Abstract
Binge-level doses of ethanol have been demonstrated to severely disrupt the cerebellum and cerebellar-dependent tasks when administered to rodent subjects during the early postnatal period. NMDA receptor-mediated excitotoxicity associated with ethanol withdrawal has been implicated as a significant component contributing to neurotoxic effects resulting from early ethanol exposure, and studies using MK-801 have reported protection from ethanol-induced damage. The present study examined whether the administration of MK-801 during ethanol withdrawal would ameliorate ethanol-associated cell death in the interpositus nucleus of the cerebellum, as well as behavioral deficits in a cerebellar dependent task. Long Evans rat pups were treated with ethanol (5.25 g/kg) in a binge-like manner on postnatal day 6 using intragastric intubation. Subjects then received an injection of MK-801 (0.5 mg/kg) or vehicle during withdrawal, 30 hours after ethanol exposure. Rats were then trained on an eyeblink classical conditioning task as juveniles (40 days of age) and cerebellar interpositus nucleus numbers were assessed following conditioning. Ethanol-exposed subjects exhibited reductions in neuronal populations as well as behavioral deficits during eyeblink conditioning. However, MK-801 administration significantly attenuated observed deficiencies, suggesting a protective effect resulting from MK-801 treatment during ethanol withdrawal. These results support the role of NMDA receptor-mediated excitotoxicity as a component mechanism by which ethanol produces teratogenicity. Additionally, our findings support previous reports which have shown correlations between dependent measures of eyeblink classical conditioning behavior and unbiased cell counts in the interpositus nucleus.
Introduction
Early developmental exposure to ethanol has been extensively linked to a variety of abnormalities in both human and non-human subjects. These abnormalities comprise various classes of disorders, such as fetal alcohol syndrome or alcohol-related neurobehavioral deficits (Green, 2004). In humans, there are an abundance of physical and behavioral alterations present in the affected individual (see Streissguth and O’Malley, 2000), however since human studies are largely limited to post facto investigation, much research has relied upon the use of animal models. Recently, animal models of binge-like ethanol exposure have offered significant contributions to the understanding of specific deficits and abnormalities observed during early development, such as the discovery of toxic dose ranges as well as temporal windows associated with heightened susceptibility to ethanol teratogenicity.
In rodent models, neural development prior to birth approximates human neuronal development during the first and second trimesters. During the first two postnatal weeks, postnatal days (PND) 4 through 9 in particular, rodents experience a period of heightened neural and glial development that is developmentally comparable to the brain growth spurt that occurs during the third trimester in humans (Bayer et al., 1993; Dobbing and Sands, 1979). This third trimester equivalent represents a period of significant dendritic arborization and synaptogenesis, as well as a heightened susceptibility to the toxic effects of ethanol exposure (Goodlett and Johnson, 1999). The postnatal rodent model has allowed for the documentation of numerous deficiencies that parallel deficits seen in fetal alcohol syndrome and alcohol-related neurodevelopmental disorders, including reductions in cell number (Goodlett et al., 1998; Goodlett et al., 1997; Goodlett et al., 1990; Green et al., 2002; Napper and West, 1995; Bonthius et al., 1992; Bonthius and West, 1990), deficiencies during cerebellar-dependent behavioral tasks such as rotarod motor performance and eyeblink classical conditioning (Goodlett et al., 1991; Green et al., 2000; Stanton and Goodlett, 1998), and abnormal physiological firing patterns from cerebellar deep nuclei and cortex, likely associated with corresponding cell loss (Green et al., 2002; Bäckman et al., 1998; Gruol et al., 2006; unpublished observations, Young, 2003).
While there is a general understanding of the effects of early ethanol exposure on development, an ongoing goal has been the identification of potential interventions that can protect or attenuate these ethanol-related deficits. Specifically, there has been an attempt to identify neurological mechanisms through which ethanol exposure impacts neural development. One such neural mechanism arises during ethanol withdrawal. One of the effects of ethanol on neural activity is a profound antagonistic action on the excitatory glutamatergic NMDA receptor (NMDAR) which results in heightened inhibitory activity in the brain. In the developing rodent brain, this effect triggers widespread apoptosis (Ikonomidou et al., 2000; Olney et al., 2000; Olney et al., 2001). Following this heightened inhibition, the ensuing neurochemical response is associated with both the development of ethanol tolerance as well as withdrawal. Briefly, NMDAR activity is increased via receptor protein upregulation and/or increased receptor activity (Dodd et al., 2000; Kang et al., 1996; Matthews et al., 1998; Sanna et al., 1993). It has been proposed that the resultant blockade of NMDAR activity could lead to a neurocompensatory upregulation of NMDAR-mediated glutamatergic function as well as secondary rebound excitability once ethanol is no longer present (Davidson et al., 1995; Faingold et al., 2000; Iorio et al., 1992).
Overactivation of NMDARs can lead to excessive intracellular calcium, and subsequent cell death (Hoffman, 1995; Lovinger, 1993). Indeed withdrawal-related excitotoxity has been associated with seizure activity, as well as neuronal cell death in several neural structures, including the cerebellum (Hoffman et al., 1995; Lovinger, 1993). Furthermore, the crucial role of NMDARs during neuronal developmental plasticity has been well-documented. Specifically, NMDARs are upregulated during fetal development in humans in both the hippocampus and the cerebellum (Represa et al., 1989; Johnson et al., 1993), and are also transiently overexpressed during the rodent neural growth spurt (Tremblay et al., 1988; McDonald and Johnston,, 1990; Chaudieu et al., 1991). This is believed to contribute to morphological differentiation by directing neuronal migration as well as mediating neuronal trophic support (Contestabile, 2000). Additionally, NMDAR populations require an optimal level of activation for normal development during this critical stage thus making the developing brain more susceptible to ethanol and NMDAR-mediated neurotoxicity (Contestabile, 2002; Monti and Contestabile, 2000; Crews et al., 1998; Bhave and Hoffman, 1997; Iorio et al., 1993).
If NMDAR-mediated excitoxicity associated with ethanol withdrawal constitutes a significant fraction of the teratogenic effects of ethanol, then NMDA antagonism during withdrawal-related hyperexitability should alleviate deficits associated with ethanol exposure (Chandler et al., 1993). In support of this hypothesis, previous reports have demonstrated that administration of the non-competitive NMDAR antagonist MK-801 (Dizocilpine) during the ethanol-withdrawal time window following acute ethanol treatment protects against ethanol-related behavioral deficits (Thomas et al., 2001; Thomas et al., 2002; Thomas et al., 1997). This effect has been shown to be highly dose and timing dependent (Thomas et al., 2001). Indeed, neonatal administration of MK-801 either alone or concurrently with ethanol has proven to be highly toxic or even lethal (Ikonomidou et al., 1999).
Cerebellar dependent behaviors appear to be particularly susceptible to ethanol exposure during the rodent brain growth spurt, in part due to substantial postnatal development in the cerebellum (Stanton et al., 1992). Therefore, classical eyeblink conditioning is a highly effective tool for the analysis of the teratogenic effects of ethanol exposure upon adult learning and memory, due in large part to the well-delineated brainstem-cerebellar circuitry that is involved (see Christian and Thompson, 2003; Steinmetz, 2000 for reviews). The elucidation of the underlying anatomical circuitry for the conditioned stimulus (CS) and unconditioned stimulus (US) pathways (Kim and Thompson, 1997; Steinmetz, 2000) have demonstrated that the key structures for conditioned response (CR)-related learning and plasticity are the Purkinje cells of Larsell’s lobules HI-III (anterior lobe) and HVI of the cerebellar cortex (e.g., Green and Steinmetz, 2005), and most importantly the anterior interpositus nucleus (INP) of the cerebellum (Clark et al., 1992; Lavond et al., 1985; Steinmetz et al., 1989; Steinmetz et al., 1992).
Previous research has shown that when rats are administered ethanol over PNDs 4–9, they exhibit learning impairments during eyeblink conditioning acquisition (Green et al., 2006; Green et al., 2000; Stanton and Goodlett, 1998). Furthermore, ethanol exposure is also associated with cell loss in the cerebellar deep nuclei (Green et al., 2002) and more specifically the INP (Tran et al., 2005). Since acquisition and retention of the conditioned response during eyeblink conditioning are known to be dependent upon an intact INP, it seems likely that protection from the glutamatergic excitoxicity present during withdrawal might save INP cells, resulting in fewer learning deficits. Therefore, in the present study neonatal rats received acute binge levels of ethanol during the brain growth spurt and were subsequently administered the NMDA antagonist MK-801 during ethanol withdrawal. As juveniles, rats were tested using a delay eyeblink conditioning paradigm to measure behavior and anatomical counts were then performed to assess the number of INP cells between treatment groups.
Materials and Methods
Subjects
Thirty-five Long Evans rats (18 male, 17 female) were bred at the Psychological and Brain Sciences department animal facilities at Indiana University. Male and female breeders (Harlan, Indianapolis) were paired, and vaginal smears were obtained daily following the first day of pairing. Gestational day (GD) 0 was determined by the presence of sperm in the vaginal smear. Following a positive smear, pregnant dams were separately housed until birth of the litter, which typically occurred on GD 22 – postnatal day (PND) 0. Litters were culled to 8–10 pups, with equal numbers of males and females when possible, by PND3.
Neonatal treatment groups
Beginning on PND 6, three randomly assigned groups of pups were initially treated and then later divided into six groups, based upon subsequent MK-801 or saline exposure. Group Ethanol (EtOH) received a binge dose of 5.25 g/kg of 11.9% ethanol in milk formula (West et al., 1984) via intragastric intubation producing mean peak blood alcohol concentrations > 300 mg/dL. Ethanol was administered over the course of two intubations delivered two hours apart (9:00 and 11:00 AM) to reach desired dosage. Two hours after the final ethanol administration, Group EtOH animals were intubated with milk formula alone (1:00 PM), and then received a final milk-alone treatment again two hours later (3:00 PM). Since the ethanol intubations induced intoxication and inefficient suckling for up to 8 hours, milk-alone supplements were administered exclusively to ethanol-treated subjects to help compensate for their lost caloric intake. Group Sham Intubation (SI) received the same schedule of intubations as Group EtOH with no fluid infusions (since caloric supplements in this group are known to produce long-lasting growth acceleration, Goodlett and Johnson, 1997). Group Unintubated Control (UC) received no intubations, but were removed from their housing and held by investigators for a duration commensurate with EtOH and SI groups. This was done to control for potential temperature and maternal separation stress effects resulting from the intubation procedures. All groups subsequently received 0.5 mg/kg of either MK-801 or saline via IP injection (injection volume: 1μl/g body weight) 30 hours following the final ethanol intubation. Additionally, treatment groups were randomly assembled from separate litters to control for possible litter effects. All Ethanol and Sham groups included animals from three separate litters, whereas both UC groups included animals from two separate litters. Final group numbers were as follows:
EtOH/MK-801 (Ethanol + MK-801), N = 7 (4 male, 3 female)
EtOH/Sal (Ethanol + saline), N = 5 (2 male, 3 female)
SI/MK-801 (Sham + MK-801), N = 6 (3 male, 3 female)
SI/Sal (Sham + saline), N = 5 (3 male, 2 female)
UC/MK-801 (Unintubated + MK-801) N = 6 (3 male, 3 female)
UC/Sal (Unintubated + saline) N = 6 (3 male, 3 female).
Determination of blood alcohol concentration (BAC)
Blood samples were taken via tail clip for SI and EtOH groups 2 hrs following the second ethanol intubation. For each pup, 20 μl of blood was collected into a heparinized capillary tube. Plasma samples were obtained by spinning down the blood samples in a microcentrifuge (Fisher Scientific, Pittsburgh) and were stored at −70° prior to BAC analysis. BACs were determined using an Analox GL5 Analyzer (Analox Instruments, Lunenberg, MA), which measures the rate of oxygen consumption resulting from oxidation of ethanol in the sample providing BACs in mg/dL.
Housing
All rats were weaned from the dam on PND 25 and housed separately by sex until surgical procedures began. Animals were provided with free access to food and water and maintained on a 12:12-h light/dark cycle. All procedures were approved by Indiana University’s Institutional Animal Care and Use Committee.
Surgery
Upon PND 40, subjects were anesthetized using intramuscular (IM) injections of an anesthetic cocktail (0.2ml/g bodyweight). The anesthetic cocktail consisted of sterile saline, ketamine (37.0 mg/ml), xylazine (1.85 mg/ml), and acepromazine (0.37 mg/ml). Each animal was surgically prepared with differential EMG eyewires to monitor eyeblink responses and a bipolar stimulation electrode for unconditioned stimulus administration (Plastics 1 Inc., Roanoke, VA). The EMG wires consisted of insulated .0003 inch stainless steel wire (Arista Surgical, New York) that was placed subdermally into the orbital musculature dorsal to the upper eyelid of the left eye. A ground wire was connected to stainless steel screws, which were attached to the skull. Both EMG wires as well as an uninsulated stainless steel ground wire were connected to gold pins housed in a 3-pin strip connector (Avnet, Indianapolis) and fixed to the skull with a headstage of Cranioplast (Plastics1, Roanoke, VA). The area around the headstage was salved with Povidone antibiotic ointment and the animals given one week to recover before the beginning of conditioning.
Conditioning Procedure
The conditioning apparatus resided inside a sound attenuating chamber. Animal headstages were plugged into a swivel commutator which carried leads for recording EMG and delivering the US. One day before acquisition training all subjects received one session of chamber adaptation, where EMG activity was recorded over the length of a typical acquisition session without CS or US presentation.
On the day following adaptation, subjects began 10 days of paired acquisition training. For each day of training, each session consisted of 10 blocks of 10 trials, with 80 of those trials consisting of the tone CS preceding the onset of the 100 msec coterminating stimulation US by 350 msec. Two trials out of each block were tone-alone presentations so an estimate of the behavioral response could be observed without contamination from the US artifact. The tone CS (450 msec, 2800 Hz, 90-dB) was delivered from an overhead speaker within the chamber. The stimulation US (100 msec, 60-Hz, 2.0 mA) was delivered through the bipolar electrode. All trials were separated by a variable 20–30 second inter-trial interval. Resultant behavioral responses were recorded, amplified, and sent to a computer-based recording system (Spike2 v 5.06, Cambridge Electronic Design).
Behavioral Data Analysis
EMG activity was sampled for 1,050 msec during each trial. The trials were then subdivided into three periods: a Pre-CS period, 350 msec before CS onset; a CS-US period, 350 msec between CS onset and US onset; and a post-US period, 350 msec following US onset. The mean activity recorded during the Pre-CS period served as a baseline for EMG activity for each independent trial.
An eyeblink was classified as a significant deflection of EMG activity from the Pre-CS baseline if behavior was 5 SDs above the average baseline activity. An eyeblink that occurred within the first 80 msec following CS onset was labeled an alpha response or “reflexive startle” response and discarded, whereas an eye blink 81–350 msec following CS onset during the CS-US period was scored as a CR. Across all trials, percentage of CRs, magnitude of CR amplitude, CR onset latency, and peak CR latency served as the dependent measures of learning. For onset and peak latency analysis, only CS-alone trials were included so as to avoid possible contamination from the US artifact.
Histology
At the conclusion of acquisition training, subjects were overdosed with 0.2 ml euthanasia solution (pentobarbital) and transcardially perfused with saline followed by 10% formalin. Brains were removed, placed in 30% sucrose/10% formalin solution for at least 10 days, and frozen-sectioned (80 μm) coronally through the cerebellar INP. Every second section was collected in 10% ethanol, mounted on gelatin-coated slides, and stained with thionin.
Interpositus nucleus cell counting
For each animal, every other section (one of two) was selected at predetermined uniform intervals through the INP, resulting in intervals of 160 mm between the rostral surfaces of saved sections. Unilateral cross-sectional areas of the INP were traced for each section and quantified using a digitizing tablet and a computer-based morphometry system (Sigmascan, Jandel Scientific). Area measurements were then used to estimate structural volumes using the Cavalieri estimator (Rosen and Harry, 1990) by the following formula:
where dbs is the real distance between analyzed sections, Σsa is the sum of all analyzed cross-sectional areas (mm2), st is the section thickness, and lsa is the largest individual cross sectional area (mm2) for a subject. Cut section thickness served as our thickness measure for area measurements. While it has been noted that tissue exhibits significant “shrinkage” during histological processing, this was not a significant concern presently due to the fact that all brains were treated identically. Therefore if there was a shrinkage issue, it applied to all brains and the relative differences in neuron number would be the same. The present concern was not necessarily the absolute number of neurons, which is what might be altered by shrinkage.
Estimates of the total number of neurons in the INP were obtained using the optical disector method outlined by Coggeshall ( Coggeshall, 1992) and a procedure similar to that of West and Gundersen (West and Gundersen, 1990). The optical disector is a 3-D sampling probe that samples particles uniformly regardless of the shape, size, or orientation of the object in space, while employing optical sectioning to sample particles (Nurcombe et al., 2001). Neurons were counted within a systematic random sample of locations throughout the INP by one of the authors (BWY), who was blind to the treatment group of the rats. The depth of the disector was calibrated to 42 μm which was adequate for visualizing neurons in multiple focal planes. Counts were made using an unbiased counting frame of 185 × 185 μm (neuronal somata touching the upper and right edges of the frame were excluded from counts) at a final magnification of 60x (63x objective, 0.85 numerical aperture).
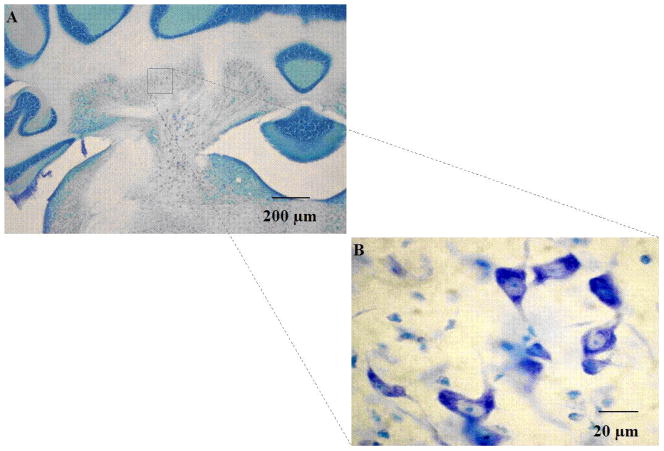
Under bright-field illumination, cells were identified as neurons using standard morphological criteria: nonhomogeneous staining, multipolar cell body containing Nissl substance, as well as a well-defined nucleus. Small, oval cells with granular staining and lacking Nissl substance were considered glia and therefore were excluded from analyses. Cells were characterized as countable objects if a nucleus or nucleolus was visible (Figure 1) and were counted if the cell was within the height of the disector and met criteria for the counting frame (Guillery, 2002). Neurons were counted as they first appeared while focusing through the z-axis. Neurons in the first focal plane (i.e., “tops”) were not included, to reduce redundant estimates. For each animal, counts were derived from an average of 7–10 sections, spaced at 160-μm intervals uniformly distributed throughout the entire extent of the INP. Within each section, 10 sampling areas were determined following a systematic random sampling scheme that allowed coverage of the entire field of interest in the x–y axis. Special attention was given to using the equivalent sampling areas across brains. INP neuron counts were then expressed as an average density (sum of neurons counted across all disectors divided by the volume the disector) Estimates of the total number of neurons in the INP were then obtained by multiplying the cell densities by the Cavalieri volume.
Fig. 1.
Thionin stained coronal section of cerebellar interpositus nucleus, shown at 4x (A) and 60x (B) magnification. Cell counts were performed at 60x, and were characterized as countable objects if a nucleus or nucleolus was visible (63x objective, 0.85 numerical aperture).
Results
Blood Alcohol Concentrations and Growth
The mean blood alcohol concentrations of ethanol groups on PND 6 were as follows: Group EtOH/MK-801 = 329.01 ± 15.43, Group EtOH/Sal = 350.4 ± 24.61. Statistical analysis showed no significant difference between ethanol/saline and ethanol/MK-801 groups. Ethanol levels recorded for sham treated subjects were below the level of reliable detection. Body weights recorded prior to surgical procedures were analyzed using repeated measures ANOVA. Analyses revealed no significant differences in body weight between treatment groups (Table 1).
Table 1.
Mean treatment group weights (g) recorded during intragastric intubations (on postnatal day 6) and subsequent saline/MK-801 injections up until surgical procedures for eyeblink conditioning.
| Animal Weights (g) | |||||||
|---|---|---|---|---|---|---|---|
| PND 6 | PND 7 | PND 10 | PND 15 | PND 21 | PND 25 | PND 30 | |
| EtOH/MK- 801 | 16.28 ± 0.28 | 17.78 ± 0.18 | 23.27 ± 2.88 | 33.96 ± 2.0 | 51.53 ± 2.19 | 74.6 ± 3.1 | 98.9 ± 3.52 |
| EtOH/SAL | 16.38 ± 0.4 | 18.4 ± 0.61 | 22.33 ± 1.65 | 35.36 ± 2.16 | 52.11 ± 2.47 | 76.4 ± 3.79 | 98.9 ± 4.4 |
| SI/MK-801 | 16.2 ± 0.1 | 19.67 ± 0.03 | 22.18 ± 2.66 | 34.67 ± 2.03 | 51.56 ± 2.54 | 75.87 ± 3.41 | 98.83 ± 4.14 |
| SI/SAL | 17.6 ± 0.3 | 21 ± 0.5 | 24.03 ± 1.74 | 36.58 ± 2.36 | 52.28 ± 2.62 | 75.9 ± 4.84 | 98.25 ± 4.35 |
| UC/MK-801 | 16.43 ± 0.56 | 20.2 ± 0.7 | 26.53 ± 0.82 | 37.19 ± 1.55 | 54.4 ± 1.4 | 78.04 ± 3.78 | 100.6 ± 8.38 |
| UC/SAL | 18.4 ± 0.1 | 19.65 ± 0.05 | 25.65 ± 0.15 | 37.62 ± 2.06 | 51.92 ± 1.63 | 75.13 ± 5.18 | 102.15 ± 6.95 |
Eyeblink Conditioning Behavior
During chamber adaptation, animals were placed in the conditioning apparatus for the duration of a typical training session (approx. 45 min.). The subjects were subjected to neither CS nor US presentation during adaptation conditioning, although EMG activity was recorded to confirm that electrodes and recording wires were functioning properly and to assess spontaneous eyeblink movement. Statistical analysis of adaptation sessions revealed no significant differences between treatment groups in regard to either rate or amplitude of spontaneous EMG activity.
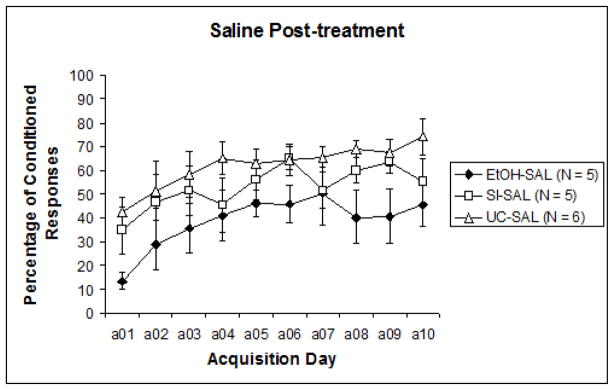
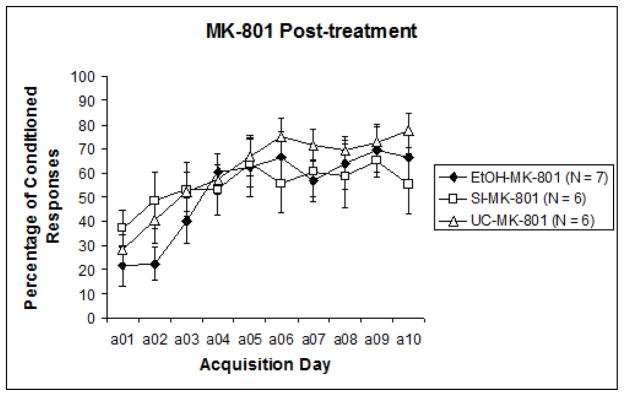
To examine acquisition versus performance stages of training, the 10 sessions were divided into two blocks of five sessions for analysis (Sessions 1–5 and Sessions 6–10). Results were analyzed with a 3 (intubation treatment) X 2 (post-intubation injection treatment) nested design repeated measures ANOVA (learning phase: acquisition days 1–5, 6–10), followed by post-hoc analyses. A significant difference was recorded between CR expression from the first half of training to the second, F1,29 = 28.396, p < .0001. This indicates that learning did occur over the course of training for all groups. Repeated measures ANOVA next revealed a significant effect of pre-treatment intubation (EtOH, SI, UC) collapsed across nested blocks, F2,32 = 3.637, p < .05. Tukey’s HSD confirmed that Group EtOH displayed significantly lower levels of learning than both Group SI and Group UC. However, when results were separated by post-treatment groups (MK-801 versus saline) to observe any individual influences of post-treatment effects, analyses revealed that significant differences were limited to the subjects that had received saline post-treatment, F2,13 = 4.173, p < .05 (Fig. 2a). Across MK-801 post-treated subjects, no significant effects of pre-treatment intubation were evident (Fig. 2b). Post-hoc analyses of post-intubation injection groups showed a significant effect of MK-801 explicitly limited to Group EtOH. Tukey’s HSD reported a significant difference between MK-801 and saline administration in Group EtOH (p < .05), but no differences in Group SI or UC. These results suggest that only subjects initially treated with ethanol were impacted by subsequent MK-801 administration, the result of which served to improve behavioral performance relative to EtOH subjects treated with saline.
Fig. 2.
Fig. 2a. Percentage of conditioned responses from subjects that received saline injections following pre-treatment intubations. Ethanol treated subjects exhibited significantly reduced measures of learning relative to control groups.,
Fig. 2b. Percentage of conditioned responses from subjects that received MK-801 following pre-treatment intubations. No group differences were apparent, suggesting an ameliorative effect of post-treatment MK-801 administration explicitly limited to ethanol treated subjects.
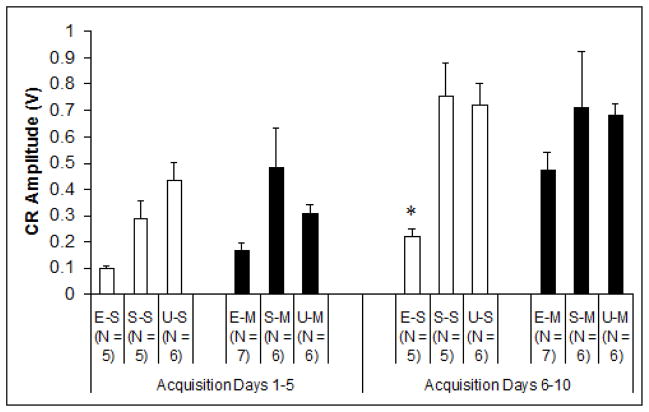
In addition to percentage of conditioned responses, CR amplitude provided another dependent measure of learning (Fig. 3). Analytical techniques were identical to those used for CR percentage. Results showed a significant post-treatment injection effect during the second acquisition phase (days 6–10), explicitly limited to EtOH treated subjects when collapsed across nested blocks, F2,14 = 4.632, p < .05. Tukey’s HSD post hoc analyses demonstrated significant differences between Group EtOH/Sal and all control groups, whereas no recorded differences between Group EtOH/MK-801 and any controls were apparent. This again was consistent with improved outcomes associated with MK-801 treatment during ethanol withdrawal. No significant group differences were observed between measures of CR onset latency or CR peak latency. Onset latency of total subject CRs increased significantly (from 203 ± 5.37 to 229 ± 5.45 msec following CS onset) across sessions, while peak latency exhibited no significant group or session changes.
Fig. 3.
Mean amplitudes (V) of behavioral conditioned responses during the first half (acquisition days 1–5) and the second half (acquisition days 6–10) of acquisition training. Group ethanol-saline exclusively exhibited significant differences to control groups over the course of conditioning. E-S = Ethanol-Saline, S-S = Sham-Saline, U-S = Unintubated-Saline, E-M = Ethanol-MK-801, S-M = Sham-MK-801, U-M = Unintubated-MK-801.
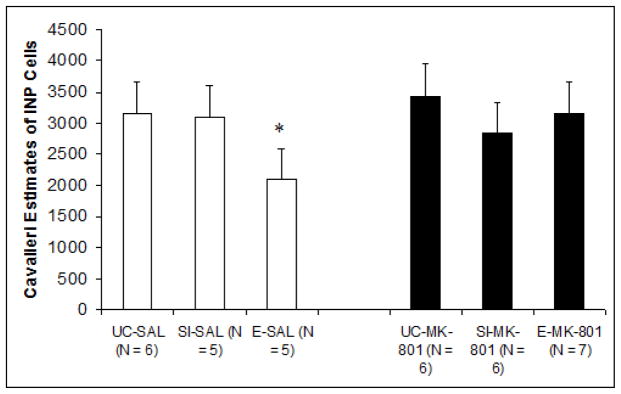
Cell Counts
Cerebella from subjects that underwent paired eyeblink conditioning were available for analysis. The mean estimated number of INP cells for each treatment group is shown in Fig. 4. A two-way ANOVA (Pre-treatment, Post-treatment) with estimated number of cells as the dependent measure revealed a significant difference between pre-treatment groups F2,29 = 4.020, p < .05. Post-hoc comparisons using Tukey’s HSD confirmed that while there were no significant differences between Group EtOH/MK-801 and controls, Group EtOH/Sal exhibited significantly reduced cellular estimates compared to both groups UC/MK-801 (p < .05; reduction of approximately 39%) and UC/Sal (p < .05; reduction of approximately 38%). Differences between groups EtOH/Sal and SI/Sal approached significance as well (p = .07). These results suggest a relative group deficiency explicit to subjects in Group EtOH/Sal, which provides support for both an ethanol-associated INP deficit, as well as MK-801-mediated protection. No additional group differences were observed.
Fig. 4.
Unbiased estimate of the total number of cerebellar interpositus nucleus cells as a function of treatment group. Estimates were calculated using principles based upon the optical disector/cavalieri combination. Results showed that ethanol-saline subjects exclusively displayed significant deficiencies relative to control groups.
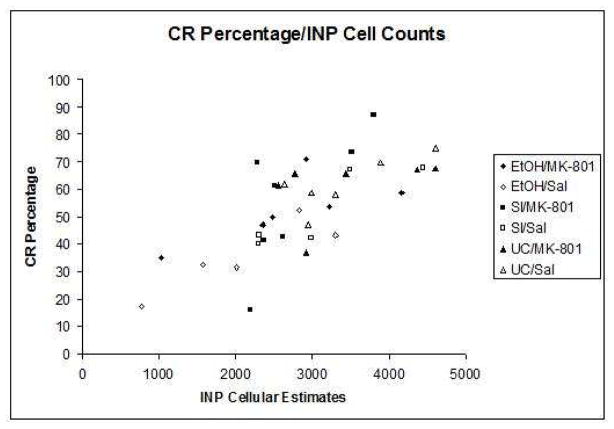
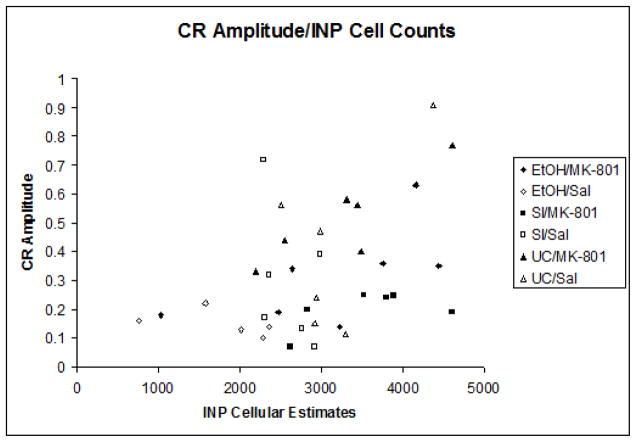
Relationship of cell counts to eyeblink conditioning
Indices of learning were calculated by computing group means for both percentage and amplitude of conditioned responses. Pearson correlation coefficients were then computed between the group means for the estimated number of neurons in the interpositus nuclei and all dependent measures of learning individually. The correlation between neuron number and percentage of CRs (r = +.528, p < .01) was highly significant (Figure 5a), while the relationship between neuron number and CR amplitude (r = +.18, p = .054) approached statistical significance as well (Figure 5b). No associations were shown between neuron number and estimates for peak latency or peak onset. The results of these analyses suggest a strong link between expressed behavior and estimated number of cells within the INP.
Fig. 5.
Fig. 5a. Scatterplot of the conditioned response percentage subject means relative to the mean estimates of INP cells.
Fig. 5b. Subject means of conditioned response amplitude relative to mean INP estimates.
Discussion
The present study examined the role of NMDAR-mediated excitotoxicity during ethanol withdrawal as a factor contibuting to the deficiencies associated with neonatal ethanol exposure. Administration of the NMDA antagonist MK-801 following acute ethanol resulted in both behavioral and neuroanatomical protection. Specifically, subjects treated with MK-801 following ethanol administration exhibited significantly improved learning, as measured both by percentage and amplitude of CRs, relative to subjects treated with ethanol alone. Additionally, MK-801 administration ameliorated the impact of neonatal ethanol exposure on INP neuronal populations. Consistent with previous reports, we found that neonatal ethanol exposure resulted in a significant decrease in INP cell number relative to control subjects. In contrast, ethanol animals subsequently treated with MK-801 exhibited no significant reduction in INP populations compared to controls. Finally, learning rates during eyeblink conditioning were significantly related to the number of INP neurons in the cerebellum.
These data presented above provide support for the role of NMDAR-mediated excitotoxicity as a mechanistic component to the damaging effects of developmental ethanol exposure. Given that NMDAR blockade during ethanol withdrawal provided behavioral protection along with concurrent neuroanatomical protection, this demonstrates strong support for a withdrawal-related excitotoxic component to the teratogenicity of ethanol (Thomas et al., 2001; Thomas et al., 2002; Lovinger, 1993), as well as supporting evidence for previous studies detailing the correlation between cerebellar deep nuclei number and eyeblink conditioning behavior (Green et al., 2002; Green et al., 2006). Since the INP serves as an integral site of learning related plasticity during eyeblink conditioning (Steinmetz et al., 1992), then one would expect any observed neuroanatomical protection to be visible through associated behavioral measures as well, which is exactly what our data suggest.
While there are numerous potential mechanisms that likely contribute to the damaging effects of early alcohol exposure (Goodlett and Horn, 2001; Goodlett et al., 2005), the focus of the present study was on glutamatergic excitotoxicity mediated by NMDAR during ethanol withdrawal. NMDARs are ionotropic glutamate receptors assembled of various subunits, the assorted compositions of which can largely affect the pharmacological properties of the receptor, including ethanol susceptibility. Studies using recombinant and native NMDARs have shown that receptors expressing the NR1/NR2A and NR1/NR2B subtypes exhibit an augmented ethanol-induced inhibition of function relative to other subunits, suggesting a greater susceptibility to ethanol (Allgaier, 2002). During the brain growth spurt, low levels of both receptor subtypes are present in the cerebellar deep nuclei (Akazawa et al., 1994). Additionally, while data further suggest that NR2A subunits may be marginally expressed throughout the cerebellum during the brain growth spurt (PND 6) only to be significantly upregulated by juvenile age (PND 40), the opposite seems to be true of NR2B subunits (Llansola et al., 2005). These studies therefore provide additional evidence that the cerebellar physiology of rats during the brain growth spurt is sufficient to support ethanol withdrawal-associated excitotoxicity as a teratogenic mechanism.
Under the current model, the neurological adaptation that occurs during acute ethanol exposure continues to cause cellular damage during a specified withdrawal period once ethanol has been metabolized. The specific time course for physiological ethanol withdrawal after an acute high-level dose has not been characterized, but it is has been demonstrated that all traces of ethanol are virtually absent from the subjects’ bloodstream at 33 hours following the final ethanol treatment (Thomas et al., 2001). Additionally, while the length of ethanol administration in the current study was relatively brief compared with earlier reports which have examined chronic exposure, a measured withdrawal reaction has been previously recorded following acute high doses of ethanol (Goldstein, 1986; Goldstein, 1974), demonstrating that physiological withdrawal is apparent even following brief ethanol exposure, given a sufficiently high dose.
It is of interest to note that while factors associated with robust learning such as percentage and amplitude of conditioned responses displayed ethanol-related impairments, other factors such as onset latency and peak latency of conditioned responses showed no differences across groups. Measures of eyeblink conditioning behavioral latency have often been described as the timing or “shape” of the conditioned response. Latency measures have also been associated with cerebellar Purkinje cells both anatomically as well as through physiological plasticity (Green and Steinmetz, 2005; Ohyama and Mauk, 2001; Medina et al., 2000; Buonomano and Mauk, 1994), particularly those cells from the cerebellar anterior lobe (Nolan and Freeman, 2006; Green and Steinmetz, 2005). It should be noted that the current study used a single day of binge exposure; most previous studies used exposures over multiple days. Since acute doses of ethanol have been shown to deplete Purkinje cells (Goodlett and Eilers, 1997; Goodlett et al., 1990) disruptions in CR timing would not have been unexpected. However, this topic has been met with seemingly variable results. A recent study describes adult subjects that received chemical Purkinje cell ablation showing little to no conditioned response timing abnormalities relative to control animals (Nolan and Freeman, 2005). This is somewhat surprising given that learning deficits associated with lesions to other structures, such as the INP, do not appear to be age dependent. While it does appear that any damage that may have occurred to the Purkinje cell population of ethanol treated subjects in the current study was insufficient to generate timing-related behavioral manifestations, further investigation of the ethanol-induced damage incurred by all relevant cellular populations is necessary to assess the degree to which each may be contributing to the deficits recorded presently.
Another intriguing finding from the present study was that sham groups demonstrated little to no learning deficiencies relative to unintubated control groups. Past studies have suggested a consistent sham-effect: on average, sham-treated animals do not learn as well as unintubated controls across behavioral measures (Green et al., 2002; Green et al., 2000; Tran et al., 2005). This sham-associated deficit has been recorded through cerebellar anatomy as well (Green et al., 2002). While this has typically not been a significant result, it has been notable and persistent enough that it has warranted mention. There have been several possible explanations offered for this finding, including increased glucocorticoid receptor populations resulting from neonatal handling and maternal separation (Liu et al., 1997; Meaney et al., 1988), as well as elevated levels of corticosterone arising post-intubation (McCormick et al., 1998). Indeed, maternal separation has been directly associated with a gender specific upregulation of glucocorticoid receptors in the INP, as well as corresponding eyeblink conditioning learning deficits (Wilbur et al., 2007). Regardless, the lack of a conspicuous SI deficit suggests that in this study, acute exposure to either intubation-induced stress or maternal separation was not related to long term learning deficiencies. It is likely that the single dose used in the current study generated insufficient stress to produce notable deficits. Further analysis of sham/separation effects following brief acute treatments could serve to elucidate any cumulative deficiencies associated with stress exposure during neonatal development.
In conclusion, this study provides support for the role of a withdrawal-related excitotoxic component to the teratogenic effects of ethanol exposure. Additionally, further support is garnered for the ability to protect against the effects of fetal ethanol exposure through pharmacological manipulation. An important area for further analysis will be to examine the physiology of cellular populations following ethanol/MK-801 administration in areas of interest such as the INP and hippocampus. Since prior reports have shown that fetal ethanol deficits are characterized by altered neuronal firing patterns due to cell loss in addition to other impairments (Green et al., 2002), it will be significant to examine whether fetal ethanol protection is evident in cellular physiology as well.
Acknowledgments
Support for this research came from NIAAA grant #AA11945 to JES. The authors would like to thank Greta Sokoloff, Eric Milner, and Randy Wilson for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bäckman C, West JR, Mahoney JC, Palmer MR. Electrophysiological characterization of cerebellar neurons from adult rats exposed to ethanol during development. Alcohol Clin Exp Res. 1998;22(5):1137–45. [PubMed] [Google Scholar]
- Bayer S, Altman J, Russo R, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14(1):83–144. [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14(1):107–18. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Bonthius NE, Napper RM, West JR. Early postnatal alcohol exposure acutely and permanently reduced the number of granule cells and mitral cells in the rat olfactory bulb: a stereological study. J Comp Neurol. 1992;324(4):557–66. doi: 10.1002/cne.903240408. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Mauk MD. Neural network model of the cerebellum: Temporal discrimination and the timing of motor responses. Neural Comput. 1994;6:38–55. [Google Scholar]
- Bhave SV, Hoffman PL. Ethanol promotes apoptosis in cerebellar granule cells by inhibiting the trophic effect of NMDA. J Neurochem. 1997;68(2):578–86. doi: 10.1046/j.1471-4159.1997.68020578.x. [DOI] [PubMed] [Google Scholar]
- Castoldi A, Barni S, Randine G, Costa L, Manzo L. Ethanol selectively interferes with the trophic action of NMDA and carbachol on cultured cerebellar granule neurons undergoing apoptosis. Brain Res Dev Brain Res. 1998;111(2):279–289. doi: 10.1016/s0165-3806(98)00135-7. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Sumners C, Crews FT. Ethanol inhibits NMDA receptor-mediated excitotoxicity in rat primary neuronal cultures. Alcohol Clin Exp Res. 1993;17(1):54–60. doi: 10.1111/j.1530-0277.1993.tb00726.x. [DOI] [PubMed] [Google Scholar]
- Chaudieu I, Mount H, Quirion R, Boksa P. Transient postnatal increases in excitatory amino acid binding sites in rat ventral mesencephalon. Neurosci Letters. 1991;133(2):267–70. doi: 10.1016/0304-3940(91)90585-h. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;11:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zhang AA, Lavond DG. Reversible lesions of the cerebellar interpositus nucleus during acquisition and retention of a classically conditioned behavior. Behav Neurosci. 1992;106:879–888. doi: 10.1037//0735-7044.106.6.879. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;15(1):9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Contestabile A. Roles of NMDA receptor activity and nitric oxide production in brain development. Brain Res Brain Res Rev. 2000;32(2–3):476–509. doi: 10.1016/s0165-0173(00)00018-7. [DOI] [PubMed] [Google Scholar]
- Contestabile A. Cerebellar granule cells as a model to study mechanisms of neuronal apoptosis or survival in vivo and in vitro. Cerebellum. 2002;1(1):41–55. doi: 10.1080/147342202753203087. [DOI] [PubMed] [Google Scholar]
- Crews FT, Steck JC, Chandler LJ, Yu CJ, Day A. Ethanol, Stroke, Brain Damage, and Excitotoxicity. Pharmacol Biochem Behav. 1998;59(4):981–91. doi: 10.1016/s0091-3057(97)00538-8. [DOI] [PubMed] [Google Scholar]
- Davidson MS, Shanley B, Wilce PA. Increased NMDA-induced excitability during ethanol withdrawal: a behavioral and histological study. Brain Res. 1995;674(1):91–96. doi: 10.1016/0006-8993(94)01440-s. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. The Brain Growth Spurt in Various Mammalian Species. Early Human Development. 1979;3:79–84. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dodd PR, Beckmann AM, Davidson MS, Wilce PA. Glutamate-Mediated Transmission, Alcohol, and Alcoholism. Neurochemistry International. 2000;37:509–533. doi: 10.1016/s0197-0186(00)00061-9. [DOI] [PubMed] [Google Scholar]
- Faingold C, Li Y, Evans M. Decreased GABA and increased glutamate receptor-mediated activity on inferior colliculus neurons in vitro are associated with susceptibility to ethanol withdrawal seizures. Brain Res. 2000;868(2):287–295. doi: 10.1016/s0006-8993(00)02342-8. [DOI] [PubMed] [Google Scholar]
- Goldstein DB. Rates of Onset and Decay of Alcohol Physical Dependence in Mice. J Pharmacol Exp Ther. 1974;190:377–383. [PubMed] [Google Scholar]
- Goldstein DB. The Alcohol Withdrawal Syndrome: A view from the Laboratory. In: Galanter M, editor. Recent Developments in Alcoholism. Plenum Press; 1986. [DOI] [PubMed] [Google Scholar]
- Goodlett C, Eilers A. Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: A stereological study of temporal windows of vulnerability. Alcohol Clin Exp Res. 1997;21:738–744. [PubMed] [Google Scholar]
- Goodlett C, Horn K. Mechanisms of Alcohol-Induced Damage to the Developing Nervous System. Alcohol Research & Health. 2001;25(3):175–184. [PMC free article] [PubMed] [Google Scholar]
- Goodlett C, Horn KH, Zhou FC. Alcohol Teratogenesis: Mechanisms of Damage and strategies for Intervention. Experimental Biology and Medicine. 2005;230(6):394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- Goodlett C, Johnson T. Temporal Windows of Vulnerability to Alcohol during the Third Trimester Equivalent: Why “knowing when” matters. In: Hannigan J, Spear L, NES, Goodlett C, editors. Alcohol and Alcoholism: Effects on Brain and Development. Lawrence Erlbaum; 1999. [Google Scholar]
- Goodlett C, Pearlman A, Lundahl K. Binge Neonatal Alcohol Intubations Induce Dose-dependent Loss of Purkinje Cells. Neurotoxicol Teratol. 1998;20:285–292. doi: 10.1016/s0892-0362(97)00102-5. [DOI] [PubMed] [Google Scholar]
- Goodlett C, Peterson S, Lundahl K, Pearlman A. Binge-like alcohol exposure of neonatal rats via intragastric intubation induces both Purkinje cell loss and cortical astrogliosis. Alcohol Clin Exp Res. 1997;21:1010–1017. [PubMed] [Google Scholar]
- Goodlett C, Thomas JD, West JR. Long-term Deficits in Cerebellar Growth and Rotarod Performance of Rats Following “binge-like” Alcohol Exposure during the Neonatal Brain Growth Spurt. Neurotoxicol Teratol. 1991;13(1):69–74. doi: 10.1016/0892-0362(91)90029-v. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: Timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19(6):435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Lundahl KR. Temporal determinants of neonatal alcohol-induced cerebellar damage and motor performance deficits. Pharmacol Biochem Behav. 1996;55(4):531–540. doi: 10.1016/s0091-3057(96)00248-1. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Marcussen B, West J. A single day of alcohol exposure during the brain growth spurt induces weight restriction and cerebellar Purkinje cell loss. Alcohol. 1990;7(2):107–114. doi: 10.1016/0741-8329(90)90070-s. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron H. Regulation of neuronal birth, migration, and death in the rat dentate gyrus. Dev Neurosci. 1996;18(1–2):22–35. doi: 10.1159/000111392. [DOI] [PubMed] [Google Scholar]
- Green JT. The effects of ethanol on the developing cerebellum and eyeblink classical conditioning. The Cerebellum. 2004;3:178–187. doi: 10.1080/14734220410017338. [DOI] [PubMed] [Google Scholar]
- Green J, Johnson T, Goodlett C, Steinmetz J. Eyeblink Classical Conditioning and Interpositus Nucleus Activity are Disrupted in Adult Rats Exposed to Ethanol as Neonates. Learning and Memory. 2002;9(5):304–320. doi: 10.1101/lm.47602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Rogers R, Goodlett C, Steinmetz J. Impairment in Eyeblink Classical Conditioning in Adult Rats Exposed to Ethanol as Neonates. Alcohol Clin Exp Res. 2000;24:438–447. [PubMed] [Google Scholar]
- Green J, Tran T, Steinmetz J, Goodlett C. Neonatal Ethanol Produces Cerebellar Deep Nuclear Cell Loss and Correlated Disruption of Eyeblink Conditioning in Adult Rats. Brain Res. 2002;956(2):302–311. doi: 10.1016/s0006-8993(02)03561-8. [DOI] [PubMed] [Google Scholar]
- Green JT, Arenos JD, Dillon CJ. The Effects of Moderate Neonatal Ethanol Exposure on Eyeblink Conditioning and Deep Cerebellar Nuclei Neuron Numbers in the Rat. Alcohol. 2006;39:135–150. doi: 10.1016/j.alcohol.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Green JT, Steinmetz JE. Purkinje cell activity in the cerebellar anterior lobe after rabbit eyeblink conditioning. Learn Mem. 2005;12(3):260–269. doi: 10.1101/lm.89505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Quina LA, Netzeband JG, Nguyen D, Gullette CE. Developmental changes in Ca2+-regulated functions of early postnatal Purkinje neurons. J Neurosci Res. 2006;83(8):1381–92. doi: 10.1002/jnr.20844. [DOI] [PubMed] [Google Scholar]
- Guillery RW. On Counting and Counting Errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Hoffman P. Glutamate receptors in alcohol withdrawal-induced neurotoxicity. Metab Brain Dis. 1995;10(1):73–79. doi: 10.1007/BF01991784. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Iorio K, Snell L, Tabakoff B. Attenuation of glutamate-induced neurotoxicity in chronically ethanol-exposed cerebellar granule cells by NMDA receptor antagonists and ganglioside GM1. Alcohol Clin Exp Res. 1995;19(3):721–726. doi: 10.1111/j.1530-0277.1995.tb01573.x. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–4. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova TI, Dikranian K, Olney JW. Ethanol-Induced Apoptotic Neurodegeneration and Fetal Alcohol Syndrome. Science. 2000;287(5455):1056. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Iorio K, Reinlib L, Tabakoff B, Hoffman P. Chronic exposure of cerebellar granule cells to ethanol results in increased N-methyl-D-aspartate receptor function. Mol Pharmacol. 1992;41(6):1142–8. [PubMed] [Google Scholar]
- Iorio K, Tabakoff B, Hoffman PL. Glutamate-induced neurotoxicity is increased in cerebellar granule cells exposed chronically to ethanol. Eur J Pharmacol. 1993;248(2):209–12. doi: 10.1016/0926-6917(93)90045-r. [DOI] [PubMed] [Google Scholar]
- Johnson M, Perry EK, Ince PG, Shaw PJ, Perry RH. Autoradiographic comparison of the distribution of [3H] MK-801 and [3H] CNQX in the human cerebellum during development and aging. Brain Res. 1993;615(2):259–66. doi: 10.1016/0006-8993(93)90036-m. [DOI] [PubMed] [Google Scholar]
- Kang M, Spigelman I, Sapp D, Olsen R. Persistent Reduction of GABAA Receptor-mediated Inhibition in Rat Hippocampus after Chronic Intermittent Ethanol Treatment. Brain Res. 1996;709:221–228. doi: 10.1016/0006-8993(95)01274-5. [DOI] [PubMed] [Google Scholar]
- Kim J, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Hembree TL, Thompson RF. Effect of kainic acid lesions of the cerebellar interpositus nucleus on eyelid conditioning in the rabbit. Brain Res. 1985;326:179–182. doi: 10.1016/0006-8993(85)91400-3. [DOI] [PubMed] [Google Scholar]
- Littleton J. Neurochemical Mechanisms Underlying Alcohol Withdrawal. Alcohol HealthRes World. 1998;22(1):13–24. [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Excitotoxicity and Alcohol-Related Brain Damage. Alcohol Clin Exp Res. 1993;17(1):19–27. doi: 10.1111/j.1530-0277.1993.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Matthews D, Devaud L, Fritschy J, Sieghart W, Morrow A. Differential Regulation of GABAA Receptor Gene Expression by Ethanol in the Rat Hippocampus Versus Cerebral Cortex. J Neurochem. 1998;70:1060–1166. doi: 10.1046/j.1471-4159.1998.70031160.x. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Kehoe P, Kovacs S. Corticosterone release in response to repeated, short episodes of neonatal isolation: evidence of sensitization. Int J Dev Neurosci. 1998;16:175–185. doi: 10.1016/s0736-5748(98)00026-4. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Reviews. 1990;15:41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Nores WL, Taylor NM, Mauk MD. Timing mechanisms in the cerebellum: Testing predictions of a large-scale computer simulation. J Neurosci. 2000;20(14):5516–5525. doi: 10.1523/JNEUROSCI.20-14-05516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti B, Contestabile A. Blockade of the NMDA receptor increases developmental apoptotic elimination of granule neurons and activates caspases in the rat cerebellum. Eur J Neurosci. 2000;12(9):3117–3123. doi: 10.1046/j.1460-9568.2000.00189.x. [DOI] [PubMed] [Google Scholar]
- Napper R, West J. Permanent Neuronal Cell Loss in the Inferior Olive of Adult Rats Exposed to Alcohol during the Brain Growth Spurt: A Sterological Investigation. Alcohol Clin Exp Res. 1995;19:1321–1326. doi: 10.1111/j.1530-0277.1995.tb01619.x. [DOI] [PubMed] [Google Scholar]
- Nolan BC, Freeman JH. Purkinje cell loss by OX7-saporin impairs excitatory and inhibitory eyeblink conditioning. Behav Neurosci. 2005;199(1):190–201. doi: 10.1037/0735-7044.119.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan BC, Freeman JH. Purkinje cell loss by OX7-saporin impairs acquisition and extinction of eyeblink conditioning. Learn Mem. 2006;13(3):359–365. doi: 10.1101/lm.168506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurcombe V, Wreford NG, Bertram JF. Stereological Estimation of Neuronal Number: The optical disector/cavalieri combination. In: Rush RA, editor. Methods in Molecular Biology: Neurotrophin Protocols. Humana Press; 2001. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Mauk M. Latent acquisition of timed responses in cerebellar cortex. J Neurosci. 2001;21:682–690. doi: 10.1523/JNEUROSCI.21-02-00682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney J, Wozniak D, Jevtovic-Todorovic V, Ikonomidou C. Glutamate signaling and the fetal alcohol syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2001;7:267–275. doi: 10.1002/mrdd.1037. [DOI] [PubMed] [Google Scholar]
- Olney JW, Ishimaru MJ, Bittigau P, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing brain. Apoptosis. 2000;5:515–521. doi: 10.1023/a:1009685428847. [DOI] [PubMed] [Google Scholar]
- Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber N, Bittigau P, Ikonomidou C. Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol. 2002;12(4):488–498. doi: 10.1111/j.1750-3639.2002.tb00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Represa A, Tremblay E, Ben-Ari Y. Transient increase of NMDA-binding sites in human hippocampus during development. Neurosci Letters. 1989;99(1–2):61–6. doi: 10.1016/0304-3940(89)90265-6. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Harry JD. Brain volume estimation from serial section measurements: a comparison of methodologies. J Neurosci Methods. 1990;35:115–124. doi: 10.1016/0165-0270(90)90101-k. [DOI] [PubMed] [Google Scholar]
- Sanna E, Serra M, Cossu A, Colombo G, Follesa P, Cuccheddu T, Concas A, Biggio G. Chronic Ethanol Intoxication Induces Differential Effects on GABAA and NMDA Receptor Function in the Rat Brain. Alcohol Clin Exp Res. 1993;17(1):115–123. doi: 10.1111/j.1530-0277.1993.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Goodlett CR. Neonatal ethanol exposure impairs eyeblink conditioning in weanling rats. Alcohol Clin Exp Res. 1998;22(1):270–275. [PubMed] [Google Scholar]
- Stanton ME, Freeman JH, Skelton RW. Eyeblink conditioning in the developing rat. Behav Neurosci. 1992;106:657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- Steinmetz J. Brain substrates of classical eyeblink conditioning: a highly localized but also distributed system. Behav Brain Res. 2000;110:13–24. doi: 10.1016/s0166-4328(99)00181-3. [DOI] [PubMed] [Google Scholar]
- Steinmetz J, Lavond D, Ivkovich D, Logan C, Thompson R. Disruption of classical eyelid conditioning after cerebellar lesions: Damage to a memory trace system or a simple performance deficit? J Neurosci. 1992;12:4403–4426. doi: 10.1523/JNEUROSCI.12-11-04403.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz J, Lavond DG, Thompson RF. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3:225–233. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, O’Malley K. Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Sem Clin Neuropsychiatry. 2000;5(3):177–190. doi: 10.1053/scnp.2000.6729. [DOI] [PubMed] [Google Scholar]
- Thomas J, Fleming S, Riley E. MK-801 can exacerbate or attenuate behavioral alterations associated with neonatal alcohol exposure in the rat, depending on the timing of administration. Alcohol Clin Exp Res. 2001;25(5):764–773. [PubMed] [Google Scholar]
- Thomas J, Weinert S, Sharif S, Riley E. MK-801 administration during ethanol withdrawal in neonatal rat pups attenuates ethanol-induced behavioral deficits. Alcohol Clin Exp Res. 1997;21(7):1218–1225. [PubMed] [Google Scholar]
- Thomas JD, Fleming SL, Riley EP. Administration of low doses of MK-801 during ethanol withdrawal in the developing rat pup attenuates alcohol’s teratogenic effects. Alcohol Clin Exp Res. 2002;26(8):1307–13. doi: 10.1097/01.ALC.0000025888.60664.D9. [DOI] [PubMed] [Google Scholar]
- Tran T, Jackson H, Horn K, Goodlett C. Vitamin E does not protect against neonatal ethanol-induced cerebellar damage or deficits in eyeblink classical conditioning in rats. Alcohol Clin Exp Res. 2005;29:117–129. doi: 10.1097/01.alc.0000150004.53870.e1. [DOI] [PubMed] [Google Scholar]
- Tremblay E, Roinsin MP, Represa A, Charriaut-Marlangue C, Ben-ari Y. Transient increased density of NMDA binding sites in developing rat hippocampus. Brain Res. 1988;461:393–396. doi: 10.1016/0006-8993(88)90275-2. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296(1):1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- Wilbur AA, Southwood CJ, Sokoloff G, Steinmetz JE, Wellman CL. Neonatal maternal separation alters adult eyeblink conditioning and glucocorticoid receptor expression in the interpositus nucleus of the cerebellum. Dev Neurobiol. 2007;67(13):1751–64. doi: 10.1002/dneu.20549. [DOI] [PubMed] [Google Scholar]