Abstract
Blunt chest trauma resulting in pulmonary contusion with an accompanying acute inflammatory response is a common but poorly understood injury. We previously demonstrated that toll-like receptor 2 participates in the inflammatory response to lung injury. We hypothesized that the toll-like receptor 4, in a MyD88-dependent manner, may also participate in the response to lung injury. To investigate this, we used a model of pulmonary contusion in the mouse that is similar to that observed clinically in humans and evaluated post injury lung function, pulmonary neutrophil recruitment and the systemic innate immune response. Comparisons were made between wild type mice and mice deficient in toll like receptor 4 or MyD88. We found toll-like receptor 4 dependent responses to pulmonary contusion that include hypoxemia, edema, and neutrophil infiltration. Increased expression of interleukin 6 and chemokine (C-X-C motif) ligand-1 in the bronchoalveolar lavage and serum was also dependent on TLR4 activation. We further demonstrated that these responses to pulmonary contusion were dependent on MyD88, an adapter protein in the signal transduction pathway mediated by toll-like receptors. These results show that toll-like receptors have a primary role in the response to acute lung injury. Lung inflammation and systemic innate immune responses are dependent on toll-like receptor activation by pulmonary contusion.
Keywords: inflammation, blunt chest trauma, mouse model, innate immunity
Introduction
Blunt chest trauma resulting in pulmonary contusion is a common injury, affecting 10-17% of all trauma admissions with estimates of mortality at 10-25% (1;2). Sequelae of pulmonary contusion vary widely and include infection (pneumonia), local organ failure (Acute Respiratory Distress Syndrome, ARDS), and remote organ failure (Multiple Organ Dysfunction/Failure, MODS/MOF) (3). In both animal and human studies, lung contusion results in activation of innate immunity with the localized production of a variety of inflammatory cytokines and chemokines, followed by neutrophil infiltration into the areas of injury (4-6). The magnitude of this response has been associated with both ARDS and infection (7;8).
The innate inflammatory response to noninfectious tissue injury is, in many ways, indistinguishable from that initiated by infection. Activation of innate immunity during infection is mediated by activation of the Toll-like receptors (TLR) (9;10). At least 13 TLRs have been identified (11), recognizing conserved components of infectious bacteria (flagella, LPS, PGN) and viruses (eg. double stranded RNA). All but TLR3 utilize the intracellular adapter protein MyD88 in order to induce NFκB activation and subsequent inflammatory mediator expression (12). An evolving paradigm has suggested that endogenous TLR ligands are liberated after cell injury or necrosis and the resulting “danger signal” serves to activate innate immune mechanisms warning the host of tissue injury (13). Indeed, evidence is accumulating that supports a role for TLR signaling after noninfectious lung injury including identification of a number of endogenous TLR ligands such as hyaluronan, heparin-sulfate, heat shock proteins (HSP) 60, HSP70), High Mobility Group Box-1 (HMGB-1) protein and surfactant protein-A which are potentially liberated after lung trauma (14-19). We have previously demonstrated a role for TLR2 induced activation of innate immunity after lung contusion involving TLR2 dependent CXCL1 expression and neutrophil influx to the injured lung (20). Complementary findings were seen after bleomycin-induced and lethal oxidant lung injury, in which TLR2 and TLR4 deficiency resulted in reduced pulmonary neutrophilia and CXC chemokine expression (14;21;22). Additionally, exogenous expression of the TLR 2 and TLR4 ligand, HSP70 in pulmonary epithelial cells has been associated with improvement of ARDS and reduced pulmonary neutrophilia (23). These data clearly indicate that TLR mediated events play an important part in the initial innate immune response to noninfectious lung injury.
Little is known about the role of TLR4 and MyD88 in the inflammatory response to pulmonary contusion. The purpose of this study was to determine, using clinically relevant critieria, if TLR4 and MyD88 participate in the response to blunt chest injury. We delivered a blunt chest injury to wild type and mice deficient in TLR4 or MyD88 expression and evaluated systemic inflammatory mediator expression, pulmonary neutrophil recruitment, and post injury lung function. We found a TLR4 dependent inflammatory response to pulmonary contusion that is characterized by edema, neutrophil infiltration, and increased expression of the innate immunity pro-inflammatory mediator interleukin 6 and the chemokine (C-X-C motif) ligand-1 (CXCL1). We further demonstrate that these responses share a common signal transduction pathway that utilizes the TLR adapter protein, MyD88. Our results indicate that lung inflammation and systemic innate immune responses are dependent on TLR activation after pulmonary contusion.
Methods
Animals
Male, age matched (8-9 weeks) wild-type (C57/BL6), TLR4-/-, and MyD88-/- mice were utilized in this study. The MyD88-/- mice were provided by S. Akira (Osaka University, Osaka Japan) and backcrossed onto a C57/BL6 background for at least 8 generations. The TLR4-/- were obtained from Jackson Laboratories (Bar Harbor, ME) and the allele introgressed into the C57/BL6 background for at least 5 generations. All animals were bred and maintained under specific pathogen-free conditions at the animal facility at Wake Forest University School of Medicine. The protocol used in this study was approved by the Animal Care and Use Committee (#A06-068).
Blunt Chest Injury Model
Injury was induced using the Cortical Contusion Impactor (CCI) as described previously (20). Briefly, mice are anesthetized with 2% isoflurane at a flow rate of 1L/min. The mouse is positioned left lateral decubitus and during inspiration, the right chest is struck with the CCI along the posterior axillary line, 1cm above the costal margin. Control animals receive anesthetic alone. Mice were followed for various times after injury as specified in the figure legends. Serum and tissue samples were collected at the time of death by isoflurane overdose and cervical dislocation.
Arterial Blood Gas
Arterial blood gas (ABG) samples were obtained from the ileac artery. The animals were induced with isoflurane anesthesia (3%) and then maintained on an admixture of 1.5% isoflurane with 100% oxygen at a flow rate of 1L/min. After 5 minutes, a midline laparotomy incision was created from just above the pubis to just below the sternum. The animal is eviscerated and the ileac artery exposed. A 26 gauge needle was then used to cannulate the iliac artery to obtain the blood. The partial pressure of oxygen (pO2) was measured in each sample using a Stat Profile pHOx Blood gas Analyzer (Nova Biomedical, Waltham, MA) according to the manufacturer's instructions.
Histopathology
Lung specimens harvested at time of death were fixed in 10% formalin, sectioned, and stained with hematoxylin and eosin (H&E). Slides were evaluated by an experienced pathologist (NAB) and graded for the presence of interstitial neutrophilic infiltrate, intra-alveolar hemorrhage, and pulmonary septal edema as described previously (20).
Bronchoalveolar lavage (BAL)
After sacrifice, BAL was performed by cannulation of the trachea and lavage is performed using 4ml of phosphate buffered saline (PBS, Sigma Biochemical, St. Louis, MO) at 4°C. BAL was centrifuged at 300 × g, 4°C for 10 minutes and supernatant collected and stored at -70°C until use. The cell pellet was counted and differentiated as previously described (20).
Lung wet:dry weights
Whole lung specimens were removed, weighed and placed in an oven at 37°C for 24 hours to dry and then reweighed. Wet weight:dry weight ratios were calculated.
Cytokine and Chemokine expression
BAL and/or serum levels of IL-6 and CXCL1 were measured using commercially available ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Samples were assayed in duplicate.
Immunoblot (Western blot) analysis
Lung tissue samples were thawed and suspended in homogenization buffer (25mM Tris-HCl pH 7.6, 150mM NaCl, 1% NP-40, 0.1% SDS, protease inhibitors) and homogenized (TissueMiser, Fisher Scientific, Pittsburgh, PA). The homogenate was centrifuged at 3000 × g, 4°C for 10 minutes and the supernatant centrifuged again at 10,000 × g, 4°C for 10 minutes. Solubilized protein concentrations were determined using Coomassie Protein Assay Reagent according to the manufacturer's instructions (Pierce, Rockford, IL). Proteins were separated by SDS-PAGE and transferred to an Immuno-Blot® PVDF membrane (Bio-Rad Laboratories, Hercules, CA). Blots were blocked for 1 hour with Odyssey Blocking Buffer (Li-Cor Biosciences, Lincoln, NE). Anti-neutrophil elastase (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-β-actin (Sigma-Aldrich, St. Louis, MO) antibodies were diluted in Odyssey Blocking Buffer with 0.1% Tween-20 and the membranes were incubated overnight at 4°C. Antibody-antigen complexes were detected using appropriately matched IRDye® secondary antibodies (Li-Cor Biosciences, Lincoln, NE) and visualized using the Odyssey Infared Imaging System (Li-Cor Biosciences, Lincoln, NE). Relative protein levels were quantitated using Odyssey v1.2 Software (Li-Cor Biosciences, Lincoln, NE) and reported as integrated intensity.
Immunohistochemistry
Lung sections were deparaffinized with three changes of xylenes and hydrated through a series of graded alcohol changes (3 washes of 100% EtOH for 5min; 95% EtOH for 5min; 75% EtOH for 5min). Endogenous peroxidase was quenched with 0.3% H2O2 in MeOH for 30min. An antigen retrieval step was performed by heating sections in 10mM citrate buffer pH6.0 for 45min. Sections were incubated with a 1:50 dilution of anti-neutrophil elastase antibody (Santa Cruz Biotechnology, Santa Cruz CA) and visualized using VECTASTAIN ABC and DAB peroxidase substrate kits (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions. Sections were counterstained with Mayer's Hematoxylin Solution (Sigma-Aldrich, St. Louis, MO) and mounted using VectaMount Mounting Medium (Vector Laboratories, Burlingame, CA).
Statistical analysis
Data are reported using GraphPad Prism (v 4.03, San Diego, CA) and expressed as the mean ± SE of independent observations as indicated in the Figure legends. One-way analysis of variance (1way ANOVA) with multiple comparison post test (Bonferroni) was used to compare the means between injury groups. A p-value <0.05 was considered to be significant.
Results
Lung dysfunction after pulmonary contusion is dependent upon the TLR4 pathway
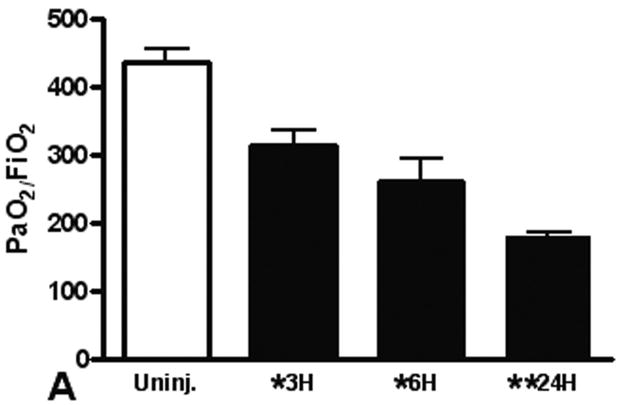
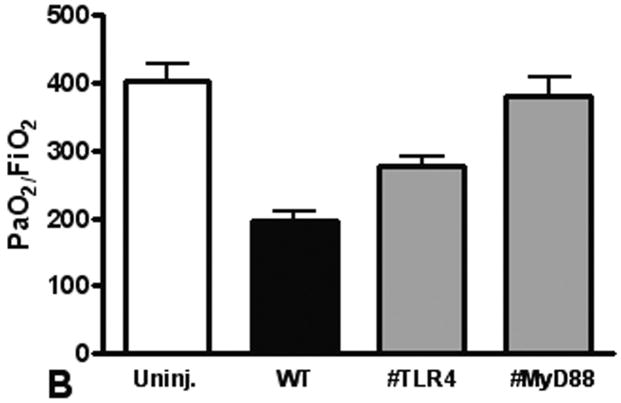
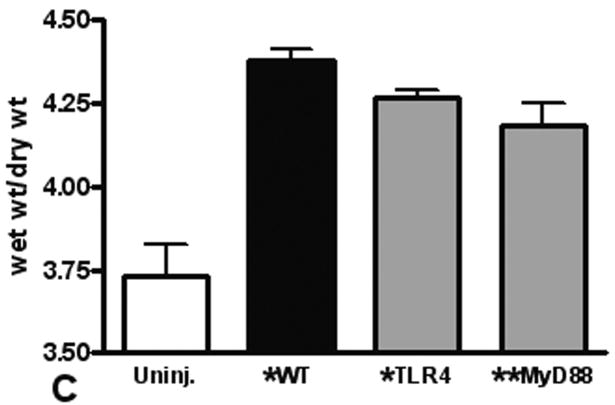
We use the CCI to induce a pulmonary contusion in our mouse model of blunt chest injury. Blood gas measurements showed that hypoxia induced by contusion was significant at 3 hours after injury and continued to increase for at least 24 hours (Figure 1A). We found significantly less hypoxia in TLR4 deficient animals suggesting that TLR4 participates in the response to acute lung injury. Consistent with this observation, we further found that MyD88 deficiency also reduced hypoxia in injured animals (compare TLR4 and MyD88 with WT, Fig. 1B). As a further indicator of acute lung injury, pulmonary edema was significantly elevated with blunt chest injury, but significantly less edema was found in MyD88 deficient mice (Figure 1C). These results showed that acute lung injury activated TLR4 and intracellular signaling through MyD88.
Figure 1. Lung dysfunction after pulmonary contusion is dependent on the TLR4 pathway.
Arterial blood from uninjured (open) and injured animals (closed) was isolated and blood gas analysis performed as described in the methods. (A) PaO2:FiO2 ratios were calculated and are shown for WT animals at 3, 6 and 24 hours after injury (n=5/time). There is a significant decrease in PaO2:FiO2 in WT animals after injury (*p≤0.01), with the most significant decrease at 24H (**p<0.01 relative to other injury times). (B) Blood gas analysis on injured TLR4 and MyD88 deficient animals (gray) at 24H shows significantly increased PaO2:FiO2 ratios when compared to WT injured animals (#p≤0.05, n=7/genotype). (C) WT (black) and TLR4 and MyD88 deficient (gray) injured lungs were isolated at 24H after injury and weighed before (wet wt.) and after drying (dry wt.) as described in the methods (n=5/genotype). Pulmonary edema (wet wt/dry wt) is significant (*p≤.05) in all animals and is significantly decreased in MyD88 deficient mice (**p<.05 compared to WT).
Lung pathology after pulmonary contusion is dependent on the TLR4 pathway
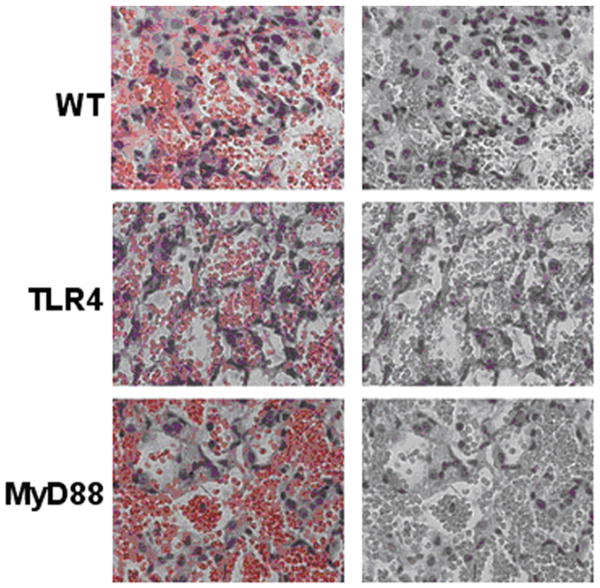
Acute lung injury is often correlated with the presence of neutrophils in the BAL of patients with pulmonary contusion. In our animal model, we evaluated pulmonary histology and BAL specimens for the presence of a neutrophilic infiltrate after injury. Histologic examination of injured lungs from WT mice showed evidence of intra-alveolar hemorrhage, alveolar septal edema and neutrophilic infiltrate as early as 3 hours, with maximum pathologies observed at 24 hours after injury (Figure 2). Injured lungs from TLR4and MyD88 deficient mice also showed evidence of hemorrhage, edema and neutrophils. Pathologic evaluation (blinded) of lung injury in knockout animals reported similar intra-alveolar hemorrhage to WT mice, but reduced alveolar septal edema and neutrophil infiltration.
Figure 2. Lung pathology after pulmonary contusion is dependent on the TLR4 pathway.
Injured lungs were formalin fixed, stained and analyzed by an experienced pathologist. Lung sections of the contusion are shown (100× magnification) at 24 hours after injury. Hemorrhage is evident in all injuries (left panels) and masked by red filter (right panels) to highlight neutrophil staining. Septal edema and neutrophil infiltration is reduced in TLR4 and MyD88 deficient mice when compared to WT. Specimens shown are representative of lung injuries in at least 3 animals/genotype.
Neutrophil recruitment to the lung after pulmonary contusion is dependent on the TLR4 pathway
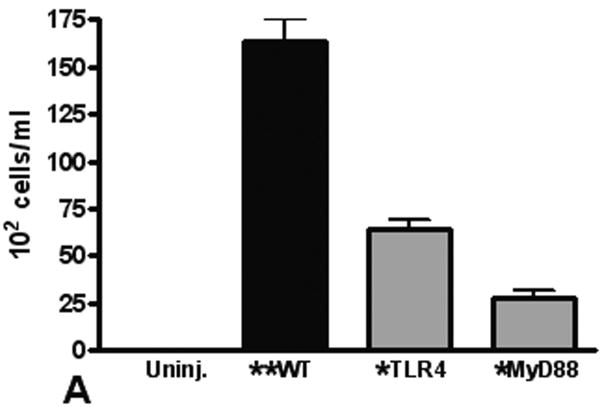
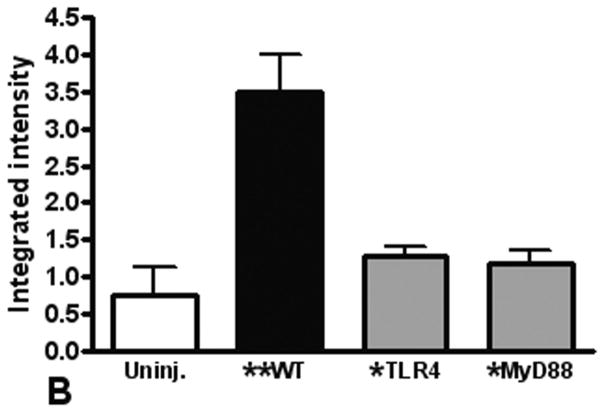
To quantitate neutrophil recruitment after injury, we used BAL to measure alveolar neutrophil infiltration. As found in our previous studies (4;20), we observed a significant increase in neutrophils in the BAL from injured mice (Figure 3A). The number of BAL neutrophils was significantly decreased in TLR4 and MyD88 deficient mice, supporting that the local inflammatory response is dependent on TLR activation. To further assess the role of neutrophils in our model of pulmonary contusion, we used immunoblot to measure neutrophil elastase levels in injured tissue. As shown in Figure 3B, we found low levels elastase in tissue homogenates from uninjured lungs. Consistent with increased neutrophil recruitment and activation by pulmonary contusion, we found increased levels of elastase in injured lung tissue. Elastase levels were decreased in TLR4 and MyD88 deficient (KO) mice. Immunohistochemical staining of lung tissue for neutrophil elastase (Figure 3C) was consistent with these results. Taken together, these results showed that blunt chest injury and pulmonary contusion significantly increases neutrophil infiltration and activation. The recruitment of neutrophils in this model of acute lung injury is dependent on the TLR4 pathway and intracellular signaling through MyD88.
Figure 3. Neutrophil recruitment to the lung after pulmonary contusion is dependent on the TLR4 pathway.
(A) The BAL from WT (closed) and TLR4 and MyD88 deficient (gray, KO) injured lungs were analyzed for neutrophil infiltration. Compared to uninjured (n=5), a significant increase in PMN in the BAL from WT mice is observed (**p≤.001) that is significantly decreased in KO mice (*p≤.001, n=6/genotype). (B) Lung homogenates from uninjured (open), WT (closed) and TLR4 and MyD88 deficient (gray) injured lungs were analyzed by immunoblot for neutrophil elastase (n=4). A significant increase in elastase in WT lung extracts is observed (**p≤.001) that is significantly decreased in KO mice (*p≤.01). (C) Immunostaining for neutrophil elastase in lungs from WT, and TLR4 or MyD88 deficient animals (100× magnification). Specimens are representative of immunohistochemistry for least 3 animals/genotype. Data shown are obtained at 24 hours after injury.
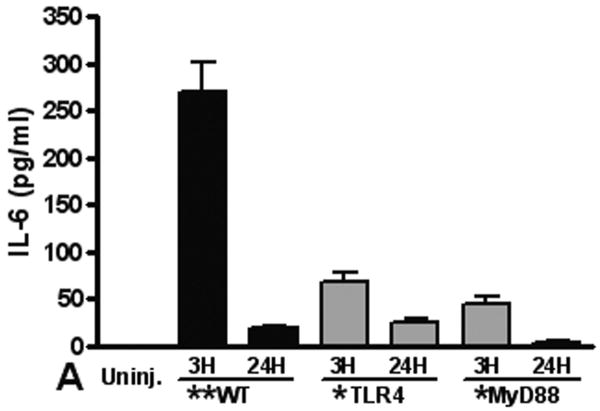
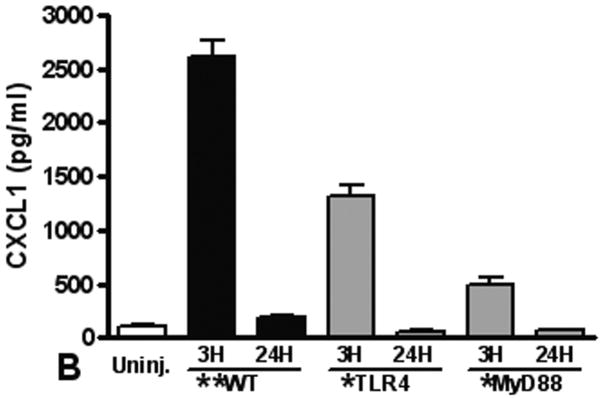
The TLR4 pathway is required for increased pulmonary and systemic IL-6 and CXCL-1 levels after pulmonary contusion
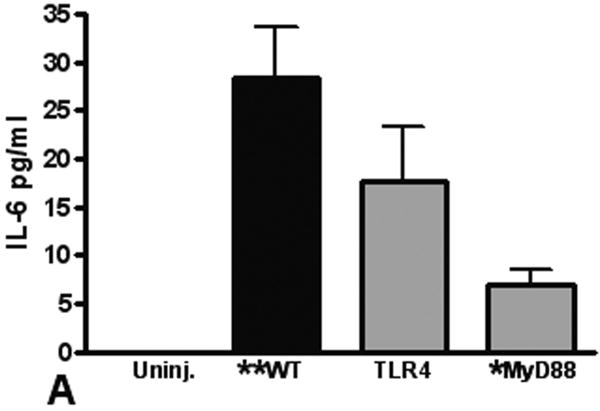
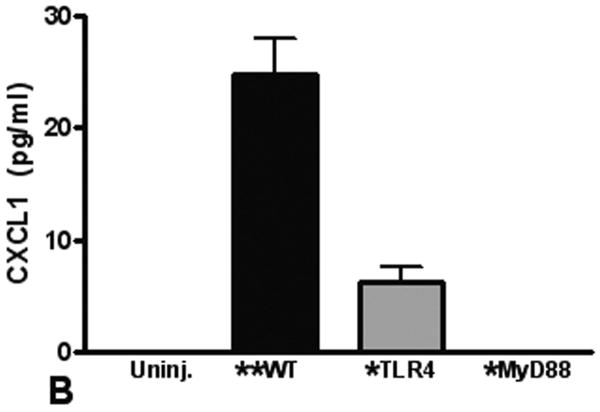
Elaboration of cytokines and chemokines are characteristic of inflammatory responses observed in patients with pulmonary contusion and in experimental models of acute lung injury. We measured IL-6, CXCL1, CXCL2/3, and MIP-1α levels in the BAL from mice at various times after injury. Overall, we found the only significant and measurable increases in IL-6 and CXCL1 in the BAL at 3H after injury (Figure 4). We observed significant increases in these mediators in the BAL from WT mice when compared to knockout mice, supporting that local inflammatory responses are dependent on the TLR4 pathway and MyD88 intracellular signaling. Finally, we measured levels of inflammatory mediators in the blood after pulmonary contusion. In the serum of injured animals we found a rapid, robust and transient increase in both IL-6 and CXCL-1 (Figure 5). These levels were significantly decreased in TLR4 and MyD88 deficient mice. CXCL2/3 and MIP-1α levels were not elevated in the BAL or the serum (data not shown) suggesting that these chemokines are not involved in the inflammatory response to lung contusion. These results show that systemic mediators of inflammation are increased in blunt chest injury with pulmonary contusion. Taken together our results show that local and systemic responses to injury are dependent, at least in part, to TLR4 activation and are mediated by the intracellular adapter, MyD88.
Figure 4. The TLR4 pathway is required for pulmonary inflammatory response to contusion.
IL-6 and CXCL-1 in the BAL from uninjured (open) and injured (filled) animals (n=5 for each genotype) were measured by ELISA as described in the methods. Levels in all samples were low and results are reported if the difference between uninjured and injured was statistically significant (**p≤0.001). (A) IL-6 levels in the BAL from injured MyD88 deficient animals (gray) at 3H shows a significant decrease when compared to WT injured animals (*p≤0.01). (B) CXCL-1 levels in the BAL from injured TLR4 and MyD88 deficient animals (gray) at 3H shows a significant decrease when compared to WT injured animals (*p≤0.001). Uninjured (IL-6 and CXCL-1) and CXCL-1 in MyD88 deficient animals' BAL was below sensitivity (2pg/ml) of the assay.
Figure 5. The TLR4 pathway is required for systemic innate immune responses to pulmonary contusion.
Innate immune proteins in the serum of uninjured (open) and after injury (closed) were measured by ELISA as described in the methods. A rapid and significant (**p≤.001) increase in IL-6 (A) and CXCL-1 (B) levels in the serum from injured WT mice at 3H is observed. IL-6 and CXCL-1 levels in the serum from knockout animals at 3H are significantly less (*p≤.001). (A) 3H n=9/genotype; 24H n=5/genotype; (B) 3H, n=8/genotype; 24H n=6/genotype.
Discussion
Mounting evidence supports a role for TLR-mediated signaling in the initial inflammatory response to noninfectious tissue injury with numerous potential endogenous TLR ligands being identified (14-19). A paradigm is evolving in which these endogenous ligands, when liberated from injured or necrotic tissue, act as “danger signals” warning the host of tissue injury and initiating the ensuing inflammatory response (13). We have used a mouse model of blunt chest trauma to study the initial innate inflammatory response as might be observed in patients with pulmonary contusion. We found that our model approximates the human injury using pathophysiological and biochemical markers. Furthermore, our previous studies showed that the innate inflammatory response to pulmonary contusion depends on TLR2 induced signaling (20). We found that TLR2 deficient animals have a less severe lung injury. The reduction in neutrophil chemotaxis to the contused lung correlated with decreased in CXCL1 expression and appeared to depend, at least in part, on TLR2 activation.
Having previously identified a role for TLR2 dependent signaling, in this report we have extended our investigations into the mechanisms regulating the response to traumatic lung injury and pulmonary contusion. As the TLR4 pathway shares many components with TLR2 signaling, we hypothesized that TLR4 may also participate in this injury response. We tested for TLR4 dependent responses in our injury model and found a clinically less severe lung injury, reduced pulmonary neutrophilia, and a diminished innate response in TLR4 deficient mice. In a manner similar to TLR2, TLR4 activation by pulmonary contusion appears to direct neutrophil migration to the lung through the expression of the chemokine, CXCL1 and independent of CXCL2/3. This is consistent with TLR4 mediated signaling that has been implicated in several other models of noninfectious tissue injury and systemic inflammation (17;18;24-29). Specifically, in bleomycin-induced and hyperoxic models of lung injury, TLR4 deficiency was associated with reduced pulmonary neutrophilia, diminished chemokine expression and increased mortality. However, our study supports a model where TLR4 deficiency is protective in a primary acute lung injury model as demonstrated by better ABG seen following lung contusion with no differences seen in mortality over the study period. Others have reported findings similar to ours in models of ischemia/reperfusion and sterile tissue injury where the absence of TLR4 is associated with a reduced innate inflammatory response and improved organ function. One explanation for the divergent findings seen in noninfectious lung models involves differing mechanisms of injury with a greater dependence upon neutrophil mediated tissue injury after contusion.
Our results support the concept that neutrophil recruitment to the lung after blunt chest injury is dependent on CXCL1 and independent of CXCL2/3. There are 4 major chemokine families, and in general, neutrophil chemotaxis is highly dependent upon CXC chemokine expression (30). CXCL1 and CXCL2/3 are the murine equivalents of IL-8, the principle chemokine involved in neutrophil chemotaxis after traumatic lung injury in humans (31). Alveolar macrophages and to a lesser extent, alveolar epithelial cells have been demonstrated to produce CXC chemokines after lung injury (32). Both CXCL1 and CXCL2/3 have been demonstrated to be elevated in other models of infectious and noninfectious lung injury, and divergent roles for both have been described (14;22;33-35). Our results showed that TLR4 and MyD88 induced neutrophil recruitment to the lung after contusion is dependent on CXCL1, raising the possibility that differing pharmacokinetics, ligand specificity, cellular involvement, and/or disruption of normal cell-cell interactions occur after pulmonary contusion when compared to other lung injury models. The involvement of other chemokines such as LTB4 or C5a cannot be excluded based upon our results.
Activation of TLR4 initiates intracellular signaling by recruitment of one or more adaptor proteins. MyD88 was the first adapter protein identified as involved in TLR4 signal transduction and NFκB activation (9;10). TLR4 utilizes both MyD88 dependent and MyD88 independent signaling pathways that result in activation of NFκB and subsequent inflammatory cytokine expression. MyD88 also participates in TLR2 signaling. In our injury model, MyD88 deficient animals showed improved oxygenation as well as reduced pulmonary neutrophil influx and innate response when compared to animals deficient in TLR4. These findings support that TLR4 utilizes the MyD88-dependent signaling pathway when activated after lung contusion; however, the MyD88 independent pathway utilizing the adapter protein, TRIF, cannot be completely excluded. Our study also supports that the response to lung contusion is not entirely dependent on TLR4 signaling, but other receptors are likely involved in mediating this response. MyD88 is a component of the intracellular signaling pathways for IL-1, IL-18, and all known TLRs with the exception of TLR3. We have previously shown that the innate immune response to pulmonary contusion is also TLR2 dependent and the more protective effect of MyD88 deficiency may reflect the sharing of this intracellular mediator by TLR2 and TLR4.
We are uncertain how TLR signaling is initiated in the lung after contusion and what, if any, coreceptors are utilized. Thus far, HMGB-1 and hyaluronan are potential TLR4 activators in models of soft tissue injury; however, a large number of endogenous TLR4 ligands exist, including surfactant, heat shock, heme, and other matrix proteins, and HMGB-1 (14-19). Many of these mediators are likely present in the lung after contusion, and some of these ligands have specificity for more than one TLR, likely with differing affinities. Thus, it is possible that several different ligands act in concert through different TLRs in order to generate a response. Ligand gradients and localization may also contribute to injury responses. Participation of coreceptors such as CD14 in the TLR4 response to pulmonary contusion also remains a possibility. For example, surfactant proteins are endogenous TLR4 ligands that require CD14 in order to activate TLR signaling (36;37). In contrast, other TLR4 ligands such as HMGB-1and hyaluronan are CD14 independent after sterile tissue injury (17;18). Low molecular weight hyaluronan fragments are thought promote formation of a TLR4 complex with MD-2 and CD44 (17) for signal transduction.
Cell type specificity of the TLR response to noninfectious injury is also not known. Alveolar macrophages, Type II alveolar epithelial cells, pulmonary endothelial cells, and tracheobronchial cells participate in the lung's innate response to both infectious and noninfectious lung injury (14;32;34;38;39). Germane to this report, Mollen (13) used chimeric mice in a model of hemorrhagic shock and bilateral femur fracture to address the question of cell type specific responses. They found that both bone marrow-derived and parenchymal cells are required in order to generate the ensuing TLR-dependent inflammatory response, suggesting that multiple cell types are involved in the generation of the innate response to noninfectious lung injury. Similar findings were reported by Wu (25) in a model of renal ischemia/reperfusion; however, it was suggested that renal parenchymal cells rather than immune cells, initiated the inflammatory response. Thus, as there are likely multiple endogenous ligands and TLRs involved in the response to pulmonary contusion, there appear to be multiple cell types acting in concert that are responsible for the inflammatory response to noninfectious lung injury.
Acknowledgments
This work was supported, in part, by the WFUSM Venture Fund, the American College of Surgeons C. James Carrico Faculty Research Fellowship, ALA RG-52711-N, and GM083154 (JJH), AI057770 (EMH), RR023570 (CEM) and AI065791 (CEM, BKY).
Reference List
- 1.Cohn SM. Pulmonary contusion: review of the clinical entity. J Trauma. 1997;42:973–979. doi: 10.1097/00005373-199705000-00033. [DOI] [PubMed] [Google Scholar]
- 2.Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. A 12-year prospective study of postinjury multiple organ failure: has anything changed? Arch Surg. 2005;140:432–438. doi: 10.1001/archsurg.140.5.432. [DOI] [PubMed] [Google Scholar]
- 3.Miller PR, Croce MA, Bee TK, Qaisi WG, Smith CP, Collins GL, Fabian TC. ARDS after pulmonary contusion: accurate measurement of contusion volume identifies high-risk patients. J Trauma. 2001;51:223–228. doi: 10.1097/00005373-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Hoth JJ, Stitzel JD, Gayzik FS, Brownlee NA, Miller PR, Yoza BK, McCall CE, Meredith JW, Payne RM. The pathogenesis of pulmonary contusion: an open chest model in the rat. J Trauma. 2006;61:32–44. doi: 10.1097/01.ta.0000224141.69216.aa. [DOI] [PubMed] [Google Scholar]
- 5.Keel M, Ecknauer E, Stocker R, Ungethum U, Steckholzer U, Kenney J, Gallati H, Trentz O, Ertel W. Different pattern of local and systemic release of proinflammatory and anti-inflammatory mediators in severely injured patients with chest trauma. J Trauma. 1996;40:907–912. doi: 10.1097/00005373-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Raghavendran K, Davidson BA, Woytash JA, Helinski JD, Marschke CJ, Manderscheid PA, Notter RH, Knight PR. The evolution of isolated bilateral lung contusion from blunt chest trauma in rats: cellular and cytokine responses. Shock. 2005;24:132–138. doi: 10.1097/01.shk.0000169725.80068.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muehlstedt SG, Richardson CJ, Lyte M, Rodriguez JL. Systemic and pulmonary effector cell function after injury. Crit Care Med. 2002;30:1322–1326. doi: 10.1097/00003246-200206000-00029. [DOI] [PubMed] [Google Scholar]
- 8.Partrick DA, Moore EE, Moore FA, Biffl WL, Barnett CC., Jr Release of anti-inflammatory mediators after major torso trauma correlates with the development of postinjury multiple organ failure. Am J Surg. 1999;178:564–569. doi: 10.1016/s0002-9610(99)00240-8. [DOI] [PubMed] [Google Scholar]
- 9.Kaisho T, Akira S. Critical roles of Toll-like receptors in host defense. Crit Rev Immunol. 2000;20:393–405. [PubMed] [Google Scholar]
- 10.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–987. doi: 10.1016/j.jaci.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Schilling D, Thomas K, Nixdorff K, Vogel SN, Fenton MJ. Toll-like receptor 4 and Toll-IL-1 receptor domain-containing adapter protein (TIRAP)/myeloid differentiation protein 88 adapter-like (Mal) contribute to maximal IL-6 expression in macrophages. J Immunol. 2002;169:5874–5880. doi: 10.4049/jimmunol.169.10.5874. [DOI] [PubMed] [Google Scholar]
- 13.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 14.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 15.Wagner JG, Harkema JR, Roth RA. Pulmonary leukostasis and the inhibition of airway neutrophil recruitment are early events in the endotoxemic rat. Shock. 2002;17:151–158. doi: 10.1097/00024382-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 17.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 18.Levy RM, Prince JM, Yang R, Mollen KP, Liao H, Watson GA, Fink MP, Vodovotz Y, Billiar TR. Systemic inflammation and remote organ damage following bilateral femur fracture requires Toll-like receptor 4. Am J Physiol Regul Integr Comp Physiol. 2006;291:R970–R976. doi: 10.1152/ajpregu.00793.2005. [DOI] [PubMed] [Google Scholar]
- 19.Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 20.Hoth JJ, Hudson WP, Brownlee NA, Yoza BK, Hiltbold EM, Meredith JW, McCall CE. Toll-like receptor 2 participates in the response to lung injury in a murine model of pulmonary contusion. Shock. 2007;28:447–452. doi: 10.1097/shk.0b013e318048801a. [DOI] [PubMed] [Google Scholar]
- 21.Qureshi ST, Zhang X, Aberg E, Bousette N, Giaid A, Shan P, Medzhitov RM, Lee PJ. Inducible activation of TLR4 confers resistance to hyperoxia-induced pulmonary apoptosis. J Immunol. 2006;176:4950–4958. doi: 10.4049/jimmunol.176.8.4950. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Shan P, Qureshi S, Homer R, Medzhitov R, Noble PW, Lee PJ. Cutting edge: TLR4 deficiency confers susceptibility to lethal oxidant lung injury. J Immunol. 2005;175:4834–4838. doi: 10.4049/jimmunol.175.8.4834. [DOI] [PubMed] [Google Scholar]
- 23.Weiss YG, Maloyan A, Tazelaar J, Raj N, Deutschman CS. Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J Clin Invest. 2002;110:801–806. doi: 10.1172/JCI15888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koedel U, Merbt UM, Schmidt C, Angele B, Popp B, Wagner H, Pfister HW, Kirschning CJ. Acute brain injury triggers MyD88-dependent, TLR2/4-independent inflammatory responses. Am J Pathol. 2007;171:200–213. doi: 10.2353/ajpath.2007.060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y, Yang H, Tracey KJ, Billiar TR, Wilson MA. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol. 2007;178:6573–6580. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 27.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 28.Frink M, Hsieh YC, Thobe BM, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. TLR4 regulates Kupffer cell chemokine production, systemic inflammation and lung neutrophil infiltration following trauma-hemorrhage. Mol Immunol. 2007;44:2625–2630. doi: 10.1016/j.molimm.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Breslin JW, Wu MH, Guo M, Reynoso R, Yuan SY. Toll-like receptor 4 contributes to microvascular inflammation and barrier dysfunction in thermal injury. Shock. 2008;29:349–355. doi: 10.1097/shk.0b013e3181454975. [DOI] [PubMed] [Google Scholar]
- 30.Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 31.Bhatia RK, Pallister I, Dent C, Jones SA, Topley N. Enhanced neutrophil migratory activity following major blunt trauma. Injury. 2005;36:956–962. doi: 10.1016/j.injury.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Lentsch AB, Ward PA. Regulation of inflammatory vascular damage. J Pathol. 2000;190:343–348. doi: 10.1002/(SICI)1096-9896(200002)190:3<343::AID-PATH522>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 33.Lomas-Neira J, Chung CS, Grutkoski PS, Dunican A, Simms HH, Cioffi WG, Ayala A. Divergent roles of murine neutrophil chemokines in hemorrhage induced priming for acute lung injury. Cytokine. 2005;31:169–179. doi: 10.1016/j.cyto.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Lomas-Neira JL, Chung CS, Wesche DE, Perl M, Ayala A. In vivo gene silencing (with siRNA) of pulmonary expression of MIP-2 versus KC results in divergent effects on hemorrhage-induced, neutrophil-mediated septic acute lung injury. J Leukoc Biol. 2005;77:846–853. doi: 10.1189/jlb.1004617. [DOI] [PubMed] [Google Scholar]
- 35.Vanderbilt JN, Mager EM, Allen L, Sawa T, Wiener-Kronish J, Gonzalez R, Dobbs LG. CXC chemokines and their receptors are expressed in type II cells and upregulated following lung injury. Am J Respir Cell Mol Biol. 2003;29:661–668. doi: 10.1165/rcmb.2002-0227OC. [DOI] [PubMed] [Google Scholar]
- 36.Sano H, Chiba H, Iwaki D, Sohma H, Voelker DR, Kuroki Y. Surfactant proteins A and D bind CD14 by different mechanisms. J Biol Chem. 2000;275:22442–22451. doi: 10.1074/jbc.M001107200. [DOI] [PubMed] [Google Scholar]
- 37.Sano H, Sohma H, Muta T, Nomura S, Voelker DR, Kuroki Y. Pulmonary surfactant protein A modulates the cellular response to smooth and rough lipopolysaccharides by interaction with CD14. J Immunol. 1999;163:387–395. [PubMed] [Google Scholar]
- 38.Neff SB, Z'graggen BR, Neff TA, Jamnicki-Abegg M, Suter D, Schimmer RC, Booy C, Joch H, Pasch T, Ward PA, Beck-Schimmer B. Inflammatory response of tracheobronchial epithelial cells to endotoxin. Am J Physiol Lung Cell Mol Physiol. 2006;290:L86–L96. doi: 10.1152/ajplung.00391.2004. [DOI] [PubMed] [Google Scholar]
- 39.Perl M, Chung CS, Lomas-Neira J, Rachel TM, Biffl WL, Cioffi WG, Ayala A. Silencing of Fas, but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am J Pathol. 2005;167:1545–1559. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]