyes showed RPE-specific expression of MMP115 in 100% of the embryos examined (Fig. 6A2), no MMP115 was detected in ectopic retina in 100% of embryos electroporated with Pax2/GFP (Fig. 6; arrow, A4). Retina-specific markers; PAX6 (Fig. 6B2, B4), HuC/D (Figs. 6C2, C4), PROX1 (Figs. 6D2, D4), visinin (Figs. 6E2, E4) and CHX10 (Figs. 6F2, F4) showed normal expression patterns in the control β-gal/GFP electroporated embryos (Figs. 6A2, B2, C2, D2, E2, F2). In comparison, Pax2/GFP electroporated eyes showed a loss of RPE marker MMP115 and in its place were cells positive for retinal markers (Figs. 6B4, C4, D4, E4, F4; arrows). As is the case with transdiffer-entiated RPE, the ectopic neural retina lay in mirror orientation to that of the primary retina (Coulombre and Coulombre, 1965). Oddly, we noted that in all the cases of the ectopic retina examined, the electroporated region did not encompass the entire ectopic retina. Rather, electroporated cells were found at the edge of the ectopic retina, and in each case the Pax2/GFP expressing cells were found in cells that had been fated to give rise to the RPE (Fig. 7). A typical example of this is shown in Fig. 7, where PAX2 expression was localized at one edge of Pax2/GFP electroporated tissue (Fig. 7D), whereas PAX6 is found in complementary expression patterns of unelectroporated primary and ectopic neural retina (Fig. 7C).
Fig. 6.
Pax2 overexpression leads to ectopic retina formation. E3 (HH13-15) chick eyes were electroporated with β-gal/GFP (left columns) and Pax2/GFP (right columns), and analyzed at E8 with cell-type specific markers. Control β-gal/GFP electroporated embryos, and Pax2/GFP electroporated embryos showing loss of pigmented epithelium (refer Fig. 3F) were sectioned and immunolabeled for RPE (MMP115; A2, A4), amacrine and ganglion cells (PAX6; B2, B4), horizontal cells (PROX1; D2, D4), amacrine and ganglion cells (HuC/D; C2, C4), photoreceptors (visinin; E2, E4) and bipolar cells (CHX10; F2, F4). Arrows in panels B4, C4, D4, E4 and F4 indicate expression of retinal markers in ectopic retina. Arrow in panel A4 indicates loss of RPE marker, MMP115. Scale bar (50 μm) in panel A1 applies to panels A1–F4.
Fig. 7.
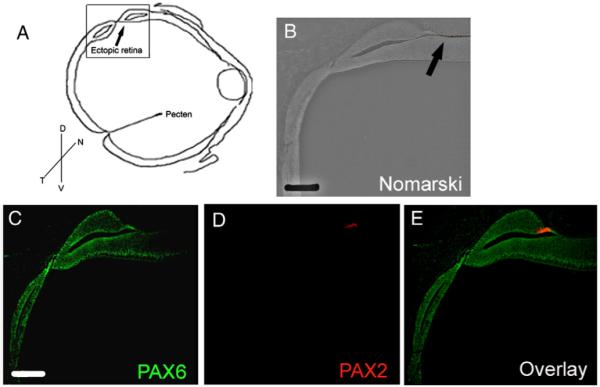
Ectopic retina is formed adjacent to the Pax2 overexpressing cells. E3 embryos electroporated with Pax2/GFP, showing loss of pigmented epithelium were sectioned at E8 and immunolabeled with PAX6 and PAX2. (A) Shows the schematic of Pax2/GFP electroporated eye; box represents the area shown in panels B–E. (B) Nomarski image of the region with ectopic retina. (C) PAX6 is expressed ectopic neural retina. (D) PAX2 expression is limited to the pigmented cells that have not formed ectopic retina (also see panel B; arrow). (E) PAX6 and PAX2 are not co-expressed in ectopic retina or in pigmented cells. Scale bar (50 μm) in panel B; scale bar (50 μm) in panel C applies to panels C–E.
Ectopic neural retina expresses Fgf8
We further investigated whether formation of an ectopic neural retina was the result of ectopic expression of growth factors or other secreted agents that may diffuse through the tissue. Because the Fgfs have been shown to be involved in transdifferentiation and Fgf8 expression is reportedly regulated by Pax2 in vivo (Bouchard et al., 2005), we first chose to determine patterns of Fgf8 expression following co-electroporation of β-gal/GFP or Pax2/GFP. Embryos were allowed to survive 24, 48, and 72 h post-electroporation, and sections through regions with ectopic neural retina were subjected to in situ hybridization with a probe specific for chick Fgf8 (Figs. 8A–F). While there did appear to be an increase in the expression of Fgf8 in the retinae adjacent to ectopic retina and even within the ectopic neural retina at the earliest time points, the Fgf8 expression in the electroporated regions within the Pax2/GFP electroporated RPE did not appear to express Fgf8 (Fig. 8G).
Fig. 8.
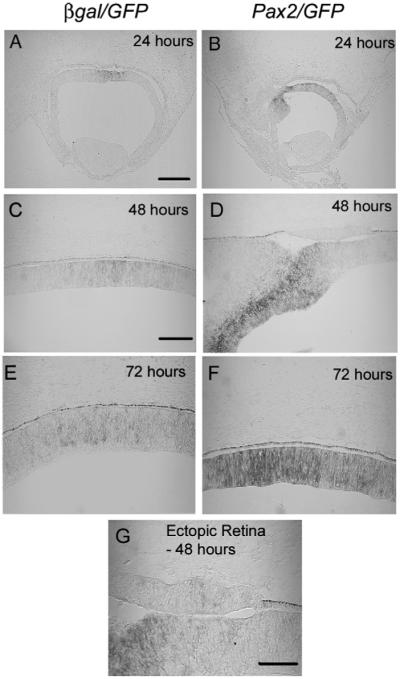
Ectopic retina overexpresses Fgf8. Fgf8 expression was observed in β-gal/GFP and Pax2/GFP electroporated embryos by in situ hybridization 24, 48 and 72 h post electroporation. (B, D, F) show increased expression of fgf8 in the retinae of Pax2/GFP electroporated embryos 24, 48 and 72 h post electroporation as compared to the β-gal/GFP control (A, C and E) respectively. (panel G) Fgf8 expression in the ectopic retina 48 h post electroporation. Scale bar (500 μm) in panel A applies to panels A, B; scale bar (50 μm) in panel C applies to panels C–F; scale bar (20 μm) in panel G.
Discussion
Summary
The present study focused on testing the hypothesis that ectopic Pax2 expression in the ventral retina may lead to improper closure of the choroid fissure, or coloboma and that the mechanism of closure deficiency may be different than those observed in Pax2 loss-of-function experiments. To address this, we used co-electroporation of Pax2 and GFP expression vectors into the chick embryo at stages 10 and 13–15. Embryos electroporated at stage 10 showed severe microphthalmia that, upon sectioning contained an optic vesicle-like structure with retinal pigmented epithelial characteristics. Since choroid fissure closure is reliant upon the formation of optic cup, and embryos electroporated at stage 10 appeared not to develop past the optic vesicle stage, we turned to testing the hypothesis in embryos that were electroporated following optic cup development, between stages 13 and 15. The following conclusions could be drawn from these experiments:1) ectopic expression of Pax2 in the ventral optic cup does indeed lead to failure of choroid fissure closure, 2) there did not appear to be any changes to the normal dorso-ventral patterning in Pax2-electroporated eyes either because of ectopic Pax2 expression or because of disruption to normal expression of patterning molecules, 3) Pax2 expression did not appear to have any effect on cellular survival and/or mitosis in comparison to normal and unelectroporated retinae, 4) the mechanism of coloboma appeared to be the development of astrocytes at the edges of the choroid fissure that extended processes into the fissure, 5) expression of Pax2 in RPE can lead to the formation of ectopic neural retina, and 6) the mechanism by which the ectopic neural retina formed was not driven by Fgf8.
BMP regulation and Pax2 expression
At least two secreted factors have been shown to regulate Pax2 expression (Macdonald et al., 1997; Pfeffer et al., 2000; Hyatt et al., 1996). For instance, a decrease in retinoic acid and sonic hedgehog (SHH), in zebrafish bring about coloboma development (Schmitt and Dowling, 1994; Ekker et al., 1995; Hyatt et al., 1996; Marsh-Armstrong et al., 1994). Our lab has shown previously that a decrease in BMP signaling brought about by overexpression of noggin in the developing optic cup leads to a persistent expression of Pax2 and a failure of choroid fissure closure (Adler and Belecky-Adams, 2002). Since there were many concomitant changes associated with noggin overexpression, it is quite possible that the failure of choroid fissure closure was unrelated to persistent Pax2 expression in the ventral hemi-retina. Clearly, our experiments here show that persistence of Pax2 expression may lead to coloboma, albeit by a slightly different mechanism than that shown in Pax2 knockout and mutant studies (see discussion below). While it is possible that electroporation of Pax2 disrupted the expression of molecules necessary for the normal dorso-ventral patterning of the developing optic cup, we were unable to detect any changes to the patterning in the early retina. In addition, no colobomas were noted in embryos electroporated in the same manner with β-gal and GFP, making it unlikely that there was a disruption to one or more of the molecules necessary for the patterning of the optic cup.
Mechanisms of coloboma development
The term coloboma refers to a transitory gap formed as a result of a defect in the closure of the choroid fissure. Loss of Pax2 expression in the eye, through either haploinsufficiency or mutation of one or both genes, has been associated in part with colobomas, malformations of the optic nerve head and associated vasculature and exiting ganglion cell axons (Otteson et al., 1998; Torres et al., 1996; Favor et al., 1996; Macdonald et al., 1997; Sanyanusin et al., 1995). The coloboma can involve various parts of the eye, for example, the choroid, ciliary body, eyelid, iris, optic nerve and retina. Although the mechanism is not completely understood, Pax2 appears to regulate, either directly or indirectly, the expression of enzymes responsible for the break down of the extracellular matrix at the lips of the optic cup juxtaposed to the choroid fissure (Torres et al., 1996). The persistence of the basal lamina at this junction is thought to prevent the fusion of the neuroepithelium (Schwarz et al., 2000). While the gain-of-function described in this study also appears to have retained the basal lamina at the edges of the optic fissure (not shown), there were several notable differences when compared to Pax2 loss-of-function, namely 1) the persistence of cells that will ultimately become astrocytes next to the choroid fissure, and 2) the development of GFAP and vimentin positive projections in the choroid fissure itself. These results lead us to believe that perhaps the mechanisms of failure of choroid fissure closure leading to coloboma, might be different in the Pax2/GFP electroporated embryos (see Fig. 9). Here we have shown that the ectopic presence of glial cells lining the choroid fissure and their processes within the fissure may inhibit the closure of the choroid fissure. Further, this also suggests that the underlying cause of colobomas in patients might be more heterogeneous at the molecular level (Schroeder et al., 1987; Heegaard et al., 2003).
Fig. 9.
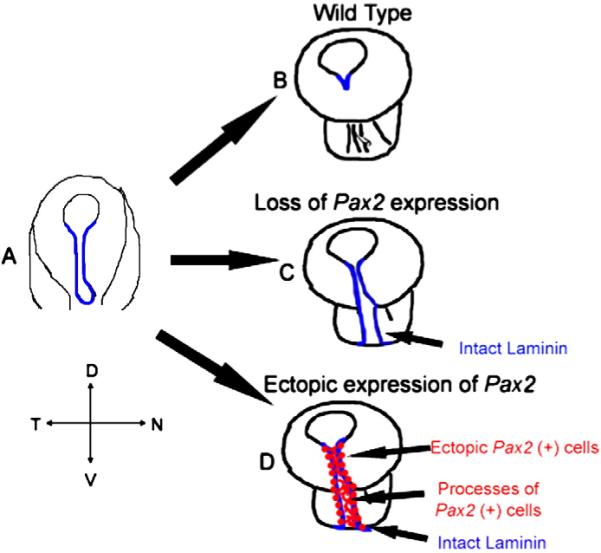
Schematic representation of normal choroid fissure closure compared to that of embryos in which Pax2 is downregulated and those in which Pax2 is ectopically expressed. (A) Optic cup stage (E3 through E6 in chick) when choroid fissure is still open and lips of the fissure express laminin (shown in blue). (B) Late optic cup stage (around E8 in chick) where laminin at the edges of the fissure has been broken down and the choroid fissure has closed. (C) With a loss of Pax2 expression in cells surrounding the choroid fissure, laminin (shown in blue) is persistent and choroid fissure fails to close. (D) When Pax2 is ectopically expressed in cells surrounding the choroid fissure past the normal stage, laminin (blue) and Pax2 expressing cells line the lips of the choroid fissure (shown in red) and their processes (shown in red) fill in the fissure, leading to failure of the choroid fissure to close.
Pax2-Pax6 dynamics
In our studies, overexpression of Pax2 in the early optic cup resulted in microphthalmia. Very similar results were obtained in studies introducing Pax6 morpholinos into HH stage 10 optic vesicle (Canto-Soler and Adler, 2006). Both Pax2 and Pax6 are co-expressed in the mouse optic vesicle, and this co-expression appears to be essential for the regulation of RPE specific transcription factor, MITF (Baumer et al., 2003). However, co-expression beyond a certain point appears to be detrimental to the development of the retina, potentially because, at least under certain conditions, Pax2 and Pax6 can repress each other’s expression (Schwarz et al., 2000). Either a lack of Pax6 expression (Canto-Soler and Adler, 2006) or aberrant Pax2 expression in the distal region of the optic vesicle leads to a morphogenetic failure of the optic cup. Electroporation of Pax2 at stage 10 also led to the development of a smaller lens (unpublished data), confirming the results of Canto-Soler and Adler (2006).
Like Pax6, Pax2 is also important in early patterning events of the optic vesicle and optic cup. Whereas Pax6 appears to play a role in the dorso-ventral and naso-temporal patterning of the retina (Baumer et al., 2003; Ziman et al., 2003; Canto-Soler and Adler, 2006; Reza et al., 2002), Pax2 functions in establishing the proximo-distal patterning of the optic vesicle and closure of the optic fissure (Morcillo et al., 2006). Pax2 appears to be necessary and sufficient to drive neuroepithelial cells into the glial cell lineage, consistent with its role in proximo-distal pattering of the optic vesicle (Soukkarieh et al., 2007). Electroporation of Pax2 into the optic cup resulted in a change of cell fate from retinal to glial, consistent with the results obtained by Soukkarieh et al. (2007), in which Pax2 ectopically expressed in the neural tube causes a switch from neuronal fate to glial. However, when Pax2 is introduced into the neural tube, Schwann cells appeared to develop, rather than the astrocytes found in our study. This result certainly suggests that Pax2 expressing cells can give rise to either astrocytes or Schwann cells, in a context-dependent manner.
Ectopic neural retina
We were fascinated by the observation that of ectopic neural retina in place of the RPE. The development of an ectopic neural retina occurred in a non cell-autonomous fashion when presumptive RPE was electroporated with Pax2 and only cells on one side of the Pax2-expressing cells underwent this change in fate or differentiation. The fact that the ectopic retina occurs in cells neighboring the Pax2-electroporated region suggests that Pax2, either directly or indirectly, regulates the expression of a molecule that then interacts with neighboring cells to bring about the change. A secreted factor would be an ideal example of a molecule that would be secreted from the Pax2 (+) cells to affect neighboring cells. The Fgfs have been implicated in the transdifferentiation process in many instances (Azuma et al., 2005; Sakaguchi et al., 1997), and in fact the transdifferentiation appears not only to be reliant on the downregulation of Mitf, but also upon the upregulation of Pax6 by the Fgfs(Spence et al., 2007; Nguyen and Arnheiter, 2000). While we did localize an increase in Fgf 8 expression in the retina as well as the secondary neural retina, the Fgf8 was not found in the Pax2 expressing cells. Whether that is because a different Fgf is involved in the process or because other factors are involved remains to be determined. In either case, it is interesting to note that to our knowledge, no one has proposed that Pax2 expression may also play a non cell-autonomous role in the optic stalk, supporting the formation of retina in surrounding cells.
The observation that only RPE on one side of the Pax2 (+) cells appears to undergo change in cell fate is somewhat novel. While there are many examples of gene expression that show asymmetry when comparing the various axes of the developing eye (for example, differential expression of ventroptin and BMP4 in the dorso-ventral axis of the optic cup), there are precious few clues as to the origin of such asymmetries. An excellent example of this is the development of left–right asymmetries that affects organ placement within developing embryos (Levin et al., 1995; Levin and Palmer, 2007). In mice, the asymmetry appears to arise from the placement of cytoskeletal motor protein, dynein, within cilia (Levin, 2005). The chirality imposed upon the cilia by the dynein causes it to beat in a clockwise motion, which generates a leftward flow of molecules within the embryo. The flow is thought to carry morphogens, such as nodal, to the left side of the embryo, where it establishes left side-specific cascades of expression patterns, including (but not limited to) BMP4, Shh, FGF8, and PITX2 (Fischer et al., 2002; Fujiwara et al., 2002; Qiu et al., 2005; Levin and Palmer 2007). Another model proposed to account for the origins of left–right asymmetry involves the polar arrangement of cytoskeletal proteins within cells, such as tubulin, that allows the transport of molecules via direction sensitive motor proteins to subcellular localization within the cell (Levin and Palmer 2007). This is proposed to allow the function of proteins that form ionic channels or transporters asymmetrically within a field of cells. The resulting voltage gradient would then allow for small charged molecules, such as serotonin, to be distributed asymmetrically to one side of the embryo (Fukumoto et al., 2005a,b; Hibino et al., 2006; Shimeld and Levin, 2006; Levin and Palmer 2007). In addition, there is also evidence that unidirectional flow of small molecules through gap junctions play a role in patterning (Levin and Mercola, 1998; Esser et al., 2006). At the surface, asymmetries that play a role in patterning may have no connection to the phenotype that is observed in our embryos; however, there are many similarities between some of the molecular components of left–right asymmetry and optic cup patterning. Further, PAX2 is a protein that is involved in optic vesicle and optic cup patterning. It would not be far-fetched to conjecture that PAX2 may regulate the expression of proteins that might be directed to a specific subcellular compartment, or that a patterning molecule regulated by PAX2 might be flowing in a directional manner either across the epithelium or through the gap junctions that link the RPE cells with one another (Pearson et al., 2004; Koh, 1989).
Supplementary Material
Acknowledgments
The authors thank the March of Dimes, the Indiana 21st Century Research and Technology fund for financial support, Harukazu Nakamura for PAX2 construct, Ruben Adler for β-gal construct, Amasaharu Noda for cRaldh3 construct, Katherine Yutzey for Tbx5 construct, Thomas M. Jessel for Bmp4 construct and Mochii M for MMP115 antibody. The authors also thank David Stocum for his support, and Bonnie Blazer Yost and Ellen Chernoff for editorial help on the manuscript.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ydbio.2008.03.041.
References
- Adler R, Belecky-Adams TL. The role of bone morphogenetic proteins in the differentiation of the ventral optic cup. Development. 2002;129(13):3161–3171. doi: 10.1242/dev.129.13.3161. [DOI] [PubMed] [Google Scholar]
- Azuma N, Yamaguchi Y, et al. Mutations of the PAX6 gene detected in patients with a variety of optic-nerve malformations. Am. J. Hum. Genet. 2003;72(6):1565–1570. doi: 10.1086/375555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma N, et al. Transdifferentiation of the retinal pigment epithelia to the neural retina by transfer of the Pax6 transcriptional factor. Hum. Mol. Genet. 2005;14:1059–1068. doi: 10.1093/hmg/ddi098. [DOI] [PubMed] [Google Scholar]
- Barbieri AM, Broccoli V, et al. Vax2 inactivation in mouse determines alteration of the eye dorsal-ventral axis, misrouting of the optic fibres and eye coloboma. Development. 2002;129(3):805–813. doi: 10.1242/dev.129.3.805. [DOI] [PubMed] [Google Scholar]
- Barres BA, et al. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992a;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Barres BA, et al. Cell death in the oligodendrocyte lineage. J. Neurobiol. 1992b;23:1221–1230. doi: 10.1002/neu.480230912. [DOI] [PubMed] [Google Scholar]
- Barthel LK, Raymond PA. Subcellular localization of alpha-tubulin and opsin mRNA in the goldfish retina using digoxigenin-labeled cRNA probes detected by alkaline phosphatase and HRP histochemistry. J. Neurosci. Methods. 1993;50(2):145–152. doi: 10.1016/0165-0270(93)90002-9. [DOI] [PubMed] [Google Scholar]
- Baumer N, Marquardt T, et al. Retinal pigmented epithelium determination requires the redundant activities of Pax2 and Pax6. Development. 2003;130(13):2903–2915. doi: 10.1242/dev.00450. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams T, et al. Pax-6, Prox 1, and Chx10 homeobox gene expression correlates with phenotypic fate of retinal precursor cells. Invest. Ophthalmol. Vis. Sci. 1997;38:1293–1303. [PubMed] [Google Scholar]
- Benetti E, et al. Renal hypoplasia without optic coloboma associated with PAX2 gene deletion. Nephrol. Dial. Transplant. 2007;22:2076–2078. doi: 10.1093/ndt/gfm187. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, et al. P0 is an early marker of the Schwann cell lineage in chickens. Neuron. 1991;7:831–844. doi: 10.1016/0896-6273(91)90285-8. [DOI] [PubMed] [Google Scholar]
- Bouchard M, et al. Identification of Pax2-regulated genes by expression profiling of the mid-hindbrain organizer region. Development. 2005;132(11):2633–2643. doi: 10.1242/dev.01833. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, et al. Implication of OTX2 in pigment epithelium determination and neural retina differentiation. J. Neurosci. 1997;17(11):4243–4252. doi: 10.1523/JNEUROSCI.17-11-04243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto-Soler MV, Adler R. Optic cup and lens development requires PAX6 expression in the early optic vesicle during a narrow time window. Dev. Biol. 2006;294(1):119–132. doi: 10.1016/j.ydbio.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu. Rev. Cell Dev. Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Cook B, et al. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest. Ophthalmol. Vis. Sci. 1995;36:990–996. [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Dev. Biol. 1965;12(1):79–92. doi: 10.1016/0012-1606(65)90022-9. [DOI] [PubMed] [Google Scholar]
- Dale K, Sattar N, et al. Differential patterning of ventral midline cells by axial mesoderm is regulated by BMP7 and chordin. Development. 1999;126(2):397–408. doi: 10.1242/dev.126.2.397. [DOI] [PubMed] [Google Scholar]
- Dressler GR, Douglass EC. Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc. Natl. Acad. Sci. U. S. A. 1992;89(4):1179–1183. doi: 10.1073/pnas.89.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles MR, Schimmenti LA. Renal-coloboma syndrome: a multi-system developmental disorder caused by PAX2 mutations. Clin. Genet. 1999;56(1):1–9. doi: 10.1034/j.1399-0004.1999.560101.x. [DOI] [PubMed] [Google Scholar]
- Ekker SC, Ungar AR, et al. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr. Biol. 1995;5(8):944–955. doi: 10.1016/s0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- Esser AT, et al. Mathematical model of morphogen electrophoresis through gap junctions. Dev. Dyn. 2006;235:2144–2159. doi: 10.1002/dvdy.20870. [DOI] [PubMed] [Google Scholar]
- Favor J, Sandulache R, et al. The mouse Pax2(1Neu) mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye, and kidney. Proc. Natl. Acad. Sci. U. S. A. 1996;93(24):13870–13875. doi: 10.1073/pnas.93.24.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, et al. FGF8 acts as a right determinant during establishment of the left–right axis in the rabbit. Curr. Biol. 2002;12:1807–1816. doi: 10.1016/s0960-9822(02)01222-8. [DOI] [PubMed] [Google Scholar]
- Fujiwara M, et al. Regulation of body size and behavioral state of C. elegans by sensory perception and the EGL-4 cGMP-dependent protein kinase. Neuron. 2002;36:1091–1102. doi: 10.1016/s0896-6273(02)01093-0. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, et al. Serotonin transporter function is an early step in left–right patterning in chick and frog embryos. Dev. Neurosci. 2005a;27:349–363. doi: 10.1159/000088451. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, et al. Serotonin signaling is a very early step in patterning of the left–right axis in chick and frog embryos. Curr. Biol. 2005b;15:794–803. doi: 10.1016/j.cub.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, et al. R- and B-cadherin expression defines subpopulations of glial cells involved in axonal guidance in the optic nerve head of the chicken. Glia. 2000;31:131–143. [PubMed] [Google Scholar]
- Gregory-Evans CY, Williams MJ, et al. Ocular coloboma: a reassessment in the age of molecular neuroscience. J. Med. Genet. 2004;41(12):881–891. doi: 10.1136/jmg.2004.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. Series of embryonic chicken growth. J. Morph. 1951;88:49–92. [PubMed] [Google Scholar]
- Heegaard S, Rosenberg T, et al. An unusual retinal vascular morphology in connection with a novel AIPL1 mutation in Leber’s congenital amaurosis. Br. J. Ophthalmol. 2003;87(8):980–983. doi: 10.1136/bjo.87.8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hero I. The optic fissure in the normal and microphthalmic mouse. Exp. Eye Res. 1989;49(2):229–239. doi: 10.1016/0014-4835(89)90093-6. [DOI] [PubMed] [Google Scholar]
- Hero I. Optic fissure closure in the normal cinnamon mouse. An ultrastructural study. Invest. Ophthalmol. Vis. Sci. 1990;31(1):197–216. [PubMed] [Google Scholar]
- Hero I, Farjah M, et al. The prenatal development of the optic fissure in colobomatous microphthalmia. Invest. Ophthalmol. Vis. Sci. 1991;32(9):2622–2635. [PubMed] [Google Scholar]
- Hibino T, et al. Phylogenetic correspondence of the body axes in bilaterians is revealed by the right-sided expression of Pitx genes in echinoderm larvae. Dev. Growth Differ. 2006;48:587–595. doi: 10.1111/j.1440-169X.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- Hughes S, Yang H, et al. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest. Ophthalmol. Vis. Sci. 2000;41(5):1217–1228. [PubMed] [Google Scholar]
- Hyatt GA, Schmitt EA, et al. Retinoic acid alters photoreceptor development in vivo. Proc. Natl. Acad. Sci. U. S. A. 1996;93(23):13298–13303. doi: 10.1073/pnas.93.23.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itasaki N, Bel-Vialar S, et al. ‘Shocking’ developments in chick embryology: electroporation and in ovo gene expression. Nat. Cell Biol. 1999;1(8):E203–E207. doi: 10.1038/70231. [DOI] [PubMed] [Google Scholar]
- Koh SW. The chick retinal pigment epithelium grown on permeable support demonstrates functional polarity. Exp. Cell Res. 1989;181:331–347. doi: 10.1016/0014-4827(89)90092-x. [DOI] [PubMed] [Google Scholar]
- Levin M. Left–right asymmetry in embryonic development: a comprehensive review. Mech. Dev. 2005;122:3–25. doi: 10.1016/j.mod.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Gap junctions are involved in the early generation of left–right assymetry. Dev. Biol. 1998;203(1):90–105. doi: 10.1006/dbio.1998.9024. [DOI] [PubMed] [Google Scholar]
- Levin M, Palmer AR. Left–right patterning from the inside out: widespread evidence for intracellular control. BioEssays. 2007;29:271–287. doi: 10.1002/bies.20545. [DOI] [PubMed] [Google Scholar]
- Levin M, et al. A molecular pathway determining left–right asymmetry in chick embryogenesis. Cell. 1995;82:803–814. doi: 10.1016/0092-8674(95)90477-8. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Barth KA, et al. Midline signalling is required for Pax gene regulation and patterning of the eyes. Development. 1995;121(10):3267–3278. doi: 10.1242/dev.121.10.3267. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Scholes J, et al. The Pax protein Noi is required for commissural axon pathway formation in the rostral forebrain. Development. 1997;124(12):2397–2408. doi: 10.1242/dev.124.12.2397. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Hallonet M, et al. Pax genes and their roles in cell differentiation and development. Curr. Opin. Cell Biol. 1996;8(6):851–857. doi: 10.1016/s0955-0674(96)80087-1. [DOI] [PubMed] [Google Scholar]
- Marsh-Armstrong N, McCaffery P, et al. Retinoic acid is necessary for development of the ventral retina in zebrafish. Proc. Natl. Acad. Sci. U. S. A. 1994;91(15):7286–7290. doi: 10.1073/pnas.91.15.7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochii M, Takeuchi T, et al. The expression of melanosomal matrix protein in the transdifferentiation of pigmented epithelial cells into lens cells. Cell Differ. 1988;23(1–2):133–141. doi: 10.1016/0045-6039(88)90045-0. [DOI] [PubMed] [Google Scholar]
- Morcillo J, Martinez-Morales JR, et al. Proper patterning of the optic fissure requires the sequential activity of BMP7 and SHH. Development. 2006;133(16):3179–3190. doi: 10.1242/dev.02493. [DOI] [PubMed] [Google Scholar]
- Nakamura H. Regionalization of the optic tectum: combinations of gene expression that define the tectum. Trends Neurosci. 2001;24(1):32–39. doi: 10.1016/s0166-2236(00)01676-3. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development. 2000;127:3581–3591. doi: 10.1242/dev.127.16.3581. [DOI] [PubMed] [Google Scholar]
- Nitsch R, et al. Human brain-cell death induced by tumour-necrosis-factor-related apoptosis-inducing ligand (TRAIL) Lancet. 2000;356:827–828. doi: 10.1016/S0140-6736(00)02659-3. [DOI] [PubMed] [Google Scholar]
- Qiu D, et al. Localization and loss-of-function implicates ciliary proteins in early, cytoplasmic roles in left–right asymmetry. Dev. Dyn. 2005;234:176–189. doi: 10.1002/dvdy.20509. [DOI] [PubMed] [Google Scholar]
- Okafuji T, Funahashi J, et al. Roles of Pax-2 in initiation of the chick tectal development. Brain Res. Dev. Brain Res. 1999;116(1):41–49. doi: 10.1016/s0165-3806(99)00073-5. [DOI] [PubMed] [Google Scholar]
- Oster SF, et al. Ganglion cell axon pathfinding in the retina and optic nerve. Semin. Cell Dev. Biol. 2004;15:125–136. doi: 10.1016/j.semcdb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Otteson DC, Shelden E, et al. Pax2 expression and retinal morphogenesis in the normal and Krd mouse. Dev. Biol. 1998;193(2):209–224. doi: 10.1006/dbio.1997.8794. [DOI] [PubMed] [Google Scholar]
- Pearson RA, et al. Ca(2+) signalling and gap junction coupling within and between pigment epithelium and neural retina in the developing chick. Eur. J. Neurosci. 2004;19:2435–2445. doi: 10.1111/j.0953-816X.2004.03338.x. [DOI] [PubMed] [Google Scholar]
- Pfeffer PL, Bouchard M, et al. Pax2 and homeodomain proteins cooperatively regulate a 435 bp enhancer of the mouse Pax5 gene at the midbrain-hindbrain boundary. Development. 2000;127(5):1017–1028. doi: 10.1242/dev.127.5.1017. [DOI] [PubMed] [Google Scholar]
- Reza HM, Ogino H, et al. L-Maf, a downstream target of Pax6, is essential for chick lens development. Mech. Dev. 2002;116(1–2):61–73. doi: 10.1016/s0925-4773(02)00137-5. [DOI] [PubMed] [Google Scholar]
- Sanyanusin P, Schimmenti LA, et al. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat. Genet. 1995;9(4):358–364. doi: 10.1038/ng0495-358. [DOI] [PubMed] [Google Scholar]
- Sakaguchi DS, et al. Basic fibroblast growth factor (FGF-2) induced transdiffer-entiation of retinal pigment epithelium: generation of retinal neurons and glia. Dev. Dyn. 1997;209:387–398. doi: 10.1002/(SICI)1097-0177(199708)209:4<387::AID-AJA6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Schmitt EA, Dowling JE. Early eye morphogenesis in the zebrafish, Brachydaniorerio. J. Comp. Neurol. 1994;344(4):532–542. doi: 10.1002/cne.903440404. [DOI] [PubMed] [Google Scholar]
- Schroeder R, Mets MB, et al. Leber’s congenital amaurosis. Retrospective review of 43 cases and a new fundus finding in two cases. Arch. Ophthalmol. 1987;105(3):356–359. doi: 10.1001/archopht.1987.01060030076030. [DOI] [PubMed] [Google Scholar]
- Schuck J, et al. The peropapillary glia of the optic nerve head in the chicken retina. Anat. Rec. 2000;259(3):263–275. doi: 10.1002/1097-0185(20000701)259:3<263::AID-AR40>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Schwarz M, et al. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development. 2000;127(20):4325–4334. doi: 10.1242/dev.127.20.4325. [DOI] [PubMed] [Google Scholar]
- Shimeld SM, Levin M. Evidence for the regulation of left–right asymmetry in Ciona intestinalis by ion flux. Dev. Dyn. 2006;235(6):1543–1553. doi: 10.1002/dvdy.20792. [DOI] [PubMed] [Google Scholar]
- Soukkarieh C, Agius E, et al. Pax2 regulates neuronal-glial cell fate choice in the embryonic optic nerve. Dev. Biol. 2007;303(2):800–813. doi: 10.1016/j.ydbio.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Spence JR, et al. Retina regeneration in the chick embryo is not induced by spontaneous Mitf downregulation but requires FGF/FGFR/MEK/Erk dependent upregulation of Pax6. Mol. Vis. 2007;13:57–65. [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Shintani T, et al. Identification of RALDH-3, a novel retinaldehyde dehydrogenase, expressed in the ventral region of the retina. Mech. Dev. 2000;98(1–2):37–50. doi: 10.1016/s0925-4773(00)00450-0. [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, et al. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development. 1996;122(11):3381–3391. doi: 10.1242/dev.122.11.3381. [DOI] [PubMed] [Google Scholar]
- Toy J, et al. Effects of homeobox genes on the differentiation of photoreceptor and nonphotoreceptor neurons. Invest. Ophthalmol. Vis. Sci. 2002;43:3522–3529. [PubMed] [Google Scholar]
- Wallace VA, Raff MC. A role for Sonic hedgehog in axon-to-astrocyte signalling in the rodent optic nerve. Development. 1999;126(13):2901–2909. doi: 10.1242/dev.126.13.2901. [DOI] [PubMed] [Google Scholar]
- Wilson JM, et al. Expression patterns of chick Musashi-1 in the developing nervous system. Gene Expr. Patterns. 2007;7:817–825. doi: 10.1016/j.modgep.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Zhang SM, et al. Myelin glycoprotein P0 is expressed at early stages of chicken and rat embryogenesis. J. Neurosci. Res. 1995;40:241–250. doi: 10.1002/jnr.490400213. [DOI] [PubMed] [Google Scholar]
- Ziman M, Rodger J, et al. A dorso-ventral gradient of Pax6 in the developing retina suggests a role in topographic map formation. Brain Res. Dev. Brain Res. 2003;140(2):299–302. doi: 10.1016/s0165-3806(02)00605-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.