Abstract
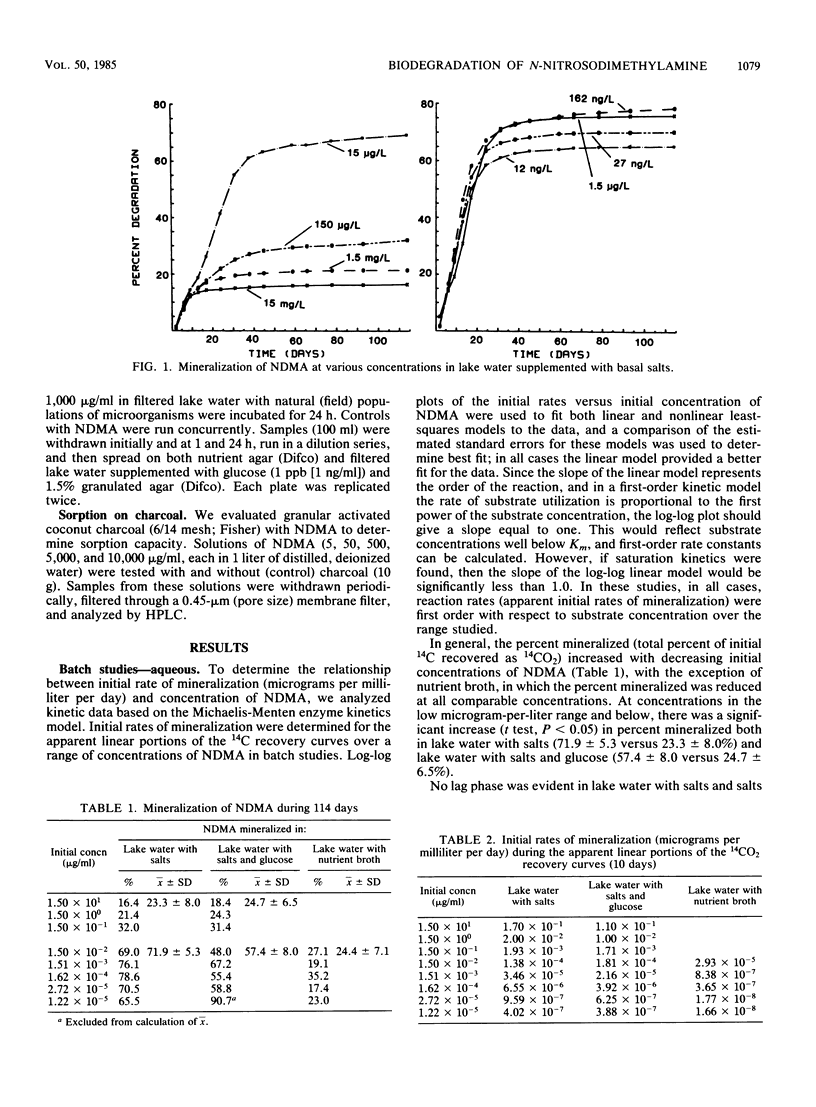
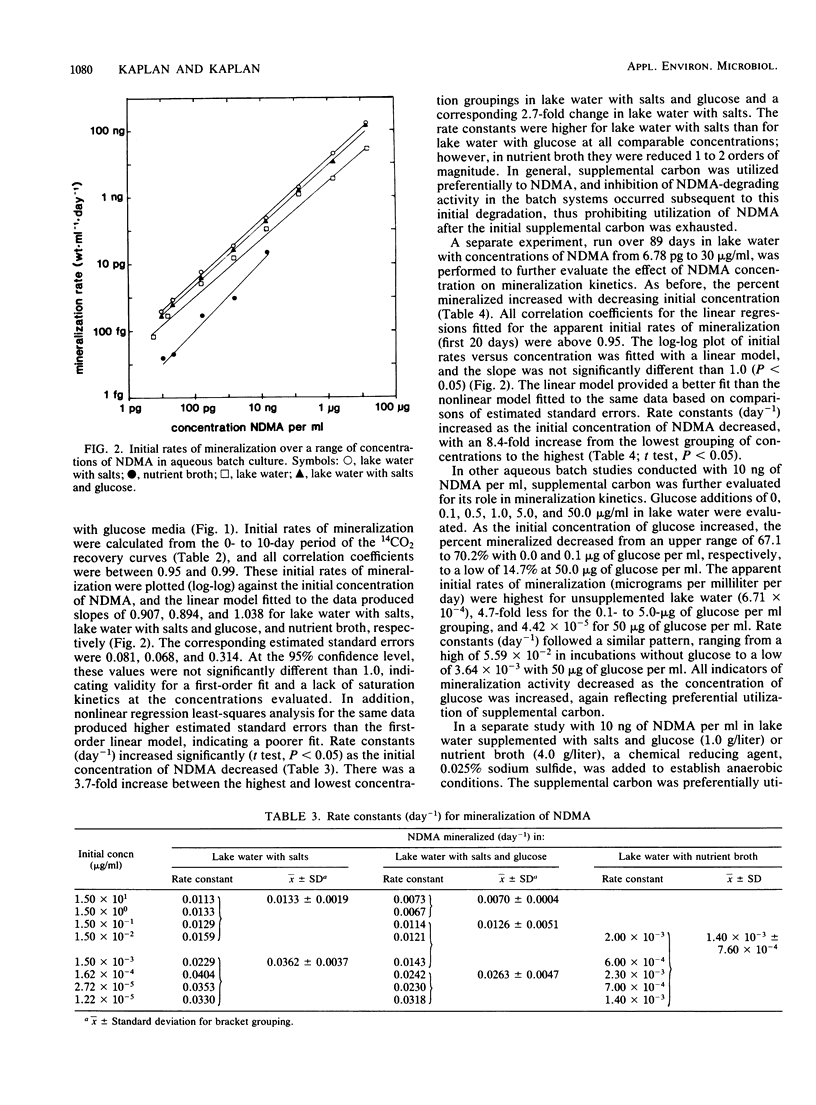
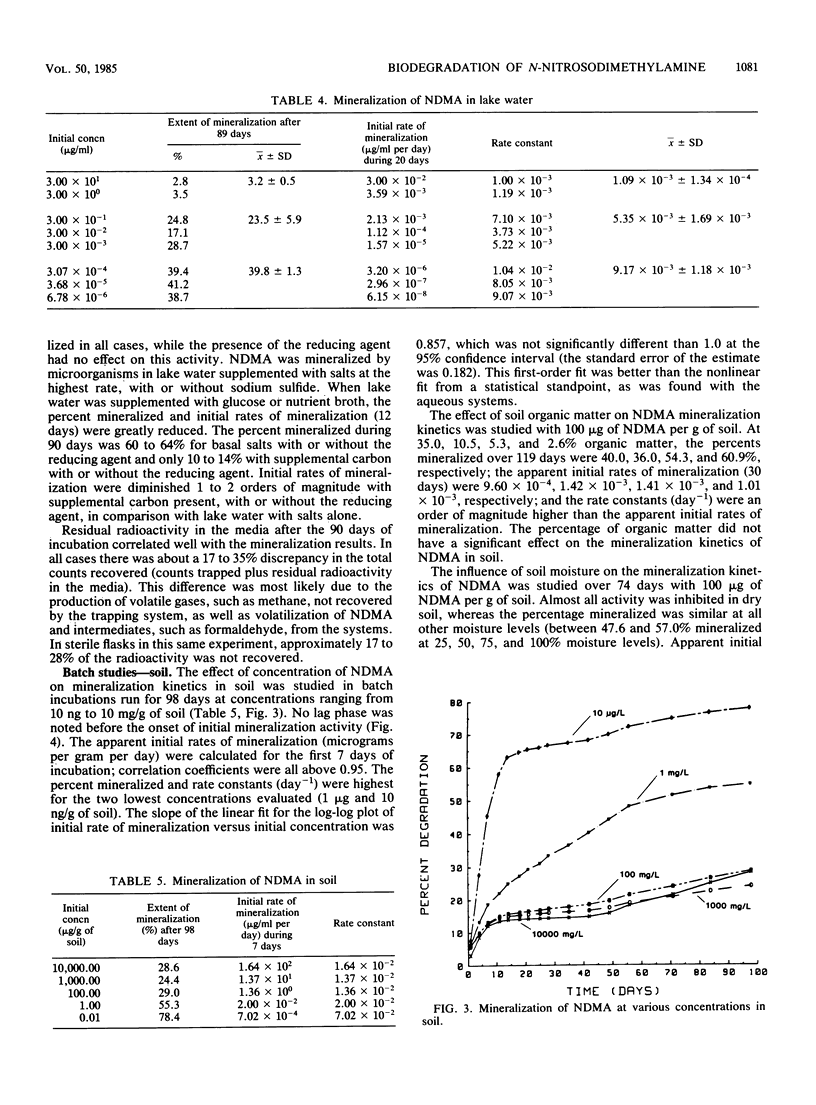
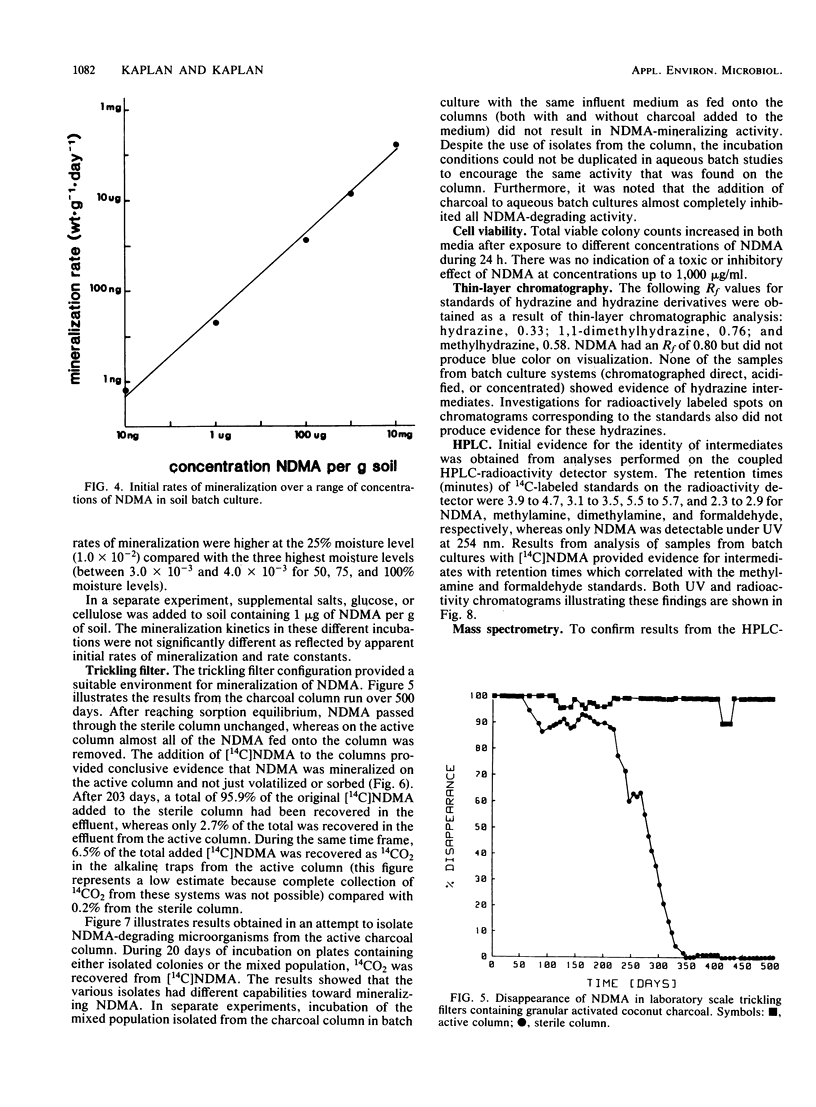
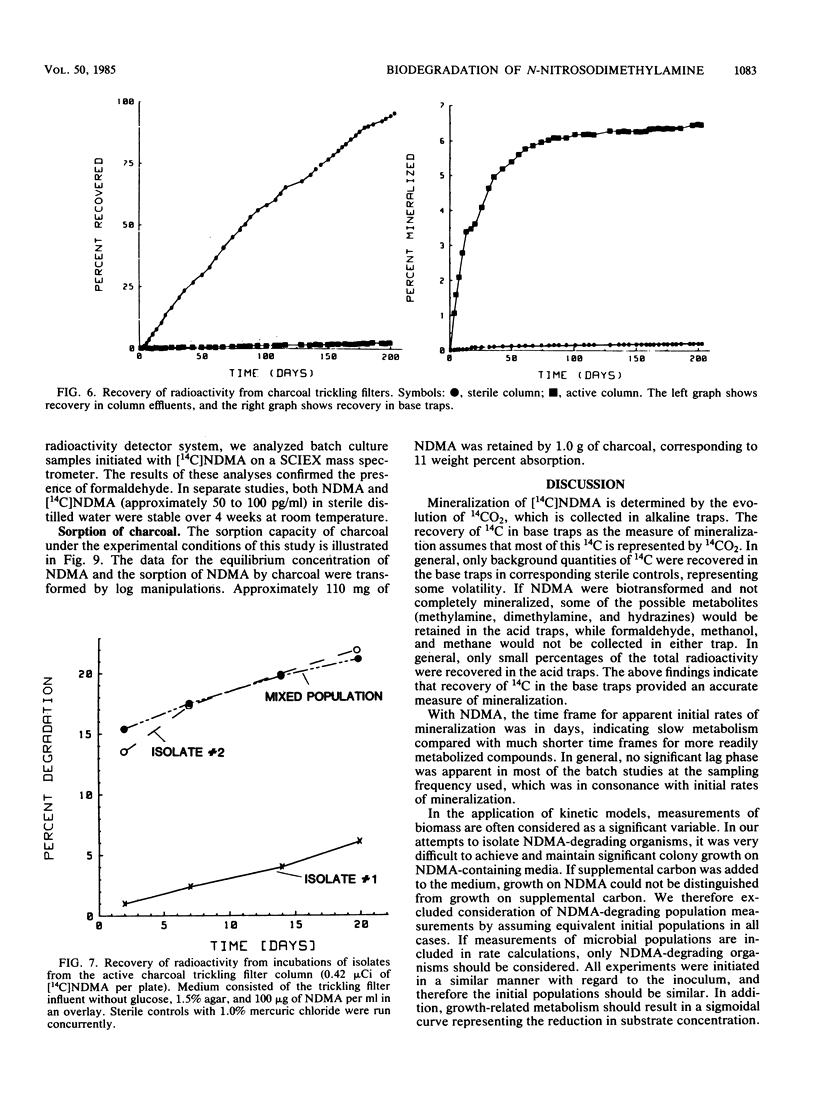
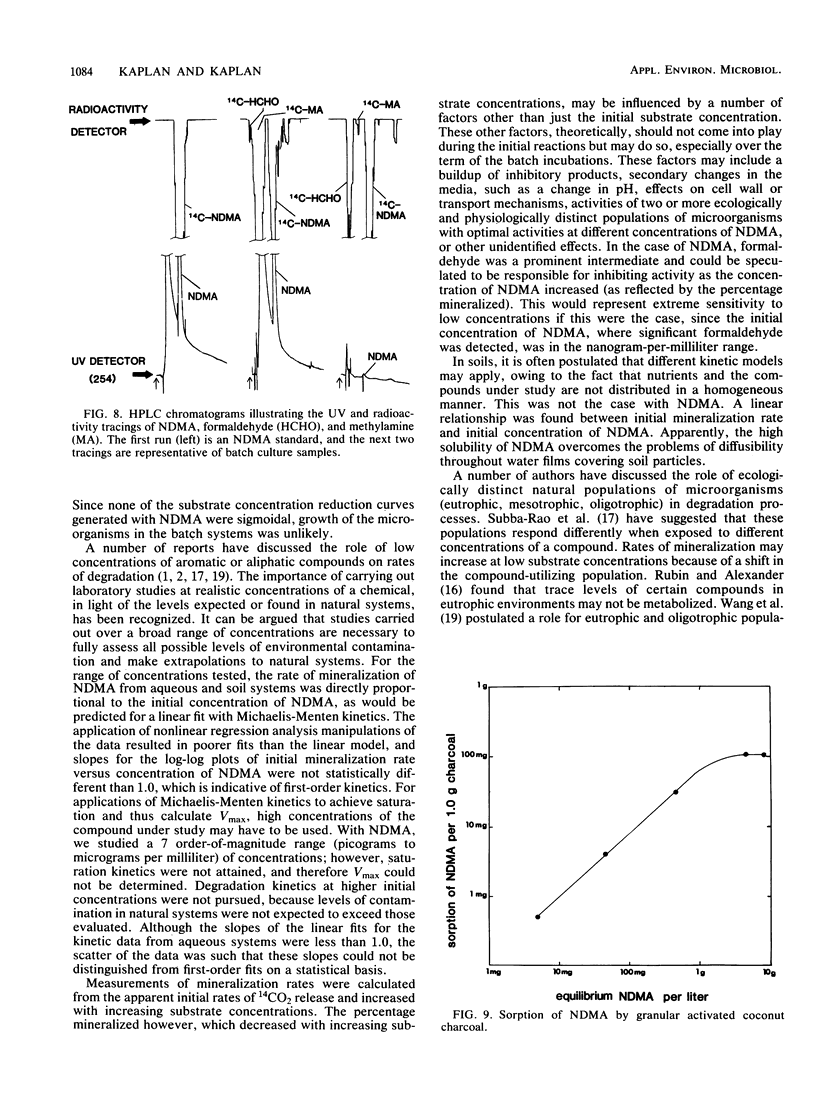
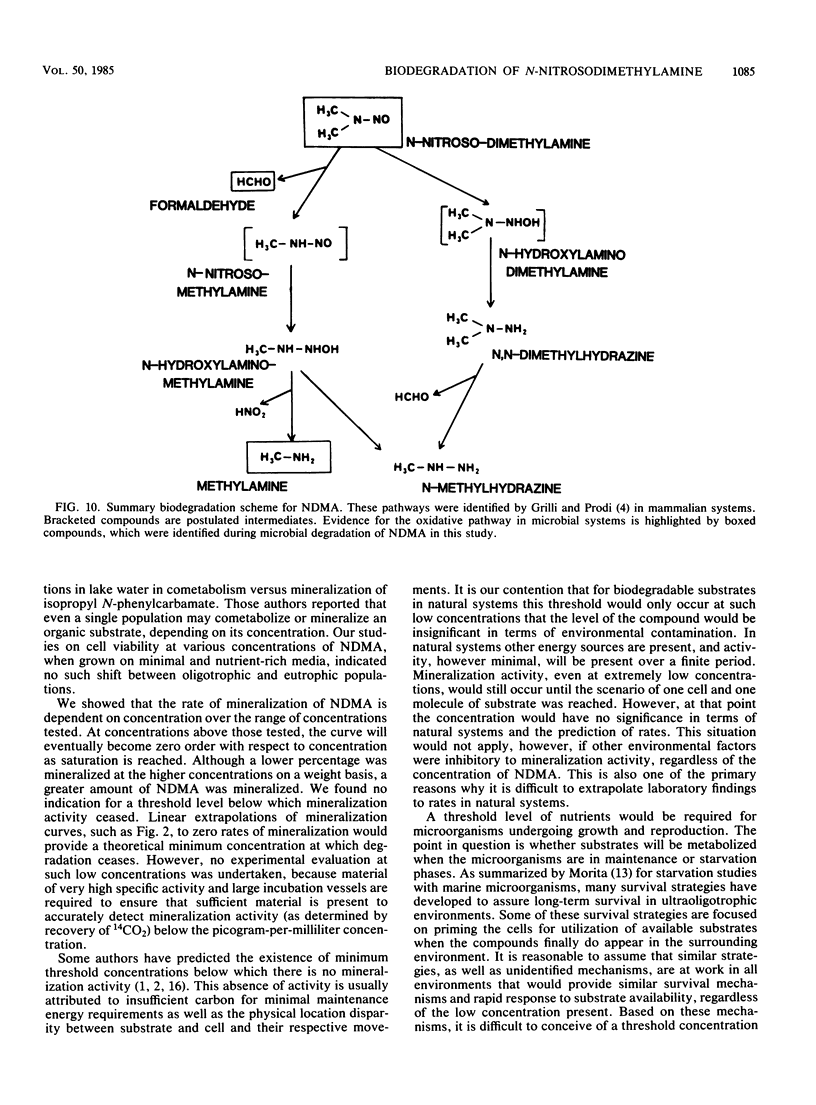
N-Nitrosodimethylamine (NDMA) was mineralized by microorganisms in aqueous and soil systems. Initial rates of mineralization (micrograms per milliliter per day) were calculated for a wide range of initial concentrations of NDMA (micrograms per milliliter to picograms per milliliter). Log-log plots of the data were fitted with both linear and nonlinear least-squares analyses; however, linear models provided better fits for the kinetic data in all cases. The slopes of the linear fits were not significantly different than 1.0 (P < 0.05); thus, first-order reaction kinetics were in effect over the range of concentrations tested, and saturation kinetics were not achieved. Rate constants (day−1) and total percent mineralized increased with decreasing initial concentrations of NDMA. Rates of mineralization were reduced in aqueous systems when supplemental carbon was available, whereas in soils, percentages of organic matter and supplemental carbon had little effect on rates of mineralization. Implications of these results for predictions of rates and threshold limits of mineralization activity in natural systems are discussed. A laboratory scale simulated trickling filter containing an activated charcoal bed provided a suitable environment for mineralization of NDMA at concentrations of 50 and 100 μg/ml on a continuous basis. NDMA was not toxic to natural populations of microorganisms at concentrations up to 10 mg/ml. Using high-pressure liquid chromatography coupled with radioactivity detection, we identified formaldehyde and methylamine as intermediates produced during the biodegradation of NDMA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boethling R. S., Alexander M. Effect of concentration of organic chemicals on their biodegradation by natural microbial communities. Appl Environ Microbiol. 1979 Jun;37(6):1211–1216. doi: 10.1128/aem.37.6.1211-1216.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli S., Prodi G. Identification of dimethylnitrosoamine metabolites in vitro. Gan. 1975 Oct;66(5):473–480. [PubMed] [Google Scholar]
- Jensen D. E., Lotlikar P. D., Magee P. N. The in vitro methylation of DNA by microsomally-activated dimethylnitrosamine and its correlation with formaldehyde production. Carcinogenesis. 1981;2(4):349–354. doi: 10.1093/carcin/2.4.349. [DOI] [PubMed] [Google Scholar]
- Lake B. G., Phillips J. C., Heading C. E., Gangolli S. D. Studies on the in vitro metabolism of dimethylnitrosamine by rat liver. Toxicology. 1976 Mar;5(3):297–309. doi: 10.1016/0300-483x(76)90049-4. [DOI] [PubMed] [Google Scholar]
- Lotlikar P. D., Baldy W. J., Jr, Dwyer E. N. Dimethylnitrosamine demethylation by reconstituted liver microsomal cytochrome P-450 enzyme system. Biochem J. 1975 Dec;152(3):705–708. doi: 10.1042/bj1520705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallik M. A., Tesfai K. Transformation of nitrosamines in soil and in vitro by soil microorganisms. Bull Environ Contam Toxicol. 1981 Jul;27(1):115–121. doi: 10.1007/BF01610996. [DOI] [PubMed] [Google Scholar]
- Milstein S., Guttenplan J. B. Near quantitative production of molecular nitrogen from metabolism of dimethylnitrosamine. Biochem Biophys Res Commun. 1979 Mar 15;87(1):337–342. doi: 10.1016/0006-291x(79)91684-x. [DOI] [PubMed] [Google Scholar]
- Subba-Rao R. V., Rubin H. E., Alexander M. Kinetics and extent of mineralization of organic chemicals at trace levels in freshwater and sewage. Appl Environ Microbiol. 1982 May;43(5):1139–1150. doi: 10.1128/aem.43.5.1139-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate R. L., 3rd, Alexander M. Stability of nitrosamines in samples of lake water, soil, and sewage. J Natl Cancer Inst. 1975 Feb;54(2):327–330. [PubMed] [Google Scholar]
- Wang Y. S., Subba-Rao R. V., Alexander M. Effect of substrate concentration and organic and inorganic compounds on the occurrence and rate of mineralization and cometabolism. Appl Environ Microbiol. 1984 Jun;47(6):1195–1200. doi: 10.1128/aem.47.6.1195-1200.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


