Abstract
The E2F family of transcription factors play an essential role in the regulation of cell cycle progression. In a screen for E2F-regulated genes we identified a novel E2F family member, E2F7. Like the recently identified E2F-like proteins of Arabidopsis, E2F7 has two DNA binding domains and binds to the E2F DNA binding consensus site independently of DP co-factors. Consistent with being an E2F target gene, we found that the expression of E2F7 is cell cycle regulated. Ectopic expression of E2F7 results in suppression of E2F target genes and accumulation of cells in G1. Furthermore, E2F7 associates with E2F-regulated promoters in vivo, and this association increases in S phase. Interestingly, however, E2F7 binds only a subset of E2F-dependent promoters in vivo, and in agreement with this, inhibition of E2F7 expression results in specific derepression of these promoters. Taken together, these data demonstrate that E2F7 is a unique repressor of a subset of E2F target genes whose products are required for cell cycle progression.
Keywords: cell cycle/E2F/microarrays/retinoblastoma/transcriptional repression
Introduction
The E2F family of transcription factors play a critical role in regulating the transcription of a number of genes required for cell proliferation. E2F function is tightly regulated by association with pRB, p107 and p130. When associated with pRB family members, the E2Fs act as transcriptional repressors, with growth suppressing activity. During the progression of cells from G1 into S phase, D-type cyclin associated kinases initiate the phosphorylation of the pRB family members, releasing them from the E2F transcription factors. This results in the activation of a series of genes involved in DNA replication and cell cycle progression (reviewed in Müller and Helin, 2000; Stevaux and Dyson, 2002; Trimarchi and Lees, 2002). In addition to controlling cell proliferation, E2F family members also play important roles in regulating the expression of genes involved in apoptosis, differentiation and development (Müller et al., 2001).
In mammals, six members of the E2F transcription factor family (E2F1–6) have been characterized so far. All six E2Fs contain two highly conserved domains that are involved in sequence-specific DNA binding and dimerization with DP proteins. Association of the E2Fs with one of the two known DP proteins is required for high-affinity, sequence-specific DNA binding, and, in the case of E2F1–5, association with members of the pRB family (Trimarchi and Lees, 2002).
The E2F family can be divided into three distinct subgroups on the basis of their structure, affinity for members of the pRB family, expression pattern and putative function. E2F1, E2F2 and E2F3 are potent transcriptional activators and are now referred to as the ‘activating’ E2Fs (Trimarchi and Lees, 2002). Their expression is cell growth regulated and at physiological levels they interact only with pRB. Consistent with an important role for these proteins in G1–S progression, their ectopic expression in quiescent immortalized cells drives cells to enter S phase (Johnson et al., 1993; Lukas et al., 1996; Lomazzi et al., 2002). Furthermore, triple knock-out mouse embryonic fibroblasts are defective for S phase entry and show a dramatic decrease in the expression of several E2F-regulated genes (Wu et al., 2001). In contrast, the role of E2F4 and E2F5 is to actively repress E2F-regulated genes through the recruitment of pocket proteins and their associated histone-modifying enzymes. E2F4 and E2F5 are expressed throughout the cell cycle and their protein products bind to all three pRB family members. Ectopic expression of these E2Fs is not sufficient for the induction of S phase in the absence of serum (Lukas et al., 1996). Finally, E2F6, the only member of the third group, lacks domains that are involved in transactivation and binding to the pRB family members. E2F6 appears to be a repressor of transcription, working independently of the pRB family members (Morkel et al., 1997; Cartwright et al., 1998; Gaubatz et al., 1998; Trimarchi et al., 1998).
Here we describe the identification of a new member of the E2F family, E2F7. E2F7 contains two separate domains exhibiting high similarity to the DNA binding domain of E2F, both of which are required for the binding to the E2F DNA consensus site. We show that E2F7 is able to repress transcription of E2F regulated promoters in vitro and that it binds to E2F-regulated promoters in vivo. Significantly, we show that E2F7 is required for the regulation of a subset of E2F-dependent promoters, and since the association of E2F7 with these promoters increases during S phase, this suggests that E2F7 has a role in controlling cell cycle progression by balancing the function of activating E2Fs.
Results
Identification of E2F7
In a screen for E2F1-regulated genes using human fibroblasts expressing an ER–E2F1 fusion protein, we identified an E2F-induced gene, the product of which showed high homology to members of the E2F transcription factor family (M.Ciro’ and K.Helin, unpublished results). In particular, the predicted protein showed high homology to the newly described E2F-like (E2L) proteins of Arabidopsis (Kosugi and Ohashi, 2002). The coding region of this novel E2F, which we have named E2F7, was cloned by RT–PCR. The expressed sequence tag (EST) corresponding to NCBI accession No. AI341146 present in the ER–E2F1 screen was used to search the human genomic sequence database. A unique human genome project working draft sequence (accession number NT019546) was found to match that of the EST, and the mRNA sequence predicted from the genomic sequence was found in the NCBI database (accession number XM084871). Amplification primers to clone the predicted cDNA were based on this mRNA sequence, which contains an in-frame STOP codon upstream of the first ATG, suggesting that the predicted cDNA contains the start codon of E2F7. Two products were obtained by RT–PCR, a shorter form containing a stop codon and a longer form truncated at the C-terminus. Inspection of the sequence of the genomic locus revealed the presence of 13 exons and the possibility of alternative splicing of exon 12, giving rise to one isoform corresponding to the shorter clone, lacking exon 12, and to a longer form including exon 12 and an alternative reading frame for exon 13. The shorter form was named E2F7a and encodes a protein of 728 amino acids; the longer form was named E2F7b and encodes a protein of 911 amino acids (Figure 1A). The two proteins differ only in the C-terminal tails from amino acids 713. A new primer was then designed to clone the full-length cDNA of the longer isoform. Both isoforms of E2F7 are expressed in all cell lines analysed so far (Figure 1D; data not shown), with E2F7b being the most abundant form.
Fig. 1. E2F7 contains two domains similar to the DNA binding domain of E2F proteins. (A) Intron–exon structure of E2F7a and E2F7b. (B) Domain organization of E2F7a and E2F7b compared with the other members of the E2F family. Number of amino acids is indicated on the right. Shaded boxes indicate homologous regions. cA, Cyclin A binding site; DB, DNA binding domain; DIM, dimerization domain; TA, transactivation domain; PB, pocket protein (pRB family) binding domain. (C) Amino acid sequence alignment of the DB1 and DB2 domains of E2F7 with the DNA binding domains of the other E2Fs and DPs. Grey highlighted characters indicate amino acids conserved among these proteins. (D) Expression of E2F7a and E2F7b. Western blot analysis of cell lysates derived from U2OS transfected with either pCDNA E2F7a (a), E2F7b (b) or pCDNA vector alone (–). Endogenous and ectopic E2F7 protein expression was detected by immunoblotting with an antibody to E2F7. Cell lysate (40 µg) was loaded to detect the endogenous proteins (–), whereas only 10 µg was loaded of the cell lysates prepared from cells overexpressing E2F7a and E2F7b.
The two forms of E2F7 both contain two domains with high homology to the DNA binding domains of the E2F family of proteins (DB1 and DB2; Figure 1B). No other region of E2F7 exhibited significant similarity to conserved E2F domains, including the dimerization domain, marked box or pRB binding domain. Alignment of the two putative E2F7 DNA binding domains with those of E2Fs and DPs showed that the highly conserved residues responsible for DNA contacts are present in E2F7 (Zheng et al., 1999; Figure 1C).
E2F7 is a direct target of E2F
To confirm that E2F7 is an E2F-regulated gene, we performed real time quantitative PCR (qPCR) analysis of mRNA isolated from WI38 cells expressing ER–E2F1 (Vigo et al., 1999; Moroni et al., 2001). In agreement with DNA oligonucleotide microarray results, activation of E2F1 by addition of 4-hydroxytamoxifen (OHT) led to a 6-fold increase in E2F7 mRNA levels (Figure 2A). Significantly, the increase in E2F7 mRNA levels is independent of de novo protein synthesis, since up-regulation was observed in the presence of cycloheximide. Furthermore, a DNA-binding mutant of E2F1 (E132) was incapable of inducing E2F7 expression (data not shown). These data indicate that the E2F7 promoter is directly regulated by E2F.
Fig. 2. E2F7 is an E2F target gene. (A) Real-time qPCR analysis of mRNA isolated from WI38 cells expressing ER–E2F1. Cells were incubated with OHT, cycloheximide (CHX) or both for 4 h. (B) E2F1 and E2F4 associate with the E2F7 promoter in vivo. Asynchronously growing U2OS cells were treated with formaldehyde and enrichment of the E2F7 promoter sequences was tested by ChIP using the indicated antibodies. β-actin and E2F1 promoters were used respectively as negative and positive controls. The percentage of the bound promoter/total promoter present in the cells is indicated. (C) WI38 cells were serum-starved and re-stimulated to enter the cell cycle by serum addition. qPCR analysis was performed at subsequent time points to determine the E2F7 expression profile. Propidium iodide (PI) FACS analysis is shown at the top of the panel. The known E2F target gene, CCNE1, is shown as control. (D) The expression of E2F7a and E2F7b is cell growth regulated. Western blot analysis of cell lysates prepared from WI38 cells starved and released as described in panel C. Antibodies specific for E2F7 and Cyclin A2 were used as indicated. Vinculin levels were analysed to check equal loading of the gel. PI/FACS analysis is presented at the top of the panel. (E) E2F7b is cell cycle regulated. U2OS cells were synchronized in metaphase and released into the cell cycle by plating in nocodazole-free medium. Cells were harvested at the indicated times after release and analysed as in (D).
Inspection of the E2F7 promoter sequence revealed the presence of four potential E2F binding sites. To determine whether E2F family members are present on the E2F7 promoter in vivo chromatin immunoprecipitation (ChIP) assays using anti-E2F1, anti-E2F4 and a non-related antibody were performed. In the osteosarcoma cell line U2OS, E2F1 and, to a lesser extent, E2F4, occupied the E2F7 promoter (Figure 2B). The β-actin and E2F1 promoters were used as negative and positive controls, respectively. Taken together, these data provide strong evidence that E2F family members in vivo transcriptionally regulate E2F7.
E2F7 expression is cell growth regulated
To investigate whether the expression of E2F7 is cell growth regulated, as most E2F target genes are, we rendered WI38 cells quiescent and subsequently re-stimulated them to enter the cell cycle. Total RNA was extracted, cDNA prepared at various times after growth stimulation and the expression of E2F7 was determined by qPCR. The E2F7 mRNA transcript (representing both isoforms) was found to be strongly cell growth regulated in human diploid fibroblasts, accumulating as cells enter the cell cycle (Figure 2C; Supplementary figure 1, available at The EMBO Journal Online). Consistent with the transcriptional regulation of E2F7 expression, we also found that the expression of both E2F7a and E2F7b proteins is significantly increased as cells enter the S phase of the cell cycle (Figure 2D).
To determine whether the expression of E2F7 is cell cycle regulated, U2OS cells were synchronized in mitosis by incubation with thymidine followed by nocodazole. Cells were released into the cell cycle by plating in nocodazole-free medium and samples were prepared for western blot and FACS analysis at different times after release. As shown in Figure 2E, E2F7a is present in all phases of the cell cycle, while E2F7b levels are undetectable in mitosis and early G1 but start to increase as cells enter S phase. These data show that the expression of E2F7b is cell cycle regulated, whereas E2F7a is not. To investigate the mechanism for the cell cycle regulated expression of E2F7b, we performed two sets of experiments. First, we treated U2OS cells, synchronized and released from mitosis, with the proteasome inhibitor MG132 for various lengths of time. This treatment did not result in any increase of E2F7b, nor in the appearance of E2F7b early in the cell cycle (data not shown), suggesting that the cell cycle-regulated expression of E2F7b is not due to its specific degradation in early G1. Next, we determined the abundance of E2F7a and E2F7b total mRNA during the cell cycle by qPCR. These experiments showed that E2F7b mRNA is cell cycle regulated, whereas only a minor regulation of total mRNA was observed (Supplementary figure 1B). Since we have only found one promoter for the two mRNAs, these data suggest that E2F7b mRNA is specifically targeted for degradation in early G1.
E2F7 binding to the E2F DNA binding consensus site requires both its DNA binding domains
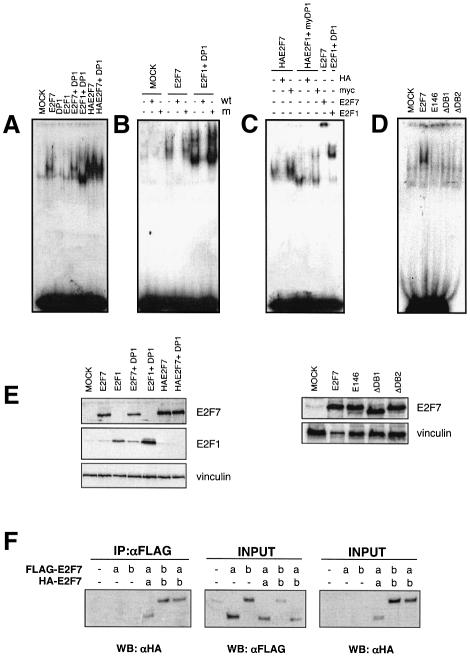
The presence of two domains similar to the E2F DNA binding domain suggested that E2F7 is capable of binding to E2F binding sites. Therefore, we assayed its DNA binding activity by electrophoresis mobility shift assay (EMSA). Cell extracts from U2OS cells transfected with E2F7, E2F1 and DP1 expression plasmids, individually or in combination, were tested for DNA binding activity. Ectopic expression of E2F7 resulted in increased DNA binding activity relative to mock-transfected control extracts (Figure 3A). Use of a more highly expressed HA-tagged E2F7 construct gave even greater DNA binding activity (Figure 3A and E). As expected, expression of either E2F1 or DP1 alone did not result in a detectable increase in E2F DNA binding activity, whereas co-expression of the two proteins led to a significant increase (Figure 3A), known to be dependent on the formation of E2F1–DP1 heterodimers (Bandara et al., 1993; Helin et al., 1993; Krek et al., 1993). Significantly, co-expression of DP1 with E2F7 did not increase the DNA binding activity of E2F7, suggesting that E2F7 does not require DP1 for DNA binding and, consistent with this, we have been unable to detect an interaction between DP1 and E2F7 (data not shown). The E2F7 DNA binding activity is specific to E2F binding sites, since a wild-type but not a mutant E2F oligonucleotide could compete for the binding to the radiolabelled probe (Figure 3B). Antibodies to either E2F7 or the HA tag were capable of abolishing or super-shifting the E2F7–DNA complex, whereas an unrelated antibody (anti-myc) did not alter the migration of the complex, demonstrating the specificity of the antibodies. As a control both anti-HA and anti-myc antibodies were capable of super-shifting the HAE2F1–mycDP1 DNA binding complex. These data show that E2F7 can specifically bind to the E2F DNA-binding consensus site. Furthermore, in contrast to the other E2Fs, E2F7 is able to bind DNA independently of DPs.
Fig. 3. Specific binding of E2F7 to E2F-binding sites independently of DP. (A) E2F7 binds E2F sites. U2OS cell extracts prepared from cells transfected with the indicated expression plasmids were incubated with E2F oligonucleotide and assayed for binding. (B) E2F7 binding requires the E2F consensus site. A 100-fold excess of either wild-type (w.t.) or mutant (m) cold competitor probe was added as indicated. (C) Antibody super-shifts were performed using anti-E2F1 (KH20), anti-HA (Babco), anti-Myc (9E10) and anti-E2F7 as indicated. (D) DNA binding requires intact DB1 and DB2 domains: mutant versions of E2F7 protein were assayed for binding. (E) Expression levels of the transfected constructs in U2OS cells were determined by western blotting using anti-E2F7 and anti-E2F1 (KH20) antibodies. Vinculin was used as a loading control. (F) E2F7 can homodimerize. U2OS cells were transfected with either Flag-tagged E2F7a or E2F7b alone or in combination with HA-tagged E2F7a or E2F7b. Anti-Flag antibody was used to immunoprecipitate the Flag-tagged E2Fs. Bound E2F7a and E2F7b proteins were detected by western blot using an anti-HA specific antibody. The levels of E2F7a and E2F7b expressed in transfected cells was measured by western blotting of aliquots taken before the immunoprecipitation (INPUT) using anti-Flag or anti-HA antibodies.
To determine whether both E2F7 DNA binding domains are required for the binding of DNA, three mutant proteins were generated. When either DB1 (E2F7ΔDB1) or DB2 (E2F7ΔDB2) domains were deleted, a complete loss of binding activity was observed (Figure 3D), suggesting that E2F7 requires both DNA binding domains to associate with E2F sites. Furthermore, a mutant containing a double amino acid substitution (E146) in DB1 was unable to bind the DNA (Figure 3D). These two amino acids (L146, G147) are part of a highly conserved motif present in all E2Fs (Figure 1C), which has been shown previously to be required for E2F1 binding to DNA (Cress et al., 1993). As shown in Figure 3E, the inability of the mutants to bind DNA was not due their different stability, since they were all expressed at levels similar to wild-type E2F7. The slower migration of E2F7–DNA complex as compared with E2F1–DP1 heterodimers bound to DNA suggested that E2F7 binds to DNA as a homodimer. To test whether E2F7 is able to homodimerize, we co-transfected various Flag- and HA-tagged forms of E2F7, and performed immunoprecipitation–western blotting experiments. As shown in Figure 3F, E2F7 forms homodimers in vivo, and taken together with the migration of the E2F7–DNA complex these data suggest that E2F7 binds to DNA as a homodimer. In summary, our data demonstrate that E2F7 binds DNA at E2F7 sites through the formation of intramolecular homodimers, involving its two DNA binding domains, but not DP binding.
E2F7 can repress E2F-regulated promoters
Given the ability of E2F7 to bind E2F consensus sites, we wished to determine whether E2F7 had transcriptional regulatory function. Therefore, U2OS cells were transfected with increasing amounts of E2F7 together with a reporter plasmid containing six E2F binding sites. A β-galactosidase expression vector was co-transfected to allow normalization for both cell number and transfection efficiency. Transfection of E2F7a expression plasmid resulted in a significant decrease in reporter gene expression compared with empty vector-transfected cells, and this decrease was proportional to the amount of E2F7a transfected (Figure 4A). The same effect was observed when an E2F7b expression plasmid was transfected (Figure 4B). A stronger repression by E2F7a and E2F7b was obtained using a reporter plasmid containing part of the human E2F1 promoter (Figure 4C). This repression was dependent on the presence of functional E2F DNA binding sites in the promoter, since a mutant version of the promoter, in which the two recognizable E2F DNA binding sites were mutated, was only partly repressed by E2F7 (Figure 4D). The DNA binding mutants of E2F7 (E2F7a–E146, E2F7b–E146 E2F7aΔDB1, E2F7aΔDB2) were not capable of repressing an E2F-dependent promoter, confirming that E2F7-mediated repression is dependent on the ability to bind DNA at E2F sites (Figure 4E). Consistent with a role in preventing transactivation of E2F-dependent promoters by the activating E2Fs, E2F7 counteracted E2F1 activation (Figure 4F). Taken together, these results demonstrate that E2F7 can act as a specific transcriptional repressor of E2F regulated promoters, possibly by competing with the activating E2Fs.
Fig. 4. E2F7 represses E2F responsive promoters. (A) Transfection assay with 6× E2F Luc reporter and increasing amounts (10, 30 and 100 ng) of E2F7a expression plasmid and (B) E2F7b expression plasmid. (C) E2F7a and E2F7b can repress transcription of the E2F1 promoter [pGL3 E2F1 (-242)].The assay was performed using 100 ng of E2F7a and E2F7b expression plasmids. (D) Repression is dependent on intact E2F sites. Transfection assay with mutant [pGL3E2F1(-242, -E2F)] E2F1constructs. (E) The DNA binding domains of E2F7 are required for repression. The effect of expressing wild-type or DNA binding mutant E2F7 proteins were evaluated on the 6× E2F luciferase promoter; 100 ng of the indicated plasmids were used for the transfections. (F) E2F7 competes with E2F1 in regulating E2F-responsive promoters. Increasing amounts of E2F1 expression plasmid (20–100 ng) were transfected in the presence or absence of constant amounts (100 ng) of E2F7 expression plasmid. All luciferase values were corrected for transfection efficiency by normalizing with β-galactosidase activity. Experiments were performed in duplicate and results were reproduced at least three times. Error bars indicate standard deviation of the mean.
E2F7 binds E2F responsive promoters in vivo
To test whether E2F7 is associated with E2F-dependent promoters in vivo, ChIP assays were performed. Extracts were prepared from asynchronously growing U2OS cells and immunoprecipitations were performed using antibodies against E2F7, E2F1 and a non-related antibody. Interestingly, our data showed that E2F7 could bind to the E2F1 and the CDC6 promoters, but not to CCNA2 (Cyclin A2) and CDC2 in vivo. In contrast, E2F1, used as positive control, binds to each of these promoters (Figure 5A). This suggests that E2F7 specifically binds to certain promoters. The specificity of the ChIP assays was verified by using a non-related antibody that did not enrich for E2F target genes, and by showing that the E2F antibodies did not enrich an unrelated target gene (β-actin) (Figure 5A).
Fig. 5. E2F7 binds to E2F responsive promoters in vivo. (A) ChIP analysis of asynchronous U2OS cells. ChIP was performed using antibodies specific for E2F1 and E2F7. Anti-Flag was used as negative control. The resulting DNA was amplified by qPCR with primers corresponding to the genes shown. β-actin promoter was used as a negative control. (B) U2OS cells were synchronized in mitosis by treating with thymidine followed by nocodazole. Cells were stimulated to re-enter the cell cycle by plating in nocodazole-free medium, harvested at the indicated time points and examined by FACS analysis. (C) ChIP analysis of U2OS cells synchronized as described in (B) was performed using the indicated antibodies as in (A).
To address whether promoter binding by E2F7 in vivo varied throughout the cell cycle, ChIP assays were performed on extracts prepared from U2OS cells at various phases of the cell cycle. As shown in Figure 5B, E2F7 primarily occupies E2F1 and CDC6 promoters in S phase (when these genes are not expressed), whereas no significant enrichment was observed on the CDC2 and CCNA2 promoters at any stage of the cell cycle. A similar experiment was performed using human diploid fibroblasts, which were made quiescent and subsequently induced to enter the cell cycle (Figure 6A). Again, E2F7 specifically associated with the E2F1 and CDC6 promoters as cells enter S phase, whereas no significant association was observed on the CCNA2 and CDC2 promoters (Figure 6B).
Fig. 6. Increased association of E2F7 with E2F responsive promoters as cells enter the S phase of the cell cycle. (A) WI38 diploid human fibroblast were rendered quiescent and subsequently induced to enter the cell cycle by the addition of serum-containing medium. Cell cycle profiles were determined by PI/FACS. (B) ChIP was performed using the indicated antibodies as described in Figure 5A.
Ectopic expression of E2F7 blocks S phase entry and cell growth
Several experiments were performed to determine whether E2F7 could affect cell proliferation. First, serum-starved Rat1 cells were microinjected with expression vectors for E2F7 or, as controls, the two CDK inhibitors p16 and p21. After injection, cells were released from quiescence by the addition of serum, and BrdU incorporation was measured 16 h later. As shown in Figure 7A and B, ectopic expression of E2F7 resulted in a significant reduction of cells in S phase. This is dependent on the integrity of the E2F7 DNA binding domains.
Fig. 7. E2F7 inhibits S phase entry and cell proliferation. (A) Ectopic E2F7 expression inhibits Rat1 cells from S phase entry. Serum-starved quiescent Rat1 cells were microinjected with expression plasmids for the indicated proteins. Immediately after microinjection, medium containing serum and BrdU was added, and the number of cells in S phase was determined 16 h later. (B) E2F7’s ability to inhibit S phase entry is dependent on DNA binding. Rat1 cells were microinjected with the indicated expression plasmids, and BrdU was determined as described in (A). p16 was used as a positive control. (C) Ectopic expression of E2F7 results in accumulation of cells in G1. GFP-positive U2OS cells transfected with pCMV vectors, as indicated, were analysed by PI/FACS. (D) Ectopic expression of E2F7 impairs growth. HeLa cells were transfected with indicated pCMV vectors. Cells were plated (50 000 cells/10-cm plate) and selected in neomycin for 14 days prior to crystal violet staining.
Secondly, we measured the effect of E2F7 overexpression on proliferating U2OS cells. Cells were transfected with E2F7, or as controls p16 or pCMV empty vector. As shown in Figure 7C, the overexpression of E2F7 resulted in a significant increase in the percentage of cells in G1, while the percentage of S phase cells was decreased.
Finally, the effects of ectopic expression were further analysed with an assay for colony-forming ability. As demonstrated in Figure 7D, overexpression of E2F7 significantly reduced the colony-forming ability of HeLa cells compared with pCMV-transfected cells. Taken together, these data are consistent with a model in which E2F7 can act as a repressor of a subset of E2F-dependent genes, expression of which is required for the entry into S phase, and that repression of these genes inhibits cell proliferation.
E2F7 regulates the expression of a subset of E2F-dependent genes
To address the physiological role of E2F in the regulation of E2F-dependent genes, the expression of E2F7 was abolished by siRNA. As shown in Figure 8A, U2OS cells transfected with siRNA to E2F7, but not luciferase (as a control), resulted in almost complete abrogation of E2F7 levels at 24 and 48 h after transfection of the siRNA. Significantly, the decreased E2F7 levels resulted in substantially increased levels of CCNE1, E2F1 and CDC6 (E2F7 target genes), whereas no change in expression was observed for CCNA2 or CDC2, which are not bound by E2F7 (Figure 8B). Taken together these data demonstrate that E2F7 is essential for maintaining the normal levels of a subset of E2F target genes in vivo.

Fig. 8. E2F7 represses a subset of E2F-dependent genes in vivo. (A) Specific depletion of E2F7a and E2F7b by siRNA in U2OS cells. Western blot analysis of U2OS cells transfected with siRNA oligos designed to interfere with E2F7 mRNA using antibodies specific for E2F7 and vinculin (loading control). An unrelated siRNA specific for luciferase (GL3) and mock-transfected cells were used as controls. Cell lysates were prepared 24 and 48 h after transfection with the siRNA oligos. (B) E2F7 depletion results in up-regulation of expression of E2F7-specific target genes. qPCR analysis of the expression of the indicated genes 48 h after transfection of U2OS cells with siRNA to E2F7. The expression level is normalized against GL3 transfected control. Determination was performed in triplicate and the standard deviation of the mean is indicated.
Discussion
Here we add a new level of complexity to the control of cell proliferation by E2F regulatory activities with the identification and characterization of a novel component of the E2F family, E2F7. E2F7 contains two copies of the highly conserved E2F DNA binding domain, which are required for the binding to the E2F DNA consensus site in vitro. E2F7 also binds to a subset of known E2F regulated promoters in vivo. Overexpression of E2F7 results in specific repression of promoters containing E2F DNA binding sites and conversely inhibition of E2F7 expression results in increased expression of target genes.
E2F7 as a transcriptional repressor
In sharp contrast to other members of the E2F transcription factor family, E2F7 cannot bind to the DP transcription factors, and it binds efficiently to the E2F DNA consensus site without DP. This binding requires both DNA binding domains of E2F7, which contain the specific residues of the E2F–DP heterodimer that make DNA base contacts (the RRXYD motif) (Zheng et al., 1999). Taken together with the prediction that the DNA binding domains of E2F7 form three α-helices and a β-sheet (like the other E2Fs and DPs) (data not shown), this suggests that the two DNA binding domains of E2F7 form an intramolecular structure that is similar to the winged helix structure of the E2F–DP heterodimer (Zheng et al., 1999). Intriguingly, we found that E2F7 binds only to a subset of E2F-dependent promoters (see below). This selectivity could be explained by the specific differences in the homodimeric E2F7 structure as compared with the heterodimeric E2F–DP structure, and may involve specific sequence requirements for E2F7 binding and/or regulation of E2F7 binding by local chromatin structure. However, future studies including crystallization of E2F7 in association with the E2F DNA binding consensus site are required to address this issue.
Interestingly, E2F7 is evolutionary conserved and is homologous to a subfamily of transcription factors, recently discovered in Arabidopsis thaliana (Kosugi and Ohashi, 2002). Unlike other known E2Fs but similar to E2F7, these E2F-like proteins, E2L1–3, have two copies of the DNA binding domain and can bind DNA independently of DP. In addition, as with E2F7, overexpression of plant E2Ls results in repression of E2F responsive promoters; however, their physiological role remains to be established.
Preliminary results suggest that, like E2F6 and plant E2L1–3, E2F7 does not bind to pRB family members (data not shown), and therefore represses transcription independently of this group of proteins. E2F6 has been shown to form several complexes containing Polycomb group proteins, and it may work as an active repressor of transcription by recruiting these proteins to E2F6 responsive promoters (Trimarchi et al., 2001; Ogawa et al., 2002). Since our data show that E2F7 is a repressor of a subset E2F target genes in vivo, it will be essential to understand the mechanism by which E2F7 represses transcription, and the mechanism by which it selectively targets a subset of E2F-dependent promoters.
Surprisingly, deletion of individual members of the E2F transcription factor family, with the exception of E2F3, has no discernible effect on expression of E2F target genes and cell proliferation (Gaubatz et al., 2000; Humbert et al., 2000; Wu et al., 2001; Storre et al., 2002). In contrast to this, inhibition of E2F7 expression results in increased levels of several E2F target genes, demonstrating that E2F7 has a unique physiological role in regulating these genes. Interestingly, we have shown by ChIP experiments that E2F7 occupies certain E2F-regulated promoters in vivo. Specifically, we have shown that E2F7 is associated with the E2F1 and CDC6 promoters, but not with the CCNA2 and CDC2 promoters. Furthermore, consistent with E2F7 being an E2F target gene the expression of which is cell cycle regulated, we found a significant increase in the association between E2F7 and E2F1 and CDC6 promoters in S phase. Given that E2F7 is a transcriptional repressor, this suggests that it is required for the down-regulation of a subset of E2F target genes in S phase, following their timely expression in late G1 and early S.
The idea that E2F7 exerts its physiological role by repressing a subset of E2F responsive promoters is supported by RNA interference experiments in which depletion of E2F7 led to increased levels of E2F1, CDC6 and CCNE1 expression. Interestingly CDC2 and CCNA2 expression levels remained constant, confirming that E2F7 regulates a specific subset of E2F-dependent promoters in vivo. Strikingly, the observation that the expression of E2F1, CDC6 and CCNE1 peaks at the G1/S transition, whereas the expression of CCNA2 and CDC2 peaks in S phase (Whitfield et al., 2002), supports the hypothesis that E2F7 is required for the correct timing of expression of early E2F target genes. Our data show that E2F1 stimulates E2F7 expression and that E2F7 represses E2F1, suggesting intricate mechanisms involving negative feedback loops for the regulation of E2F activity during the cell cycle.
In agreement with E2F7 being a repressor of E2F-regulated promoters, we found that ectopic expression of E2F7 led to accumulation of cells in G1 and lack of S phase entry. This result is consistent with ectopically expressed E2F7 competing with the activating E2Fs for binding to E2F responsive promoters, leading to lack of transcription of genes essential for cell cycle progression.
In current models for the regulation of E2F-dependent transcription, E2F4 and E2F5 bind to pRB family members in quiescent and early G1 cells and act as active repressors of transcription (Müller and Helin, 2000; Stevaux and Dyson, 2002; Trimarchi and Lees, 2002). As cells enter the cell cycle, the pRB family members are inactivated by phosphorylation, leading to the derepression and subsequent activation of E2F-dependent promoters. While E2F4 and E2F5 are exported to the cytoplasm, E2F1–3 remain nuclear and active, until inactivated during S phase by Cyclin A–CDK2. Based on our results, we propose that at this stage of the cell cycle E2F7 binds to a subset of E2F dependent promoters, resulting in their repression and allowing correct progression into the following phases of the cell cycle.
E2F7, a possible tumour suppressor gene?
The emerging concept from our study is that E2F7 is a negative regulator of cell proliferation, and that abrogation of E2F7 expression results in increased levels of several E2F-regulated genes. Among these genes is CCNE1, which is believed to contribute to tumorigenesis and is overexpressed in several types of cancer as a consequence of gene amplification or reduced proteolysis (Courjal et al., 1996; Moberg et al., 2001; Strohmaier et al., 2001). Thus, E2F7 qualifies as a putative tumour suppressor gene. So far, we have only been able to abrogate E2F7 expression in short-term assays and have not observed any significant changes on cell proliferation in these assays (data not shown). Interestingly, however, E2F7 is located at chromosome 12q21, a region the deletion of which is associated with poor prognosis for pancreatic cancer patients (Kimura et al., 1998). To investigate whether E2F7 is indeed a tumour suppressor gene, we will need to analyse the effects of deleting E2F7 in mouse, and to screen a number of primary tumours for lack of E2F7 expression. While the role of E2F7 as a growth suppressor still needs to be elucidated in detail, our data clearly demonstrate that it is an important component in the regulation of E2F-dependent promoters in vivo.
Materials and methods
cDNA cloning and plasmids
E2F7a and E2F7b were cloned by RT–PCR amplification of cDNA isolated from WI38 human fibroblasts, based on the sequence present in the database (NCBI accession No. XM084871). PCR was performed with the Expand long template PCR kit (Roche) using primers designed to contain SnaBI sites (upstream primer, TAC GTA TAG GAA AGC AGG GAT GGA; downstream primer A, TAC GTA TCA CGA TGT GTG CGT TGG; downstream primer B, TAC GTA GAC TCT CAG GGC GTT TGA TC). The amplified fragments were cloned into pCR2.1 Topo cloning vector and then subcloned as blunt-end fragments into pCMV HA-tagged vector, and as EcoRI-cut fragments into pCDNA1 HA, pCDNA3 Flag-tagged vectors and sequenced. Point mutations as described below were introduced into the sequence of E2F7a and E2F7b. The point mutant E146 was generated by PCR using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). Deletion mutants of E2F7a were prepared as described below. The primers were: MUT146F, ACA GAA AAG TGA ATT CCT CCT GTG CC; MUT146R, GGC ACA GGA GGA ATT CAC TTT TCT GT. The deletion mutants ΔDB1 and ΔDB21 were generated by PCR using the following primers: ΔDB2E2F7F, GTA ACA GAA GAG CGA GGT CGT; ΔDB2E2F7R, ACT ATG GTC TGG GGC ATC TTG; ΔDB1E2F7F, GCT AAG AAT CAG TAT GGC TGG; ΔDB1E2F7R, AGC ACT GTC CCC AAC AAC ATC. The vectors pCMV-βgal, pCMVHAE2F1, pBabeHAER-E2F1 and pCMVHADP1 have been described previously (Helin et al., 1993; Vigo et al., 1999b). Luciferase reporter construct pGL3TATA basic 6× E2F was as previously described (Müller et al., 1997). pCMVHAp16 and pCMVHAp21 were cloned by amplifying the full-length open reading frame (ORF) of the genes into pCMVHA. pCMVMYC was generated by cloning the full-length ORF into pCMVneoBam. pGL3E2F1(-242), and pGL3E2F1(-242, -E2F) were generated by subcloning the fragments from the corresponding CAT reporter plasmids (Johnson et al., 1994).
Tissue culture
WI38 human fibroblast, TIG3 human fibroblast, U2OS and Rat1 cells were grown in DMEM plus 10% (v/v) FCS. Transfections, infections and cell cycle synchronization experiments were performed as described previously (Petersen et al., 1999; Lomazzi et al., 2002).
Generation of antibodies to E2F7
Polyclonal antibodies were generated by immuninizing rabbits with an affinity-purified C-terminal glutathione S-transferase E2F7 protein (amino acids 419–728).
EMSAs
EMSAs were performed as described previously (Helin et al., 1992).
Real-time qPCR
Total RNA was prepared using RNeasy extraction kit (Qiagen). RT–PCR was performed using PE Applied Biosystems Taq Man® Reverse Transcription reagents, according to the manufacturer’s instructions. Real-time PCR was performed using an ABI prism 7700 Sequence Detection system. Primer sequences are available upon request.
Northern blot analysis
Northern blot analysis was performed as described previously (Müller et al., 2001).
Immunoprecipitations
U2OS cells were transfected with Flag-tagged E2F7a or E2F7b alone or together with HA-tagged E2F7a or E2F7b constructs. Transfected cells were lysed in E1A lysis buffer. The lysates were incubated at 4°C for 2 h with 20 µl packed gel volume of anti-Flag (M2 affinity gel freezer safe; Sigma). The bound proteins were resolved by SDS–PAGE. Western blots were performed as described below.
Western blot analysis
Total cell extracts were made in E1A buffer. Proteins (20–80 µg) were separated on SDS 8% (w/v) polyacrylamide gels and processed for western blotting. The blots were probed with antibodies specific for E2F1 (KH95), HA (Babco), Flag (Sigma), E2F7, Cyclin A2 (Sc-C19-G) and vinculin (hVin1; Sigma).
Luciferase assay
U2OS cells were transfected with 200 ng of reporter construct [pGL3 6XE2F, pGL3 hE2F1(-242) and hE2F1(-242, -E2F)], 20–200 ng of the expression plasmid and 100 ng of pCMV-β-gal plasmid. Cells were harvested for luciferase and β-galactosidase activitity 48 h after transfection. Normalized luciferase activity was determined as described previously (Müller et al., 1997).
ChIP
ChIP and data analysis were carried out as described previously (Frank et al., 2001). Briefly, U2OS cells were cross-linked by the addition of formaldehyde to 1% final concentration, the reaction was stopped by addition of glycine, cells washed in TBS and harvested into SDS buffer. Following centrifugation cells were resuspended in immunoprecipitation buffer and chromatin was sonicated to an average size of 500–1000 bp. Lysates were subsequently precleared with protein A–Sepharose beads (Amersham). Precleared chromatin was incubated at 4°C overnight with antibodies specific for E2F1 (Sc-193), E2F4 (Sc-866) or E2F7, or with an unrelated anti-Flag antibody (F3165; Sigma). Immunocomplexes were recovered with protein A–Sepharose beads. After extensive washes immunocomplexes were eluted from the beads, cross-links reversed, and material recovered by phenol/chloroform extraction and ethanol precipitation. DNA was resuspended in 200 µl of water and 7.5 µl used per real time qPCR using 200 nM of primers in 25 µl SYBR Green Reaction Mix (Perkin Elmer). The primer sequences are available upon request.
Microinjection experiments
Microinjection experiments were performed as described previously (Petersen et al., 1999; Vigo et al., 1999).
FACS analysis
For FACS analysis, U2OS cells were grown in a 10-cm dish and transfected with 9 µg of the indicated expression constructs and 1 µg of pCMVNLS-EGFP. The FACS profile of the gated transfected cells was determined as described previously (Petersen et al., 1999; Vigo et al., 1999).
Colony assay
HeLa cells were transfected with the indicated pCMV plasmids. Transfected cells were plated at different dilutions in 10-cm dishes and were selected in neomycin 300 µg/ml, the media was changed every 3–4 days. After 2 weeks, the ability to form colonies was assayed by counting crystal violet stained cells.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Marco Ciro’ for providing unpublished data and Emmanuela Frittoli for help with microinjection experiments. We thank Bruno Amati, Andrea Musacchio and Claire Attwooll for helpful comments on the manuscript and members of the Helin laboratory for discussions. This work was supported by grants from the Association for International Cancer Research (AICR), Associazione Italiana per la Ricerca sul Cancro (AIRC), Fondazione Italiana per la Ricerca sul Cancro (FIRC) and The Italian Health Ministry.
References
- Bandara L.R., Buck,V.M., Zamanian,M., Johnston,L.H. and La Thangue,N.B. (1993) Functional synergy between DP-1 and E2F-1 in the cell cycle-regulating transcription factor DRTF1/E2F. EMBO J., 12, 4317–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright P., Muller,H., Wagener,C., Holm,K. and Helin,K. (1998) E2F-6: a novel member of the E2F family is an inhibitor of E2F-dependent transcription. Oncogene, 17, 611–623. [DOI] [PubMed] [Google Scholar]
- Courjal F., Louason,G., Speiser,P., Katsaros,D., Zeillinger,R. and Theillet,C. (1996) Cyclin gene amplification and overexpression in breast and ovarian cancers: evidence for the selection of cyclin D1 in breast and cyclin E in ovarian tumors. Int. J. Cancer, 69, 247–255. [DOI] [PubMed] [Google Scholar]
- Cress W.D., Johnson,D.G. and Nevins,J.R. (1993) A genetic analysis of the E2F1 gene distinguishes regulation by Rb, p107 and adenovirus E4. Mol. Cell. Biol., 13, 6314–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S.R., Schroeder,M., Fernandez,P., Taubert,S. and Amati,B. (2001) Binding of c-Myc to chromatin mediates mitogen-induced acetylation of histone H4 and gene activation. Genes Dev., 15, 2069–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaubatz S., Wood,J.G. and Livingston,D.M. (1998) Unusual proliferation arrest and transcriptional control properties of a newly discovered E2F family member, E2F-6. Proc. Natl Acad. Sci. USA, 95, 9190–9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaubatz S., Lindeman,G.J., Ishida,S., Jakoi,L., Nevins,J.R., Livingston,D.M. and Rempel,R.E. (2000) E2F4 and E2F5 play an essential role in pocket protein-mediated G1 control. Mol. Cell, 6, 729–735. [DOI] [PubMed] [Google Scholar]
- Helin K., Lees,J.A., Vidal,M., Dyson,N., Harlow,E. and Fattaey,A. (1992) A cDNA encoding a pRB-binding protein with properties of the transcription factor E2F. Cell, 70, 337–350. [DOI] [PubMed] [Google Scholar]
- Helin K., Wu,C.-L., Fattaey,A.R., Lees,J.A., Dynlacht,B.D., Ngwu,C. and Harlow,E. (1993) Heterodimerization of the transcription factors E2F-1 and DP-1 leads to cooperative transactivation. Genes Dev., 7, 1850–1861. [DOI] [PubMed] [Google Scholar]
- Humbert P.O., Verona,R., Trimarchi,J.M., Dandapani,S. and Lees,J.A. (2000) E2f3 is critical for normal cellular proliferation. Genes Dev., 14, 690–703. [PMC free article] [PubMed] [Google Scholar]
- Johnson D.G., Schwarz,J.K., Cress,W.D. and Nevins,J.R. (1993) Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature, 365, 349–352. [DOI] [PubMed] [Google Scholar]
- Johnson D.G., Ohtani,K. and Nevins,J.R. (1994) Autoregulatory control of E2F1 expression in response to positive and negative regulators of cell cycle progression. Genes Dev., 8, 1514–1525. [DOI] [PubMed] [Google Scholar]
- Kimura M. et al. (1998) Identification of two common regions of allelic loss in chromosome arm 12q in human pancreatic cancer. Cancer Res., 58, 2456–2460. [PubMed] [Google Scholar]
- Kosugi S. and Ohashi,Y. (2002) E2Ls, E2F-like repressors of Arabidopsis that bind to E2F sites in a monomeric form. J. Biol. Chem., 277, 16553–16558. [DOI] [PubMed] [Google Scholar]
- Krek W., Livingston,D.M. and Shirodkar,S. (1993) Binding to DNA and the retinoblastoma gene product promoted by complex formation of different E2F family members. Science, 262, 1557–1560. [DOI] [PubMed] [Google Scholar]
- Lomazzi M., Moroni,M.C., Jensen,M.R., Frittoli,E. and Helin,K. (2002) Suppression of the p53- or pRB-mediated G1 checkpoint is required for E2F-induced S phase entry. Nat. Genet., 31, 190–194. [DOI] [PubMed] [Google Scholar]
- Lukas J., Petersen,B.O., Holm,K., Bartek,J. and Helin,K. (1996) Deregulated expression of E2F family members induces S-phase entry and overcomes p16INK4A-mediated growth suppression. Mol. Cell. Biol., 16, 1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg K.H., Bell,D.W., Wahrer,D.C., Haber,D.A. and Hariharan,I.K. (2001) Archipelago regulates Cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature, 413, 311–316. [DOI] [PubMed] [Google Scholar]
- Morkel M., Wenkel,J., Bannister,A.J., Kouzarides,T. and Hagemeier,C. (1997) An E2F-like repressor of transcription. Nature, 390, 567–568. [DOI] [PubMed] [Google Scholar]
- Moroni M.C., Hickman,E.S., Denchi,E.L., Caprara,G., Colli,E., Cecconi,F., Müller,H. and Helin,K. (2001) APAF-1 is a transcriptional target for E2F and p53. Nat. Cell Biol., 3, 552–558. [DOI] [PubMed] [Google Scholar]
- Müller H. and Helin,K. (2000) The E2F transcription factors: key regulators of cell proliferation. Biochim. Biophys Acta, 1470, M1–M12. [DOI] [PubMed] [Google Scholar]
- Müller H., Moroni,M.C., Vigo,E., Petersen,B.O., Bartek,J. and Helin,K. (1997) Induction of S-phase entry by E2F transcription factors depends on their nuclear localization. Mol. Cell. Biol., 17, 5508–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. et al. (2001) E2Fs regulate the expression of genes involved in differentiation, development, proliferation and apoptosis. Genes Dev., 15, 267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Ishiguro,K.-I., Gaubatz,S., Livingston,D.M. and Nakatani,Y. (2002) A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science, 296, 1132–1136. [DOI] [PubMed] [Google Scholar]
- Petersen B.O., Lukas,J., Sorensen,C.S., Bartek,J. and Helin,K. (1999) Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO J., 18, 396–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevaux O. and Dyson,N.J. (2002) A revised picture of the E2F transcriptional network and RB function. Curr. Opin. Cell Biol., 14, 684–691. [DOI] [PubMed] [Google Scholar]
- Storre J., Elsässer,H.-P., Fuchs,M., Ullmann,D., Livingston,D.M. and Gaubatz,S. (2002) Homeotic transformations of the axial skeleton that accompany a targeted deletion of E2f6. EMBO rep., 3, 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmaier H., Spruck,C.H., Kaiser,P., Won,K.A., Sangfelt,O. and Reed,S.I. (2001) Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature, 413, 316–322. [DOI] [PubMed] [Google Scholar]
- Trimarchi J.M. and Lees,J.A. (2002) Sibling rivalry in the E2F family. Nat. Rev. Mol. Cell. Biol., 3, 11–20. [DOI] [PubMed] [Google Scholar]
- Trimarchi J.M., Fairchild,B., Verona,R., Moberg,K., Andon,N. and Lees,J.A. (1998) E2F-6, a member of the E2F family that can behave as a transcriptional repressor. Proc. Natl Acad. Sci. USA, 95, 2850–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi J.M., Fairchild,B., Wen,J. and Lees,J.A. (2001) The E2F6 transcription factor is a component of the mammalian Bmi1-containing polycomb complex. Proc. Natl Acad. Sci. USA, 98, 1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigo E., Müller,H., Prosperini,E., Hateboer,G., Cartwright,P., Moroni,M.C. and Helin,K. (1999) CDC25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol. Cell. Biol., 19, 6379–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield M.L. et al. (2002) Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol. Biol. Cell, 13, 1977–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. et al. (2001) The E2F1-3 transcription factors are essential for cellular proliferation. Nature, 414, 457–462. [DOI] [PubMed] [Google Scholar]
- Zheng N., Fraenkel,E., Pabo,C.O. and Pavletich,N.P. (1999) Structural basis of DNA recognition by the heterodimeric cell cycle transcription factor E2F–DP. Genes Dev., 13, 666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]