Abstract
The nicotinic acetylcholine receptor (AChR) is in intimate contact with the lipids in its native membrane. Here we analyze the possibility that it is the intrinsic properties of the AChR that determine its partition into a given lipid domain. Torpedo AChR or a synthetic peptide corresponding to the AChR γM4 segment (the one in closer contact with lipids) was reconstituted into “raft”-containing model membranes. The distribution of the AChR was assessed by Triton X-100 extraction in combination with fluorescence studies, and lipid analyses were performed on each sample. The influence of rapsyn, a peripheral protein involved in AChR aggregation, was studied. Raft-like domain aggregation was also studied using membranes containing the ganglioside GM1 followed by GM1 crosslinking. The γM4 peptide displays a marked preference for raft-like domains. In contrast, AChR alone or in the presence of rapsyn or ganglioside aggregation exhibits no such preference for raft-like domains, but it does cause a significant reduction in the total amount of these domains. The results indicate that the distribution of the AChR in lipid domains cannot be due exclusively to the intrinsic physicochemical properties of the protein and that there must be an external signal in native cell membranes that directs the AChR to a specific membrane domain.
Keywords: AChR, raft, model membrane, FRET, lipid analysis, detergent partition
The nicotinic acetylcholine receptor (AChR) is a pentameric transmembrane glycoprotein composed of four different but homologous subunits in the stoichiometry α2βγδ in vertebrate embryonic skeletal muscle and in the electromotor synapse of electric fish. Each subunit contains a relatively large extracellular domain and four hydrophobic membrane-spanning segments referred to as M1-M4, ending in a short extracellular carboxyl terminal domain. Three concentric rings can be distinguished in the AChR transmembrane (TM) region (1, 2): the M2 TM segments of all subunits outline the inner ring and form the walls of the ion channel proper; M1 and M3 constitute the middle ring; and the M4 segments form the outer ring, which is in closest contact with the AChR lipid microenvironment.
One outstanding characteristic of the mature neuromuscular junction (NMJ) is the high density of AChR clusters at the postsynaptic membrane (3). Neural agrin, an extracellular proteoglycan, initiates the cascade of events in the AChR clustering process in the myotubes by first activating MuSK (muscle-specific receptor tyrosine kinase), which subsequently induces the activation of several other intracellular enzymes. Finally, association of AChR with rapsyn, a cytoplasmic peripheral membrane protein colocalized with AChR in vivo, mediates binding to the cytoskeleton (3, 4), leading in turn to efficient receptor clustering (5). Lipids have been postulated to be involved in AChR nanodomain organization and clustering, presumably at early, preinnervation stages of development (6). The plasma membrane organization of living cells is currently considered to be a mosaic of macroscopic and stable or transient and short-scale segregated domains (reviewed by Ref. 7). Lipid domains termed lipid “rafts” are highly enriched in both cholesterol (Chol) and sphingolipids. Lipid rafts display distinct biophysical and biochemical characteristics, and contain selected integral or peripheral membrane proteins (8–10). These domains are considered functionally dynamic lateral platforms embedded in the membrane and functionally involved in the segregation of particular proteins, presumably increasing the efficiency of certain signal-transduction processes (11–14). In particular, there is considerable evidence to support the notion that this type of membrane domain plays an important role in the organization and compartmentalization of the different components of neurotransmitter signaling, including the ligand-gated ion channel superfamily (see Ref. 15).
Cartaud et al. suggested that raft lipid domains were involved in the sorting and subsequent delivery of AChR and rapsyn to the plasma membrane (16) and that AChR was constitutively associated with raft lipid domains together with syntrophin, rapsyn, and other postsynaptic components, independently of agrin stimulation (17). Agrin activation of MuSK was necessary, however, for inducing the coalescence of rafts and for AChR clustering. Likewise, Willmann et al. (18) found that the AChR and the majority of the agrin-signaling pathway was concentrated in raft-like domains and that incubation of cells with agrin produced maximal AChR clustering but did not alter the association with lipid rafts. In contrast, Zhu et al. (19), working with the same cells, found the majority of AChR associated with nonraft domains, but both AChR and MuSK translocated into lipid rafts upon neural agrin stimulation. Similarly, Campagna and Fallon (20) indicated that although some AChR occurred constitutively in lipid rafts in C2C12 myotubes, agrin signaling increased the number of AChR associated with these domains by effectively translocating AChR into these platforms. Stetzkowski-Marden et al. (19) assigned a key role to cholesterol in determining the formation, size, and structure of the coalesced domains. Application of exogenous cholesterol to denervated NMJ protected AChR clusters against denervation-induced disassembly (21).
The wealth of information on the biochemical and physicochemical properties of the AChR protein has been obtained using Torpedinidae species (reviewed in Refs. 1 and 2). The present work investigates whether the Torpedo AChR protein spontaneously localizes in raft-like lipid domains. To this end, we compared the behavior of a synthetic peptide corresponding to the outermost ring of lipid-contacting transmembrane segments (1), the AChR γM4 segment, with that of the AChR purified from T. californica electroplax plasma membranes upon reconstitution into model membranes with defined lipid composition, i.e., containing different proportions of cholesterol, sphingomyelin (SM), and phosphatydilcholine (PC). The role of lipid raft patching in AChR distribution was also analyzed using model lipid mixtures containing the ganglioside GM1 (a bona fide raft lipid), and inducing GM1 aggregation by cholera toxin B plus anti-cholera toxin antibody-mediated crosslinking. Our data suggest that the transmembrane segment γM4 displays preferential partition into lipid rafts, being found almost exclusively in this type of domain, whereas the AChR alone or together with rapsyn does not. In addition, ganglioside aggregation modifies the detergent partition profile of the receptor protein. Furthermore, the presence of γM4 induces an increase in the total amount of DRMs in the model membranes, whereas the presence of AChR has the opposite effect.
MATERIALS AND METHODS
Materials
A peptide corresponding to the T. californica AChR TM segment γM4 and the two extramembranous regions and having the sequence DKACFWIALLLFSIGTLAIFLTGHFNQV (28-mer peptide) was purchased from Biosynthesis, Inc. (Lewisville, TX). The peptide was estimated to be over 90% pure (22) and was kept lyophilized at −80°C until use. T. californica specimens were obtained from the Pacific coast of California, killed by pithing, and their electric organs were dissected and stored at −70°C until further use. Laurdan and dehydroergosterol (DHE) were purchased from Molecular Probes (Eugene, OR). Affi-Gel 10 Gel, dithiothreitol, and SM2 Biobeads were obtained from Bio-Rad (Hercules, CA). POPC was from Avanti Polar Lipids, Inc. (Birmingham, AL). Chol, SM, cholera toxin B subunit (CTxB), ganglioside GM1, Tween 20, and Triton X-100 were obtained from Sigma-Aldrich (St. Louis, MO). The monoclonal antibody mAb 210 against the major immunogenic region in the α subunit of the AChR and the mAb 124 monoclonal antibody against the B subunit of the AChR were kindly provided by Dr. J. Lindstrom, University of Pennsylvania School of Medicine. The mAb 1234 monoclonal antibody against rapsyn was kindly provided by Dr. S. Frohener, University of Washington.
Methods
Preparation of unilamellar lipid vesicles containing γM4 peptide.
Aliquots of POPC, SM, and Chol dissolved in chloroform:methanol (2:1 v/v) were mixed to obtain the following lipid compositions: POPC:SM:Chol (1:1:1 molar relation) and POPC:SM:Chol (0.35:1:0.87 molar ratio), followed by the addition of the γM4 peptide dissolved in ethanol (250:1, lipid:peptide molar relation). The samples were first dried under nitrogen for 1 h and then large unilamellar vesicles were obtained following conventional procedures. Briefly, dialysis buffer (100 mM NaCl, 0.1 mM EDTA, 0.02% NaN3, 10 mM phosphate, pH 7.4) with 1% cholate was added to the dried lipid/peptide mixture (1:1 lipid:buffer w/v), incubated at 45°C for 10 min, vortexed for 1 min, and dialyzed against 1 l of dialysis buffer with five changes of buffer (every 12 h) at 4°C.
Preparation of AChR membranes.
Crude AChR-membranes were prepared from the electric organ of T. californica as previously described (6, 23). The electric tissue was first dissected into small pieces, and then homogenized at 4°C using a Virtis 60 glass (The Virtis Co., Inc.) until an homogeneous suspension was obtained. The homogenate was subjected to a first centrifugation at 2,500 g for 10 min at 4°C, and the obtained supernatant was then centrifuged at 30,000 g for 1 h at 4°C. Crude membranes were obtained by resuspending the pellet (24).
AChR-rich membranes were obtained by centrifuging the crude membrane fractions on a sucrose gradient (50%, 39%, and 35% sucrose) at 30,000 g for 1 h at 4°C (24). The middle fraction corresponds to the AChR-rich membranes containing the highest specific activity [in the order of 2.0–2.8 nmol α-bungarotoxin sites/mg protein (23)].
Affinity chromatography purification of the AChR protein.
T. californica crude membranes (2 mg/ml protein) were solubilized in 1% sodium cholate for 45 min. The insoluble material was discarded after centrifugation for 1 h at 74,000 g. Purification of the AChR by affinity chromatography was done in the presence of synthetic lipids as reported by da Costa et al. (25, 26). Affinity column preparation required first the coupling of cystamine to Affi-Gel 10, followed by reduction with dithiothreitol and coupling of bromoacetylcholine bromide to the gel. The supernatant was applied to the affinity column. Total replacement of endogenous lipids required washing the column with a linear gradient of specific lipids in a defined molar ratio, dissolved in dialysis buffer containing 1% cholate. Briefly, the column was washed first with a lipid solution of 1.3 mM, followed by a step gradient to a lipid solution of 3.2 mM and finally a step gradient to 0.13 mM lipid solution (26). The AChR was then eluted from the column with a 0.13 mM lipid solution in 250 mM NaCl, 0.1 mM EDTA, 0.02% NaN, 5 mM phosphate, pH 7.8, containing 1% cholate and 10 mM carbamoylcholine. AChR was next dialyzed against 1 l of dialysis buffer with five changes of buffer (every 12 h) at 4°C. Different lipid systems were prepared (POPC: SM:Chol 1:1:1 and POPC:SM:Chol 0.35:1:0.87 molar ratio). A POPC:SM:GM1:Chol (1:0.98:0.02:1 molar ratio) system was prepared by adding GM1 immediately before dialysis. Where a higher lipid:AChR molar ratio was required, further lipids were added immediately before dialysis. The AChR purification was checked by SDS-PAGE, and protein concentration was determined by the method of Lowry (27). The samples were stored at −70°C until use.
Preparation of rapsyn-enriched alkaline extract from T. californica postsynaptic membranes.
Rapsyn and other peripheral membrane proteins were extracted from AChR-rich membranes by incubation at pH 11 (28). Briefly, AChR-rich membranes were sedimented by centrifugation at 85,000 g for 1 h and resuspended in 10 mM sodium phosphate buffer, pH 7.4, to a final concentration of 3 mg protein/ml. The pH was adjusted to 11.0 by dropwise addition of 1 N NaOH, and the preparation was incubated at 4°C for 1 h under gentle stirring. Membranes were then centrifuged at 85,000 g for 20 min, after which the supernatant extract was neutralized by adding 1 M HCl. Any insoluble material was removed from the extract by another centrifugation at 85,000 g for 20 min. The supernatant was stored at −70°C until use. The rapsyn-enriched extract was subjected to SDS-PAGE and stained with Coomassie blue. The protein bands were quantified by scanning the gels, and further analysis of the images using Image J software (a freely available application in the public domain for image analysis and processing developed and maintained by Wayne Rasband at the Research Services Branch, National Institutes of Health). About 80% of the protein in the pH 11 extract consisted of rapsyn, with little or no detectable contamination with AChR subunits, in accordance with literature data (29).
Preparation of rapsyn-AChR liposomes.
Rapsyn-enriched extract was added to purified AChR reconstituted in POPC: SM:cholesterol 1:1:1 liposomes in different rapsyn:AChR molar stoichiometries (1:1, 2:1, 4:1 rapsyn:AChR). The rapsyn:AChR mixture was prepared in dialysis buffer containing 1% cholate and dialyzed against 1 l of dialysis buffer with five buffer changes (every 12 h) at 4°C. The samples were then centrifuged at 104,000 g for 45 min at 4°C in a TLA 100.4 rotor with a Beckman Optima TLX centrifuge to separate unbound rapsyn. The samples were stored at −70°C until use.
Crosslinking of GM1 with CTxB and anti-CTx.
GM1-containing membranes were treated with CTxB (1:1000) for 30 min at 4°C with gentle agitation or with CTxB, same time and temperature, followed by addition of anti-CTx (1:500) for 30 min at 4°C to induce ganglioside crosslinking before detergent treatment.
Preparation of DRM and DSM fractions.
From 50 to 300 µg of purified AChR (or 20 µg of γM4 peptide) reconstituted in a defined lipid composition were treated with 1% Triton X-100 (or 2.5% Tween 20, when indicated) for 20 min at 4°C. In the case of liposomes alone or liposomes containing the γM4 peptide, the sample was separated into detergent-soluble membrane (DSM) and detergent-resistant membrane (DRM) fractions by high-speed centrifugation at 104,000 g for 3 h at 4°C in a TLA 100.4 rotor using a Beckman Optima TLX centrifuge. The supernatant (DSM) was removed, and the pellet (DRM) was resuspended in an equal volume of buffer (10 mM phosphate, pH 7). In the case of proteoliposomes (AChR-containing liposomes), we used two different methods to separate DRM and DSM fractions: high-speed centrifugation and sucrose density gradients. Although the latter is a routine practice used to separate these fractions from cell membranes, there is little experience with proteoliposomes systems. Following Ayuyan and Cohen (30), the same differences were introduced to the classical protocol. DRM and DSM fractions were separated on a step sucrose gradient consisting of 4 ml layers of 40% and 30% sucrose in buffer (10 mM phosphate, pH 7), loading the sample at the top of the sucrose gradient (2 ml of the detergent-treated sample were mixed with 2 ml of 10% sucrose in phosphate buffer to achieve a final concentration of 5% sucrose). After centrifugation at 260,000 g at 4°C for 20 h, 12 1-ml fractions were taken from top to bottom of the centrifuge tube. Raft fractions from the density gradients were identified by dot blotting of the raft lipid GM1 and probed with labeled cholera toxin (31). Aliquots from each fraction were dried on a nitrocellulose membrane, blocked with 2% milk in Tris-buffered saline with 0.05% Tween 20 (TTBS), and then transferred to 0.5% BSA in TTBS buffer containing 1:20,000 diluted horseradish peroxidase-coupled cholera toxin subunit. After washing with TTBS buffer and a final wash with TBS (TTBS without Tween 20), immunoreactive dots were detected by enhanced chemoluminescent detection (ECL, Amersham Pharmacia Biotech) using X-ray films (Kodak Biomax).
SDS-PAGE and Western blot assays.
The AChR protein in the DRM and DSM fractions was precipitated by TCA treatment, washed with acetone, dissolved in Laemmli buffer at 100°C for 5 min (32), and separated by SDS-PAGE on 10% polyacrylamide gels (Mini Protean II, Bio Rad, Richmond, CA). γM4 peptide was precipitated by a methanol-chloroform-water method (33), dissolved in Laemmli buffer, and separated by electrophoresis on a Tricine gel. In some cases, both types of gel were developed using Coomassie blue or silver nitrate staining, depending on the amount of protein. In other cases, the proteins were electrotransferred to an Inmobilon-P membrane (Millipore, Billerica, MA). Membranes were blocked with 5% nonfat dry milk in TTBS buffer (20 mM Tris-HCl, pH 7.4, 100 mM NaCl, and 0.1% (w/v) Tween 20) for 2 h at room temperature followed by incubation with primary antibodies [anti-AChR α-subunit (mAb 210) (1:5000), anti-AChR β-subunit (mAb124) (1:2000), and anti-rapsyn (mAb 1234) (1:500)] overnight at 4°C. Membranes were washed five times with TTBS buffer and then exposed to the appropriate HRP-conjugated secondary antibody [anti-rat (1:3000) or anti-mouse (1:5000)] for 2 h at room temperature. Membranes were washed again with TTBS four times followed by an additional wash with TBS. Immunoreactive bands were detected by enhanced chemoluminescent detection (ECL, Amersham Pharmacia Biotech) using X-ray films (Kodak Biomax). Protein distribution between soluble or insoluble fractions in gels stained with Coomassie blue or Western blots were quantified by densitometry using image analysis software (Image J).
Lipid characterization of the DSM and DRM fractions.
Triton X-100 was eliminated from the DSM and DRM fractions by washing the samples for 2 h with SM-2 Biobeads (0.2 mg/ml, batch method). Lipids were subsequently extracted from DRM and DSM fractions according to the procedure of Bligh and Dyer (34), resolved into classes by TLC on silica gel G-plates, and spotted under UV light after spraying with dichlorofluorescein. TLC was performed using chloroform-methanol-acetic acid-0.15 M NaCl (20:10:3.2:1, v/v/v/v) as the first developing solvent to separate SM from PC, followed by ether as the second one up to the top of the plates to separate Chol from the phospholipids. The spots corresponding to SM and PC were scraped off, and the lipids were subjected to lipid phosphorus determination using the chemicals and reactions described by Rouser et al. (35). The spots corresponding to Chol were scraped off, and Chol was eluted by thoroughly mixing this silica three times with chloroform:methanol:water (5:5:1 by vol.), centrifuging, pooling the three eluates, mixing with four volumes of water, separating into phases, and recovering the Chol from the organic phase. Chol analysis was performed using the cholesterol oxidase assay (Wiener Laboratories, Rosario, Argentina).
Fluorescence measurements.
All fluorimetric measurements were performed in an SLM model 4800 fluorimeter (SLM Instruments, Urbana, IL) using a vertically polarized light beam from Hannovia 200-W mercury/xenon arc obtained with a Glan-Thompson Polarizer (4 nm excitation and emission slits) and 1 ml quartz cuvettes. The temperature was set with a thermostated circulating water bath (Haake, Darmstadt, Germany). Stock solutions of Laurdan and DHE were prepared in ethanol and stored at −20°C until use. Each fluorescent probe was added to the liposomes (5% of total lipids) in two different forms: (a) added prior to the dialysis step, which, in this case, was done in the dark, and (b) added directly to the liposomes from an ethanol solution, followed by 45 min stabilization, keeping the amount of organic solvent below 0.5% all along the experiment. Similar results were obtained with both forms of addition. For fluorescence measurements, the liposomes were suspended in 20 mM HEPES buffer, 150 mM NaCl, and 0.25 mM MgCl2 (pH 7.4) to obtain 8 µg AChR or 20 µg γM4 peptide in 700 µl.
Förster resonance energy transfer (FRET) measurements.
The energy transfer efficiency (E) in relation to all other deactivation processes of the excited donor depends on the sixth power of the distance between donor and acceptor. According to Förster's theory (36) E can be calculated as follows:
| (Eq. 1) |
where I and ID are the emission intensities in the presence and in the absence of the acceptor, respectively. Here, I corresponds to the maximal intrinsic protein emission intensity, which is 330 nm. The excitation wavelength was set at 290 nm. When E was measured in the presence of different amounts of acceptor molecules, a further correction was introduced to compensate for any modification of the intrinsic fluorescence of Trp by any other quenching mechanism, as follows:
| (Eq. 2) |
where Ecorr is the calculated value of E corrected by the quenching of the intrinsic fluorescence by the acceptor molecules. E(+acceptor) and E(−acceptor) values were calculated using Equation 1 in the presence or absence of acceptor molecules (Laurdan or DHE).
Generalized polarization.
Excitation GP (exGP) (37, 38) was calculated as follows:
| (Eq. 3) |
where I434 and I490 are the emission intensities at the characteristic wavelength of the gel phase (434 nm) and the liquid crystalline phase (490 nm), respectively. Excitation GP values were obtained from emission spectra obtained with an excitation wavelength of 360 nm.
Data analysis.
Intergroup comparisons were carried out using one-way ANOVA test with the values representing the mean ± SD of the total number of samples indicated in each figure legend.
RESULTS
Detergent solubility of the γM4 peptide in a POPC:SM:Chol 1:1:1 (“raft-containing membrane”) lipid system
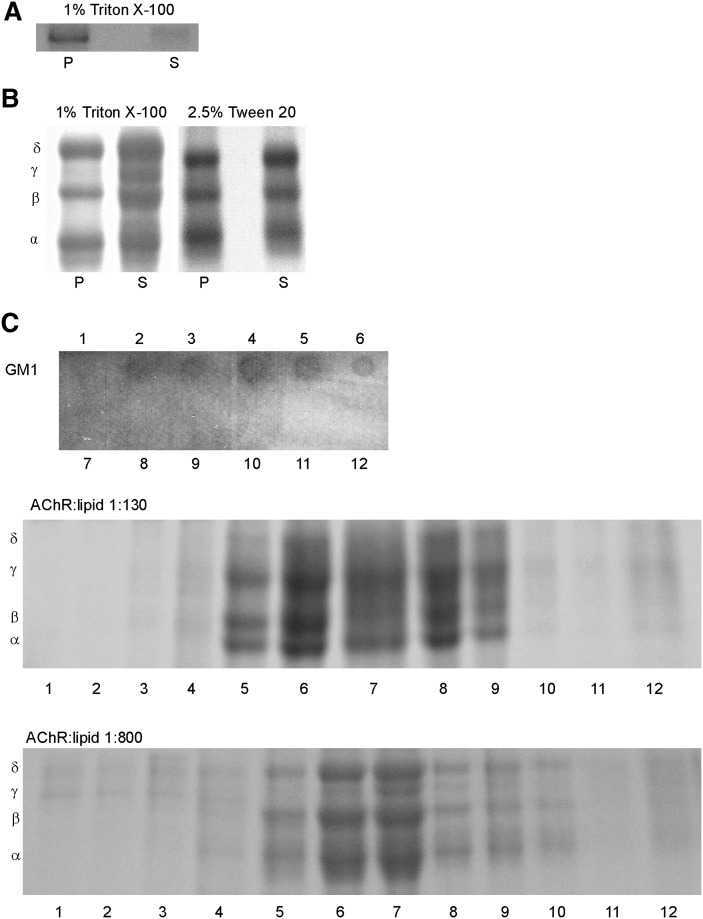
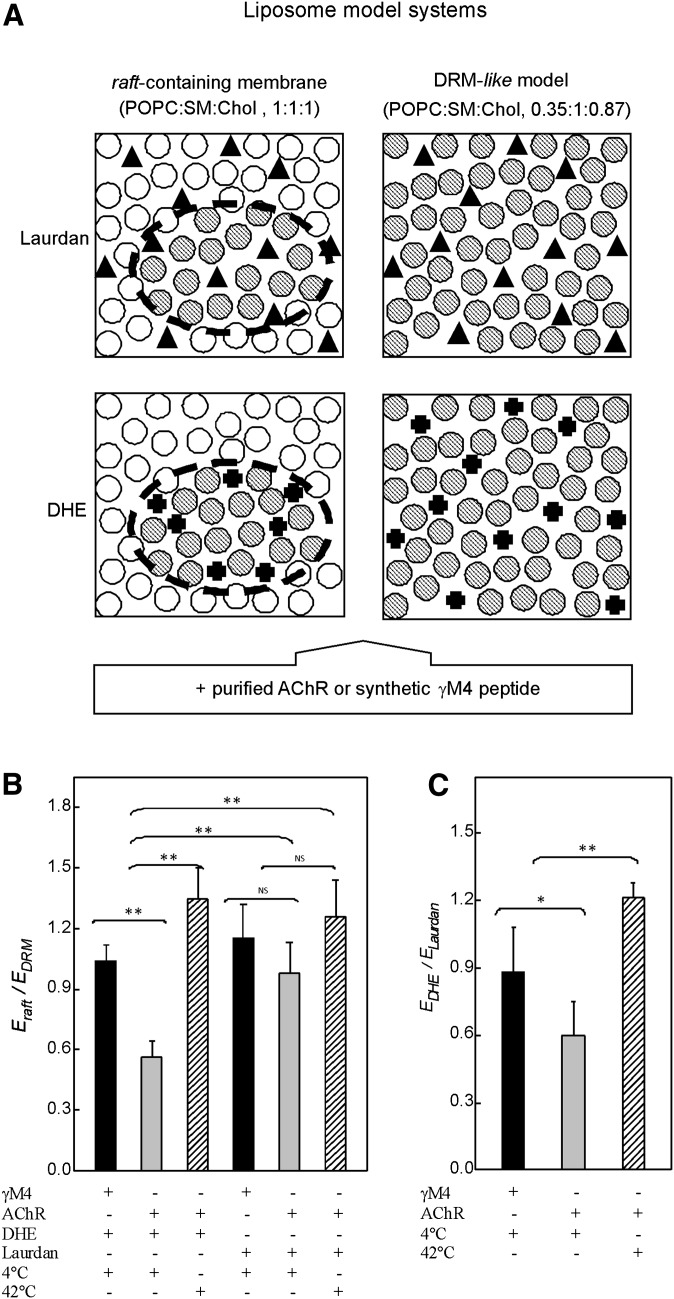
Previous studies from our laboratory showed that γM4 peptide displayed a linear α-helical configuration in the membrane (39) with the helix aligned at an angle of 15° with respect to the membrane normal (40). Fluorescence and molecular modeling studies showed lipid-dependent changes in the torsion angle of γM4, which we interpreted as resulting from a hydrophobic mismatch between peptide length and bilayer thickness (22). This is precisely one of the fundamental differences between the so-called raft and nonraft organization of lipids in a bilayer: the two types of domain differ in thickness (41, 42), and hence, the hydrophobic match (or mismatch) between membrane thickness and the length of the protein transmembrane domain could be a conditioning factor for preferential partitioning of the AChR peptide or the whole AChR into either domain. We investigated the sorting of the γM4 peptide into raft or nonraft domains having different membrane widths by reconstitution of γM4 in a model lipid system consisting of canonical raft-containing lipid mixtures (POPC:SM:Chol, 1:1:1 molar ratio) (8). The sample was submitted to Triton X-100 treatment at 4°C and subsequently separated into DSM and DRM fractions by centrifugation. Upon centrifugation, the resulting fractions were subjected to Tricine gel electrophoresis. The γM4 peptide was preferentially present in the DRM fraction (Fig. 1A and Table 1).
Fig. 1.
Liposomes of (A) γM4 synthetic peptide in POPC:SM:Chol (1:1:1) and (B) purified AChR reconstituted in a POPC:SM:Chol (1:1:1) at a 1:130 AChR:lipid molar ratio were subjected to 1% Triton X-100 or 2.5% Tween 20 extraction at 4°C and subsequent high-speed centrifugation as described in “Materials and Methods.” DSM and DRM fractions were collected as supernatant (S) and pellet (P). C: Liposomes of purified AChR reconstituted in POPC: SM:Chol (1:1:1) at a 1:130 and 1:800 AChR:lipid molar ratios were subjected to 1% Triton X-100 extraction and subsequent sucrose density separation as described in “Materials and Methods.” A representative GM1 dot blot of fractions 1–12 is shown, indicating that the DRMs were located in fractions 3–6. Panels A–C are representative Coomassie blue-stained SDS-PAGE of at least three independent experiments. AChR, nicotinic acetylcholine receptor; Chol, cholesterol; DRM, detergent-resistant membrane; DSM, detergent-soluble membrane; POPC, palmitoyloleylphosphatydilcholine; SM, brain sphingomyelin.
TABLE 1.
Presence of γM4 peptide and AChR in DRM and DSM fractions
| Liposome Model System | DRM Fraction (%) | DSM Fraction (%) |
|---|---|---|
| γM4 Peptide Distribution | ||
| γM4/PC:SM:Chol (1:1:1) | 75.9 ± 16.1 | 24.1 ± 16.1 |
| AChR Distribution | ||
| AChR/PC:SM:Chol (1:1:1) (AChR:lip 1:130) | 45.5 ± 7.4 | 54.0 ± 7.4 |
| AChR/PC:SM:Chol (1:1:1) (AChR:lip 1:130)a | 49.0 ± 12.7 | 51.5 ± 12.7 |
| AChR/PC:SM:Chol (1:1:1) (AChR:lip 1:400) | 55.8 ± 12.5 | 44.2 ± 12.5 |
| AChR/PC:SM:Chol (1:1:1) (AChR:lip 1:800)a | 43.8 ± 4.2 | 56.2 ± 4.2 |
| AChR/PC:SM:GM1:Chol (1:0.98:0.02:1) | 57.5 ± 9.9 | 42.5 ± 9.9 |
| AChR/PC:SM:GM1:Chol (1:0.98:0.02:1) + CTxB | 46.9 ± 6.6 | 53.1 ± 6.6 |
| AChR/PC:SM:GM1:Chol (1:0.98:0.02:1) + CTxB + anti-CTx | 33.5 ± 6.2b | 66.6 ± 6.2b |
| AChR:rapsyn (1:1)/PC:SM:Chol (1:1:1) | 50.8 ± 11.0 | 49.2 ± 11.0 |
| AChR:rapsyn (1:2)/ PC:SM:Chol (1:1:1) | 52.1 ± 14.1 | 47.9 ± 14.1 |
| AChR:rapsyn (1:4)/PC:SM:Chol (1:1:1) | 49.2 ± 14.6 | 50.8 ± 14.6 |
Values represent the mean ± S.D.; n = 4.
Abbreviations: AChR, nicotinic acetylcholine receptor; Chol, cholesterol; CTx, cholera toxin; CTxB, cholera toxin B-subunit; DRM, detergent-resistant membrane; DSM, detergent-soluble membrane; PC, phosphatydilcholine; SM, brain sphingomyelin.
DRM and DSM separated by sucrose density gradients.
Statistically significant differences with respect to the control condition (without CTxB and anti-CTx) (P < 0.01).
Detergent solubility of purified AChR in a POPC:SM:Chol 1:1:1 (“raft-containing membranes”) lipid system
To investigate whether the whole AChR macromolecule behaves in a manner similar to its most lipid-exposed transmembrane domain (i.e., preferentially associated with Chol-enriched domains), affinity purified AChR from T. californica was reconstituted into liposomes composed of POPC:SM:Chol (1:1:1 molar ratio) in a 1:130 molar ratio AChR:lipid stoichiometry. The liposomes were treated with 1% Triton X-100 at 4°C for 20 min followed by DRM and DSM separation. One point to be considered was whether detergent micelles containing large proteins were dense enough to pellet at high speeds mixing DSM and DRM fractions. Toward this end, we compared the results obtained at a high-speed centrifugation (Fig. 1B) with those obtained with sucrose density gradients of the Triton-treated proteoliposomes (see “Materials and Methods”). The resulting DSM and DRM fractions were subjected to SDS-PAGE electrophoresis (Fig. 1B, C). Independently of the separation method used, densitometric analysis of the bands corresponding to the receptor subunits showed that nearly half of total AChR was in the DRM fraction in both lipid:protein ratios, contrary to what was observed with the γM4 peptide, which was mainly in the DRM fraction (Table 1).
One outstanding characteristic of the mature neuromuscular junction is the high density of AChR molecules at the postsynaptic membrane, which leads to the formation of receptor supramolecular aggregates or “clusters” (reviewed in Refs. 1, 3). To test the effect of AChR concentration on the partition profile, the density gradient distribution of Triton-treated samples containing the tested receptor concentration (AChR:lipid molar ratio 1:130, “concentrated sample”) was compared with a sample containing AChR:lipid at a molar ratio of 1:800 (“diluted sample”). The distribution of the AChR into DSM and DRM in the two samples was similar (Fig. 1C and Table 1); furthermore, a sample having an intermediate ratio (AChR:lipid 1:400 molar ratio) showed an analogous concentration-independent behavior (Table 1). In the subsequent experiments, the DRM/DSM separation was achieved by high-speed centrifugation and performed with a 1:130 AChR:lipid molar ratio.
When liposomes with purified AChR in raft-containing membranes were extracted with the nonionic detergent Tween 20 (2.5% v/v final concentration), a slight increase in the amount of AChR in the DRM fraction was observed (from 45.5% to 56%, Fig. 1B). This difference may be due to the lesser solubilization strength of Tween 20 compared with Triton X-100 (43).
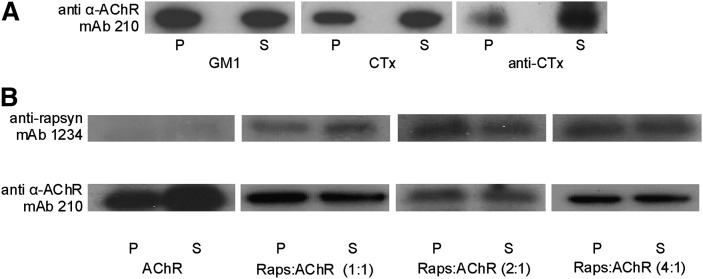
The AChR was also reconstituted in liposomes containing the ganglioside GM1, a purported component of lipid rafts in living cells (9, 44), at a concentration of 2% relative to that of total sphingolipids (POPC:SM:GM1:Chol, 1:0.98:0.02:1 molar ratio). In this case, similar amounts of AChR were found in the DRM and DSM fractions; i.e., no significant changes were observed in the AChR partition profile with the inclusion of GM1 (Fig. 2A and Table 1). A commonly used strategy to concentrate lipid rafts and, hence, to aggregate molecules present in these domains and facilitate their visualization is to crosslink GM1 with cholera toxin B subunit (CTxB) (45, 46). CTxB is pentavalent for GM1, and the patching of the ganglioside leads to formation of larger GM1 clusters (47, 48). Exposure of GM1-containing liposomes to CTxB, followed by Triton X-100 treatment and SDS electrophoresis showed that the aggregation of GM1 induced a slight increase in the proportion of AChR in the DSM fraction (Fig. 2A and Table 1). Addition of a secondary antibody against CTxB resulted in a significant increase in AChR in the DSM fraction (Fig. 2A and Table 1).
Fig. 2.
A: Immunoblot with mAb 210 (monoclonal antibody against the α subunit of the T. californica AChR) of DRM (P) and DSM (S) fractions obtained from purified AChR reconstituted in POPC:SM:Chol containing GM1 (i) without further treatment, (ii) treated with cholera toxin B subunit (CTxB) for GM1 crosslinking, and (iii) treated with CTxB for GM1 crosslinking followed by anti-cholera toxin antibody (anti-CTx) for crosslinking of CTxB. B: Immunoblot of DSM (S) and DRM (P) fractions obtained from purified AChR reconstituted in a POPC:SM:Chol 1:1:1 system in the presence of a rapsyn-enriched extract in three different rapsyn:AChR molar stoichiometries (1:1, 2:1, and 4:1). Blots were probed with mAb 1234 (monoclonal antibody against rapsyn) and mAb 210 (monoclonal antibody against the α subunit of the T. californica AChR). Panels A and B are representative of at least three independent experiments. AChR, nicotinic acetylcholine receptor; Chol, cholesterol; CTx, cholera toxin; CTxB, cholera toxin B-subunit; DRM, detergent-resistant membrane; DSM, detergent-soluble membrane; GM1, ganglioside GM1; POPC, palmitoyloleylphosphatydilcholine; SM, brain sphingomyelin.
AChR-rapsyn interaction and detergent solubility
Rapsyn is a myristoylated peripheral protein anchored to the cytoplasmatic face of the postsynaptic muscle membrane. It is purported to reside in raft domains and is ultimately responsible for AChR clustering. Porter et al. (49) indicated that radiolabeled rapsyn in alkaline extracts obtained from T. nobiliana membranes (28) binds tightly to pure liposomes. In this work, a similar fraction, highly enriched in rapsyn, was isolated from T. californica membranes by alkaline treatment and added during the reconstitution process, such that rapsyn was targeted to the inner face of the POPC:SM:Chol (1:1:1 molar ratio) liposomes. Three rapsyn:AChR mixtures with different molar stoichiometries were prepared (1:1, 2:1, and 4:1). The rapsyn-loaded liposomes were treated with 1% Triton X-100, and the DRM and DSM fractions were obtained by ultracentrifugation. Two different antibodies were used in the Western blot analysis of these fractions: anti-rapsyn and anti-AChR antibodies. Fig. 2B shows that the distribution of rapsyn and AChR was quite similar. Both rapsyn and the AChR localize in raft and nonraft domains, and the presence of rapsyn had no effect on the partition of the AChR protein in the DRM and DSM, as revealed by densitometric analysis of the Western blots (Table 1).
Lipid composition and biophysical properties of model membranes with different lipid compositions
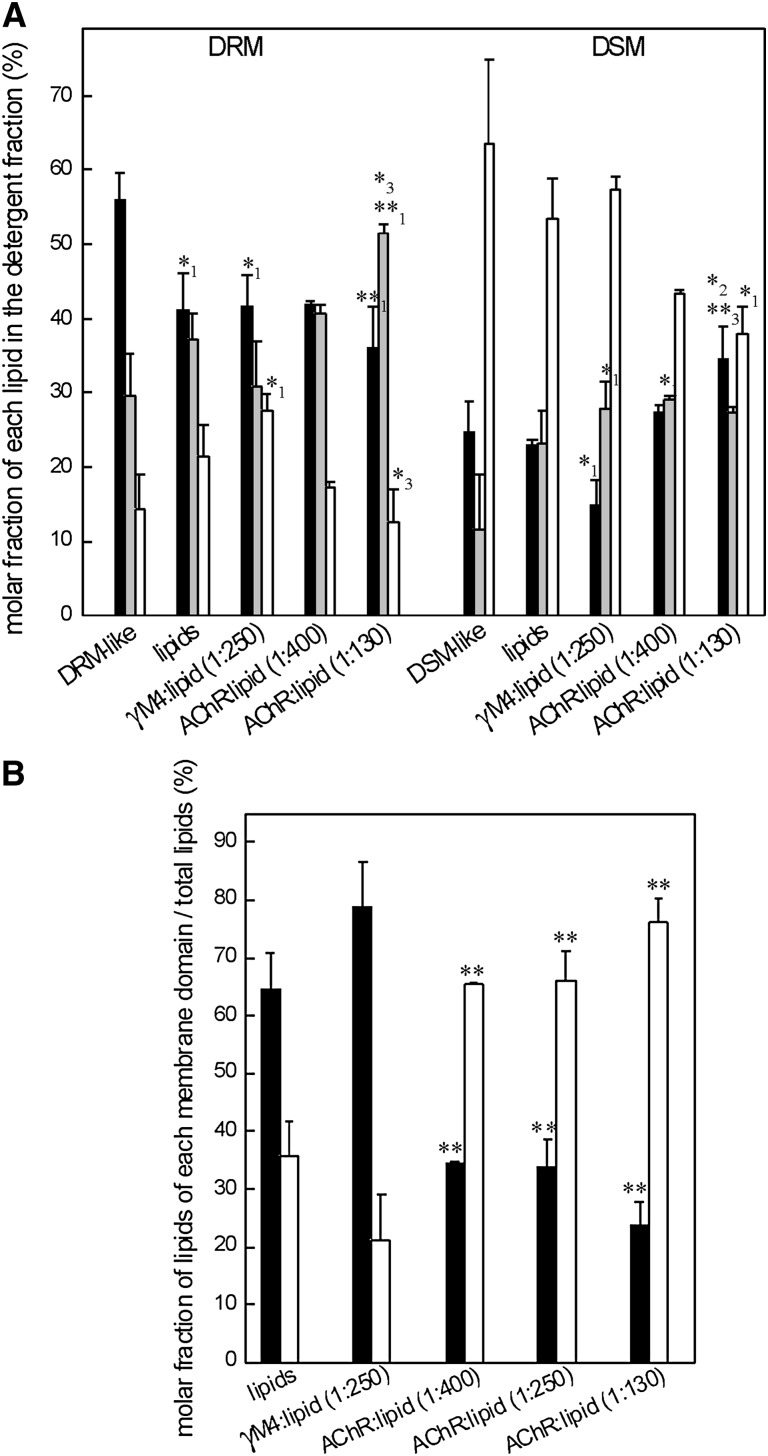
To investigate the lipid composition of the two lipid domains in the presence or absence of the γM4 peptide or the AChR, liposomes of POPC:SM:Chol (1:1:1 molar ratio) were subjected to Triton-X100 treatment at 4°C for 20 min, and DRM and DSM fractions were obtained. The lipid composition of both samples was analyzed as described in “Material and Methods” (a TLC plate of the lipid extracts stained with iodine vapor is shown in supplementary Fig. I). As expected, the lipid analyses revealed that the DRM fraction was highly enriched in Chol and SM (42, 50), whereas the DSM fraction was enriched in POPC (Fig. 3A). In the absence of protein, the lipid distribution of the DRM and DSM fractions was similar when the liposomes included GM1 (POPC:SM:GM1:Chol 1:0.98:0.02:1 molar ratio) (data not shown). Two additional samples were analyzed: one corresponding to a DRM-like model system [POPC:SM:Chol 0.35:1:0.87 molar ratio, without AChR (51)], and one to a DSM-like model system poor in SM and Chol [POPC:SM:Chol 1:0.11:0.24 molar ratio, without AChR (51)]. DRM-like liposomes were found to have practically no detergent-soluble phase. As expected, the insoluble phase was enriched in Chol and SM, showing a lipid composition slightly different from that of the DRM obtained from the raft-containing membrane after Triton X-100 treatment, although these differences were not statistically significant. The DSM-like system was essentially completely detergent-soluble and highly enriched in POPC, with a lipid composition comparable to that observed in the DSM obtained from the raft-containing membrane (Fig. 3A).
Fig. 3.
A: Molar fractions of Chol (filled bars), SM (gray bars), and PC (empty bars) in DRM and DSM fractions obtained from a DRM-like model and a DSM-like model (PC:SM:Chol 0.35:1:0.87 and 1:0.11:0.24 molar ratio, respectively, both without AChR) and from four different raft-containing membranes (POPC:SM:Chol, 1:1:1 molar ratio) in the absence of AChR (lipids), in the presence of γM4 peptide (1:250), and in the presence of different AChR:lipid molar ratios (1:400 and 1:130). Each bar corresponds to the average ± SD of at least three independent measurements. Statistically significant differences: *P < 0.05; **P < 0.01. Subscripts indicate the lipid system with which the comparison showed statistically significant differences: (1) DRM- or DSM-like system, (2) raft-containing membrane, and (3) γM4:lipid model. B: Molar fractions of the lipids of DRM (black columns) or DSM (white columns) with respect to the total lipid of different model membranes in the absence of AChR (lipids), in the presence of γM4 peptide (1:250), and in the presence of different AChR:lipid molar ratios (1:400, 1:250, and 1:130). Each bar corresponds to the average ± SD of at least three independent measurements. Statistically significant differences with respect to the lipid system without AChR: *P < 0.05; **P < 0.01. AChR, nicotinic acetylcholine receptor; Chol, cholesterol; DRM, detergent-resistant membrane; DSM, detergent-soluble membrane; POPC, palmitoyloleylphosphatydilcholine; SM, brain sphingomyelin.
Analysis of the lipid composition of DRM and DSM fractions obtained from a lipid model system that contained the γM4 peptide indicated that the molar fractions of Chol, SM, and PC were similar to the ones obtained from pure lipid model membranes, though Chol and SM showed differences with respect to the DRM-like and the DSM-like models (Fig. 3A). The lipid distribution in the soluble and insoluble fractions after detergent treatment in liposomes of POPC:SM:Chol (1:1:1 molar ratio) was also analyzed in the presence of AChR, at two different AChR:lipid molar ratios (1:130 and 1:400). The lipid composition of the DRM and DSM fractions was similar in both AChR:lipid molar ratios and was also similar to that corresponding to liposomes without AChR, indicating that the lipid composition of these phases depends mainly on the lipid properties and not on the presence of the protein (Fig. 3A). Some differences were observed when these results were compared with the DRM- and DSM-like phases. In the case of liposomes with 1:400 AChR:lipid molar ratio, the lipid composition of DRM and DSM fractions obtained was similar to that in the DRM- and DSM-like systems. In contrast, in the case of liposomes with an AChR:lipid ratio of 1:130, the lipid composition of the two phases differed from that of the DRM- and DSM-like systems (Fig. 3A).
Although the lipid composition of DRM and DSM fractions was not modified by the presence of either the whole AChR or the γM4 peptide in the liposome, the amount of each fraction was modified. The presence of the γM4 peptide caused an increment in the amount of DRM fraction with a concomitant decrease in the DSM fraction. On the other hand, the mere presence of the AChR induced a statistically significant increase in the relative proportion of the DSM fraction which did not depend on the lipid:AChR molar ratio (Fig. 3B). Thus, the γM4 peptide appears to behave as a DRM promoter and the AChR as a DRM disruptor.
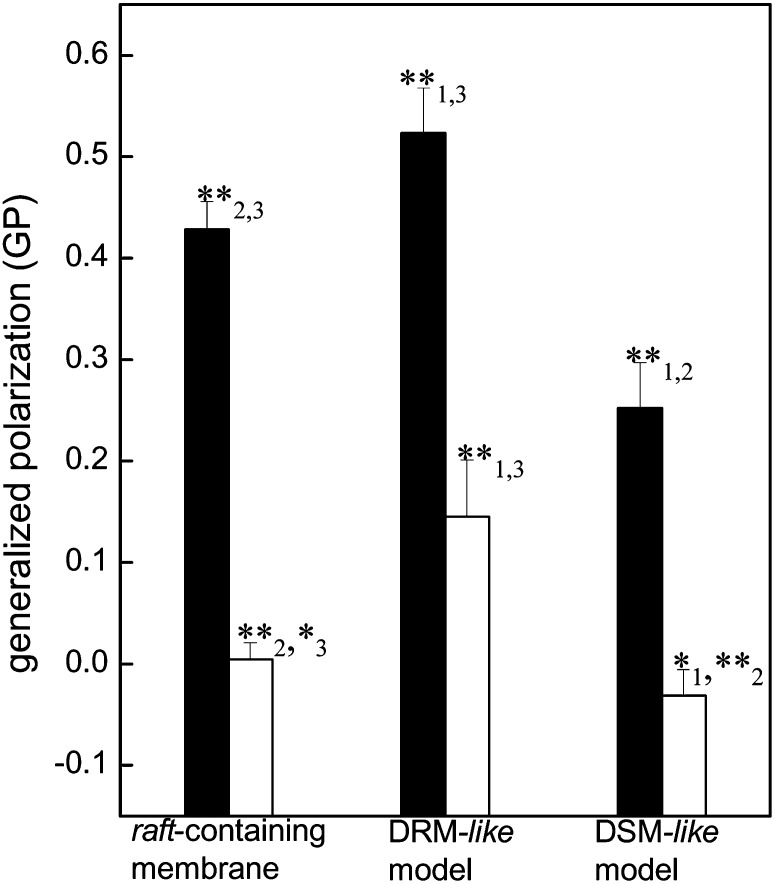
The so-called generalized polarization (GP) of the Laurdan probe provides an indirect measure of the degree of membrane order or membrane polarity (higher GP values, higher membrane order) (37, 38, 52, 53). Fig. 4 shows that at 4°C the lipid mixture corresponding to a raft-containing membrane exhibits an intermediate GP value between the nonraft, DSM-like model system (POPC:SM:Chol, 1:0.11:0.24) having the lowest membrane order, and the highly ordered DRM-like model system enriched in Chol and SM. The presence of the ganglioside GM1 in the raft-containing model did not induce changes in GP (data not shown). Increasing the temperature to 42°C produced a diminution of the GP values in the three systems tested, indicating a decrease in their membrane order; however, the lipid composition-related differences among the models persisted.
Fig. 4.
Excitation GP of Laurdan of purified AChR reconstituted in a raft-containing membrane (POPC:SM:Chol, 1:1:1 molar ratio), a DRM-like model (PC:SM:Chol, 0.35:1:0.87 molar ratio), and a DSM-like model (PC:SM:Chol, 1:0.11:0.24 molar ratio) at 4°C and 42°C (black and white columns, respectively). Each column corresponds to the average ± SD of at least four independent measurements. Statistically significant differences between black columns or white columns: *P < 0.05; **P < 0.01. The subindices indicate the lipid system with which the comparison showed statistically significant differences: (1) raft-containing membrane, (2) DRM-like model, and (3) DSM-like model. Although it is not shown in the figure, the differences between the black and white columns of each lipid system are statistically significant (P < 0.01). AChR, nicotinic acetylcholine receptor; Chol, cholesterol; DRM, detergent-resistant membrane; DSM, detergent-soluble membrane; GP, generalized polarization; PC, phosphatydilcholine; POPC, palmitoyloleylphosphatydilcholine; SM, brain sphingomyelin.
AChR-lipid interactions revealed by FRET
The study of lipid rafts is complicated because of their nanoscopic size and dynamic behavior. One of the most promising direct methodologies for studying raft domains is Förster resonance energy transfer (FRET), which has a nanometer scale resolution (54, 55). Here we complemented the results obtained using Triton X-100 with FRET measurements between the single-tryptophan intrinsic fluorescence of the γM4 peptide or the 51 tryptophans of the whole AChR acting as donor, and the fluorescent probes DHE or Laurdan as acceptors. Laurdan, a fluorescent free fatty acid analog, has no preference for any lipid phase (56, 57) and was used as a control fluorescent probe. Two lipid model systems were compared: a raft-containing model (POPC:SM:Chol, 1:1:1 molar ratio) and a DRM-like model system (POPC:SM:Chol, 0.35:1:0.87 molar ratio).
The efficiency of the FRET process (E) is related to the extent of the overlap between the emission spectrum of the donor and the absorption spectrum of the acceptor, the quantum yield of the donor, the relative orientation of the transition dipoles of both the donor and the acceptor, and—of most relevance here—the distance between the donor and the acceptor molecules (58). Here we used the ratio between two calculated E values (E-ratio) as a measure of the degree of localization of the AChR in the plane of the membrane. The ratio between the E obtained with the raft-containing model and with the DRM-like model (Eraft/EDRM) was calculated using DHE as the acceptor probe. In the DRM-like model, there is only one phase, and therefore, donor and acceptor molecules share the same membrane environment and are close to one another; consequently, the calculated E value is the highest possible value. What happens in the raft-containing model with coexistence of phases? As DHE is predominantly located in the raft domain, if the donor is also in this domain (i.e., donor and acceptor molecules share the domain), the calculated E value should be similar to that obtained with the DRM-like model, and hence, the E-ratio must be equal to or near unity. A significantly lower E-ratio should result if the donor is located in both lipid domains (i.e., a fraction of the donor molecules would not interact with the acceptor molecules) and a near-zero E-ratio if the donor partitions preferentially in the nonraft domain (see Fig. 5A). The Eraft/EDRM ratio obtained was somewhat higher than unity for the γM4 peptide and below unity for the AChR-containing system (Fig. 5B and Table 1). These results show that the γM4 peptide has a preference for raft (Chol-enriched) domains, whereas the AChR protein appears to display no preference for either domain. In another series of experiments, the two reconstituted lipid model systems, in this case containing AChR protein, were incubated at 42°C, at which temperature only a single phase occurs in the raft-containing model (59), resulting in an E-ratio close to unity. As shown in Fig. 5B, the E-ratio obtained at 42°C was significantly different from the one obtained at 4°C, suggesting that at higher temperatures DHE molecules establish contact with all the AChR macromolecules, as is the case with the DRM-like model. The E-ratio was also measured using Laurdan as acceptor probe instead of DHE. Laurdan molecules partition evenly in both lipid environments, and thus, the E-ratio is near unity independently of whether the intervening donor partitions into one or both phases.
Fig. 5.
A: Lipid model systems used in FRET studies: raft-containing membrane (POPC:SM:Chol, 1:1:1 molar ratio), where there is a coexistence of two different lipid domains (⊘, ○ for DRM and DSM-like domains, respectively), and DRM-like model (POPC:SM:Chol, 0.35:1:0.87 molar ratio) consisting of only one lipid domain (⊘). Laurdan fluorescent probe (▴), used as a control probe, shows no preferential partition into a particular domain. DHE (✚), used as a fluorescent Chol mimetic, labels DRM domains. FRET measurements were performed between the intrinsic fluorescence of the γM4 peptide or the AChR, donor molecules, and Laurdan or DHE as acceptor probes. B: Ratio of the FRET efficiencies between the intrinsic fluorescence of the purified AChR (or the γM4 peptide) as donor molecules and DHE (or Laurdan) as acceptor molecules obtained with the raft-containing membrane (Eraft) and with the DRM-like model (EDRM) at 4°C and 42°C. C: Ratio of the FRET efficiencies obtained between the intrinsic fluorescence of the purified AChR (or the γM4 peptide) as donor molecules and DHE (EDHE) and Laurdan (ELaurdan) as acceptor molecules in the raft-containing membrane at 4°C and 42°C. Each column corresponds to the average ± SD of at least four independent measurements. *, ** Statistically significant differences: *P < 0.05; **P < 0.01. NS (nonsignificant) differences: P < 0.05. AChR, nicotinic acetylcholine receptor; Chol, cholesterol; DHE, dehydroergosterol; DRM, detergent-resistant membrane; DSM, detergent-soluble membrane; E, energy transfer efficiency; FRET, Förster resonance energy transfer; POPC, palmitoyloleylphosphatidilcholine; SM, brain sphingomyelin.
A further parameter, the ratio of the E values obtained with the two probes (EDHE/ELaurdan) was applied next to the data obtained with the raft-containing model. Account was taken of the difference in quantum yield and in the overlap between the absorption spectra of each of the two acceptor molecules with the fluorescence emission spectrum of the donor, and a correction for these differences was applied. The two donor-acceptor pairs were assumed to have the same probability of FRET in the DRM-like system, and hence, the EDHE/ELaurdan ratio in this system should be equal to unity. The two calculated E values differed, as a consequence of the different characteristics of the two donor-acceptor pairs; hence, the E-ratio was divided by itself to obtain a value of unity. This E-ratio was subsequently used as a correction factor for the raft-containing model. In this system, when the donor molecules reside in both phases, EDHE should be close to half of ELaurdan, and EDHE/ELaurdan about 0.5; if the donor molecules localize in raft domains, EDHE would be significantly higher than ELaurdan, and their ratio higher than one. If on the other hand, the donors prefer the nonraft domains (see Fig. 5A for an extended explanation), the ratio will be near zero. The near-unity EDHE/ELaurdan ratio obtained for the γM4 is in agreement with the information derived from the Eraft/EDRM ratio and also with the data obtained in the detergent-extraction experiments, indicating that the γM4 peptide localizes preferentially in Chol-enriched domains. In the case of the whole AChR, the E-ratio was significantly lower than that obtained with γM4, indicating that the AChR has no preferential affinity for a Chol-enriched domain (Fig. 5C and Table 1). When the measurements with the raft-containing model were taken at 42°C, a near-unity EDHE/ELaurdan ratio was observed, indicating that at higher temperatures both DHE and Laurdan establish contact with all the AChR molecules and the Chol-enriched domains disappear.
DISCUSSION
The aim of this study was to characterize the relationship between the AChR protein and its lipid environment with regard to the possible occurrence of the receptor in laterally segregated lipid domains in the membrane. For this purpose, we chose the best-characterized AChR, that from Torpedo electric tissue. The whole AChR was reconstituted into model membranes with defined proportions of Chol, SM, and PC, and they were analyzed by biochemical and biophysical techniques. The synthetic γM4 peptide was similarly studied to learn about the contribution of the lipid-contacting TM outer ring to the putative lateral segregation of the AChR in the membrane.
Biochemical analysis showed that the γM4 peptide reconstituted in equimolar POPC:SM:Chol mixtures was preferentially partitioned in the DRM fraction (Fig. 1A), whereas the whole AChR showed no preferential partition between DRM and DSM fractions (Fig. 1B, C). Although cold Triton X-100 insolubility is in general an accepted criterion to assess phase state in model membranes (60), extrapolation of the phase state in native biomembranes from the detergent solubility data is more controversial. For this reason, the Triton X-100 experiments were challenged using another strategy that does not involve physical separation of lipid fractions. We employed FRET experiments using DHE, a Chol-mimetic probe, as acceptor of the intrinsic fluorescence of the whole AChR or the γM4 peptide to measure domain coexistence in model systems. Various controls were necessary: liposomes exhibiting only one lipid domain, use of a fluorescence probe that partitions in both lo and ld domains, exposure to different temperatures, etc. (Fig. 5). FRET experiments unambiguously pointed to the preferential localization of the γM4 peptide in Chol-enriched domains, in agreement with the results of biochemical experiments. In contrast, the whole AChR in a model system with lipid domain coexistence (4°C) exhibited no preferential affinity for a Chol-enriched domain, as was also observed in the detergent experiments. At 42°C, the model system has only one mixed lipid phase, and donor and acceptor molecules share the same environment, as reflected in the FRET ratios (Fig. 5B, C). In the experiments of Zhu et al. (19), under “basal” conditions the major pool of AChR is associated with nonraft membrane domains, and certain stimuli like neural agrin translocate a fraction of the AChR and MuSK into lipid rafts.
Previous work from our laboratory showed that the orientation of the γM4 peptide in the membrane is sensitive to bilayer thickness and lipid composition, its tilt angle varying as a function of bilayer width and Chol content (22). In subsequent work, we found that patches of γM4 peptide are formed in a Chol-enriched lo phase in binary lipid model systems (61, 62). In all these cases, we postulated that the adaptation of the peptide to bilayer thickness entailed overcoming the hydrophobic mismatch. DRM fractions prepared from the 1:1:1 POPC:SM:Chol system containing the peptide are enriched in Chol and SM (Fig. 3A), in agreement with other studies (50). Moreover, lipids present in DRM fractions appear to occur in the lo phase (57, 63).
GM1 monosialoganglioside contains a lipid tail that tethers it to the outer leaflet of the cell membrane, and its presence has long been associated with lipid rafts. Previous results from our laboratory (64) showed that GM1 is one of the major gangliosides in AChR-rich membranes purified from T. marmorata electric tissue and that GM1 colocalizes with the ventral, innervated membrane of the electrocytes. GM1 has also been shown to colocalize with AChR and Chol at the NMJ of rat sternomastoid (65) and rat diaphragm muscles and C2C12 myotubes (19). Replacement of 2% SM by GM1 in the 1:1:1 POPC:SM:Chol system produced no statistically significant effects on the partition behavior of the AChR in DRM and DSM fractions (Table 1). Lipid clustering has been used as a tool to characterize the possible association of proteins and lipids with rafts in model membranes (46, 66) and cells (45, 67). In this study, GM1 crosslinking by CTxB and an antibody against CTx increased the amount of AChR in the DSM fraction (Fig. 2A and Table 1). One possibility is that CTxB crosslinking decreases AChR affinity for DRM domains. Alternatively, steric repulsion could prevent binding of the AChR to aggregated GM1-containing domains. Note that the affinity and selectivity of gangliosides for the AChR in its native membrane is rather low (68).
The mature neuromuscular junction is characterized by the high density of AChR molecules at the postsynaptic membrane, associated with the occurrence of large supramolecular clusters (reviewed in Refs. 1, 3). Here we show that the AChR alone, in a highly concentrated sample, does not appear to form high-order aggregates (Fig. 1C). It has been postulated that the clustering process is a mechanism that involves extracellular, cytoplasmic-facing, and integral membrane proteins, and that the nonreceptor protein rapsyn plays a key role in AChR clustering in the postsynaptic membrane. Rapsyn has been postulated to be raft-associated, together with the AChR, in late exocytic compartments (16) and at the cell membrane (17–19). Rapsyn is myristoylated (29), and this modification is required for membrane binding (69). Marchand et al. (16) postulated that this protein modification could be responsible for its association with lipid rafts, as many acylated proteins, some of them myristoylated, are raft-associated. Liposomes of 1:1:1 POPC:SM:Chol with AChR and a rapsyn-enriched extract with different rapsyn:AChR proportions (1:1, 2:1, 4:1) were subjected to detergent extraction. Independently of their relative amounts, after Triton X-100 treatment, rapsyn and AChR partitioned together showed no significant differences with respect to the same system without rapsyn (Fig. 2B and Table 1). Therefore, incorporation of rapsyn to the system produced no effect on AChR-DRM association.
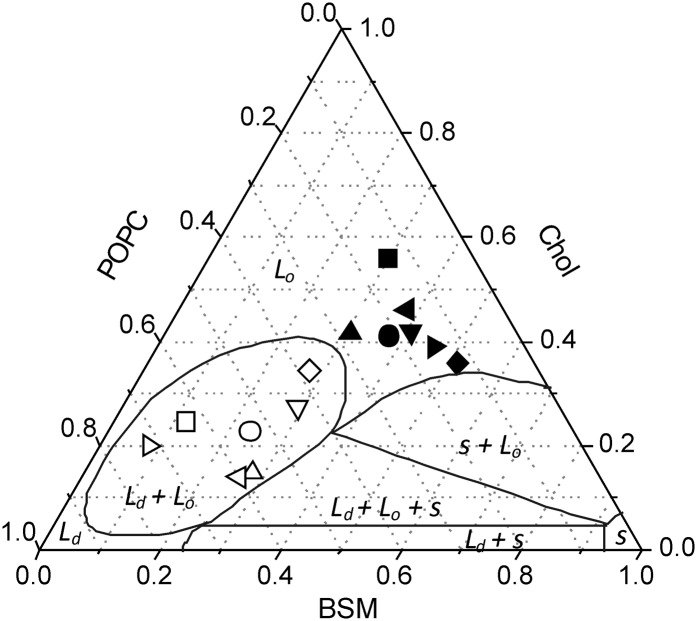
The distribution of lipids between DRMs and DSMs after detergent extraction of 1:1:1 POPC:SM:Chol liposomes in the absence of protein revealed that the DRM fraction is highly enriched in Chol and SM, whereas the DSM fraction is highly enriched in POPC (Fig. 3A), in agreement with previous studies on model systems (42, 50, 51). When purified AChR or γM4 was included in different lipid:protein ratios in the reconstituted system, no significant effects were apparent on the lipid composition of DRM and DSM fractions, suggesting that lipid composition is independent of protein content. Recent studies using lipid vesicles having the same composition as in the present study (Chol/SM/POPC) reported the occurrence of (ld + lo) domain coexistence (70, 71) or only one type of domain (72). It is interesting to compare the DRM and DSM lipid compositions obtained from the different liposomes studied (Fig. 3A) with the phase diagram postulated by Pokorny et al. (70) (Fig. 6), bearing in mind that detergent extraction at 4°C results in the formation of a quaternary phase system. As shown in Fig. 6, the composition of the DRM fractions obtained for all the systems tested fall within the lo domain region, as other DRMs obtained from liposomes formed with different types of lipids (bovine liver PC, Chol, and SM (50), and DOPC, Chol, and SM (51)). In contrast, all the DSM fractions tested fall within the ld + lo coexistence region, suggesting that this fraction is not a homogeneous phase.
Fig. 6.
Gibbs triangle showing DRM (solid) and DSM (open) compositions for all the systems tested in this work: DRM-like (▪), DSM-like (□), different raft-containing membranes (i) in the absence of AChR (•, ○), (ii) in the presence of γM4 peptide (1:250 ▴, △), and (iii) in the presence of different AChR:lipid molar ratios (1:400, ▾, ▽; and 1:130, ♦, ⋄). The DRM and DSM lipid compositions proposed in the work of Schroeder et al. (50) (◂, ◃) and McIntosh et al. (51) (▸, ▹) are indicated. Also shown are the lines that divide the phase diagram in the different pure phases (solid, s; liquid-ordered, lo; and liquid disordered, ld) and the different coexistence regions at 22°C (s + lo; ld + lo + s; ld + s; and ld + lo) proposed by Pokorny et al. (70). AChR, nicotinic acetylcholine receptor; BSM, brain sphingomyelin; Chol, cholesterol; DRM, detergent-resistant membrane; DSM, detergent-soluble membrane; POPC, palmitoyloleylphosphatydilcholine.
To sum up, the γM4 segment of the paradigm Torpedo AChR protein, the lipid-contacting TM segment, prefers ordered lipid domains, whereas the whole AChR displays no preference for a liquid-ordered or disordered lipid domain. Furthermore, the AChR behaves in vitro as a DRM-disruptor, its presence inducing a significant diminution in the amount of the DRM fraction. We can further infer that the predilection of the AChR for a given lipid domain in its natural milieu cannot be solely ascribed to the physicochemical properties of the two partners or to the interactions between the protein TM domain and the lipid milieu.
Supplementary Material
Footnotes
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure.
This work was supported by grants from the Agencia Nacional de Promoción Científica y Técnológica (FONCYT) (S.S.A. and F.J.B.), the Universidad Nacional del Sur (F.J.B.), and the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (F.J.B.).
Abbreviations:
- AChR
- nicotinic acetylcholine receptor
- CDx
- methyl-β-cyclodextrin
- Chol
- cholesterol
- CTx
- cholera toxin
- CTxB
- cholera toxin B-subunit
- DHE
- dehydroergosterol
- DOPC
- dioleoylphosphatidylcholine
- DRM
- detergent-resistant membrane
- DSM
- detergent-soluble membrane
- E
- energy transfer efficiency
- FRET
- Förster resonance energy transfer
- GM1
- ganglioside GM1
- GP
- generalized polarization
- NMJ
- neuromuscular junction
- PC
- phosphatydilcholine
- POPA
- palmitoyloleylphosphatidic acid
- POPC
- palmitoyloleylphosphatydilcholine
- SM
- sphingomyelin
- TM
- transmembrane
REFERENCES
- 1.Barrantes F. J. 2004. Structural basis for lipid modulation of nicotinic acetylcholine receptor function. Brain Res. Brain Res. Rev. 47: 71–95. [DOI] [PubMed] [Google Scholar]
- 2.Barrantes F. J. 2003. Modulation of nicotinic acetylcholine receptor function through the outer and middle rings of transmembrane domains. Curr. Opin. Drug Discov. Devel. 6: 620–632. [PubMed] [Google Scholar]
- 3.Sanes J. R., Lichtman J. W. 2001. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2: 791–805. [DOI] [PubMed] [Google Scholar]
- 4.Moransard M., Borges L. S., Willmann R., Marangi P. A., Brenner H. R., Ferns M. J., Fuhrer C. 2003. Agrin regulates rapsyn interaction with surface acetylcholine receptors, and this underlies cytoskeletal anchoring and clustering. J. Biol. Chem. 278: 7350–7359. [DOI] [PubMed] [Google Scholar]
- 5.Borges L. S., Ferns M. 2001. Agrin-induced phosphorylation of the acetylcholine receptor regulates cytoskeletal anchoring and clustering. J. Cell Biol. 153: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrantes F. J. 2007. Cholesterol effects on nicotinic acetylcholine receptor. J. Neurochem. 103 (Suppl. 1): 72–80. [DOI] [PubMed] [Google Scholar]
- 7.Mayor S., Rao M. 2004. Rafts: scale-dependent, active lipid organization at the cell surface. Traffic. 5: 231–240. [DOI] [PubMed] [Google Scholar]
- 8.Brown D. A., Rose J. K. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 68: 533–544. [DOI] [PubMed] [Google Scholar]
- 9.Simons K., Ikonen E. 1997. Functional rafts in cell membranes. Nature. 387: 569–572. [DOI] [PubMed] [Google Scholar]
- 10.Brown D. A., London E. 1998. Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 164: 103–114. [DOI] [PubMed] [Google Scholar]
- 11.Janes P. W., Ley S. C., Magee A. I., Kabouridis P. S. 2000. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin. Immunol. 12: 23–34. [DOI] [PubMed] [Google Scholar]
- 12.Tansey M. G., Baloh R. H., Milbrandt J., Johnson E. M., Jr 2000. GFRalpha-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron. 25: 611–623. [DOI] [PubMed] [Google Scholar]
- 13.Ma L., Huang Y. Z., Pitcher G. M., Valtschanoff J. G., Ma Y. H., Feng L. Y., Lu B., Xiong W. C., Salter M. W., Weinberg R. J., et al. 2003. Ligand-dependent recruitment of the ErbB4 signaling complex into neuronal lipid rafts. J. Neurosci. 23: 3164–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki S., Numakawa T., Shimazu K., Koshimizu H., Hara T., Hatanaka H., Mei L., Lu B., Kojima M. 2004. BDNF-induced recruitment of TrkB receptor into neuronal lipid rafts: roles in synaptic modulation. J. Cell Biol. 167: 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen J. A., Halverson-Tamboli R. A., Rasenick M. M. 2007. Lipid raft microdomains and neurotransmitter signalling. Nat. Rev. Neurosci. 8: 128–140. [DOI] [PubMed] [Google Scholar]
- 16.Marchand S., Devillers-Thiery A., Pons S., Changeux J. P., Cartaud J. 2002. Rapsyn escorts the nicotinic acetylcholine receptor along the exocytic pathway via association with lipid rafts. J. Neurosci. 22: 8891–8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stetzkowski-Marden F., Gaus K., Recouvreur M., Cartaud A., Cartaud J. 2006. Agrin elicits membrane lipid condensation at sites of acetylcholine receptor clusters in C2C12 myotubes. J. Lipid Res. 47: 2121–2133. [DOI] [PubMed] [Google Scholar]
- 18.Willmann R., Pun S., Stallmach L., Sadasivam G., Santos A. F., Caroni P., Fuhrer C. 2006. Cholesterol and lipid microdomains stabilize the postsynapse at the neuromuscular junction. EMBO J. 25: 4050–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu D., Xiong W. C., Mei L. 2006. Lipid rafts serve as a signaling platform for nicotinic acetylcholine receptor clustering. J. Neurosci. 26: 4841–4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campagna J. A., Fallon J. 2006. Lipid rafts are involved in C95 (4,8) agrin fragment-induced acetylcholine receptor clustering. Neuroscience. 138: 123–132. [DOI] [PubMed] [Google Scholar]
- 21.Pun S., Sigrist M., Santos A. F., Ruegg M. A., Sanes J. R., Jessell T. M., Arber S., Caroni P. 2002. An intrinsic distinction in neuromuscular junction assembly and maintenance in different skeletal muscles. Neuron. 34: 357–370. [DOI] [PubMed] [Google Scholar]
- 22.Antollini S. S., Xu Y., Jiang H., Barrantes F. J. 2005. Fluorescence and molecular dynamics studies of the acetylcholine receptor gammaM4 transmembrane peptide in reconstituted systems. Mol. Membr. Biol. 22: 471–483. [DOI] [PubMed] [Google Scholar]
- 23.Barrantes F. J. 1982. Oligomeric forms of the membrane-bound acetylcholine receptor disclosed upon extraction of the Mr 43,000 nonreceptor peptide. J. Cell Biol. 92: 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antollini S. S., Barrantes F. J. 2007. Laurdan studies of membrane lipid-nicotinic acetylcholine receptor protein interactions. Methods Mol. Biol. 400: 531–542. [DOI] [PubMed] [Google Scholar]
- 25.daCosta C. J., Wagg I. D., McKay M. E., Baenziger J. E. 2004. Phosphatidic acid and phosphatidylserine have distinct structural and functional interactions with the nicotinic acetylcholine receptor. J. Biol. Chem. 279: 14967–14974. [DOI] [PubMed] [Google Scholar]
- 26.daCosta C. J., Ogrel A. A., McCardy E. A., Blanton M. P., Baenziger J. E. 2002. Lipid-protein interactions at the nicotinic acetylcholine receptor. A functional coupling between nicotinic receptors and phosphatidic acid-containing lipid bilayers. J. Biol. Chem. 277: 201–208. [DOI] [PubMed] [Google Scholar]
- 27.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 28.Neubig R. R., Krodel E. K., Boyd N. D., Cohen J. B. 1979. Acetylcholine and local anesthetic binding to Torpedo nicotinic postsynaptic membranes after removal of nonreceptor peptides. Proc. Natl. Acad. Sci. USA. 76: 690–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musil L. S., Carr C., Cohen J. B., Merlie J. P. 1988. Acetylcholine receptor-associated 43K protein contains covalently bound myristate. J. Cell Biol. 107: 1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayuyan A. G., Cohen F. S. 2008. Raft composition at physiological temperature and pH in the absence of detergents. Biophys. J. 94: 2654–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong J., Briggs M. M., Mlaver D., Vidal A., McIntosh T. J. 2009. Sorting of lens aquaporins and connexins into raft and nonraft bilayers: role of protein homo-oligomerization. Biophys. J. 97: 2493–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 33.Wessel D., Flugge U. I. 1984. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138: 141–143. [DOI] [PubMed] [Google Scholar]
- 34.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 35.Rouser G., Fkeischer S., Yamamoto A. 1970. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 5: 494–496. [DOI] [PubMed] [Google Scholar]
- 36.Förster T. 1948. Intermolecular energy migration and fluorescence Ann. Phys. (Leipzig). 2: 55–75. [Google Scholar]
- 37.Parasassi T., De Stasio G., d'Ubaldo A., Gratton E. 1990. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys. J. 57: 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parasassi T., De Stasio G., Ravagnan G., Rusch R. M., Gratton E. 1991. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 60: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrantes F. J., Antollini S. S., Blanton M. P., Prieto M. 2000. Topography of nicotinic acetylcholine receptor membrane-embedded domains. J. Biol. Chem. 275: 37333–37339. [DOI] [PubMed] [Google Scholar]
- 40.Williamson P. T., Zandomeneghi G., Barrantes F. J., Watts A., Meier B. H. 2005. Structural and dynamic studies of the gamma-M4 trans-membrane domain of the nicotinic acetylcholine receptor. Mol. Membr. Biol. 22: 485–496. [DOI] [PubMed] [Google Scholar]
- 41.Sprong H., van der Sluijs P., Van Meer G. 2001. How proteins move lipids and lipids move proteins. Nat. Rev. Mol. Cell Biol. 2: 504–513. [DOI] [PubMed] [Google Scholar]
- 42.Gandhavadi M., Allende D., Vidal A., Simon S. A., McIntosh T. J. 2002. Structure, composition, and peptide binding properties of detergent soluble bilayers and detergent resistant rafts. Biophys. J. 82: 1469–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babiychuk E. B., Draeger A. 2006. Biochemical characterization of detergent-resistant membranes: a systematic approach. Biochem. J. 397: 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simons K., Toomre D. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1: 31–39. [DOI] [PubMed] [Google Scholar]
- 45.Harder T., Scheiffele P., Verkade P., Simons K. 1998. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141: 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammond A. T., Heberle F. A., Baumgart T., Holowka D., Baird B., Feigenson G. W. 2005. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc. Natl. Acad. Sci. USA. 102: 6320–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merritt E. A., Sarfaty S., van den Akker F., L'Hoir C., Martial J. A., Hol W. G. 1994. Crystal structure of cholera toxin B-pentamer bound to receptor GM1 pentasaccharide. Protein Sci. 3: 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spiegel S., Kassis S., Wilchek M., Fishman P. H. 1984. Direct visualization of redistribution and capping of fluorescent gangliosides on lymphocytes. J. Cell Biol. 99: 1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porter S., Froehner S. C. 1985. Interaction of the 43K protein with components of Torpedo postsynaptic membranes. Biochemistry. 24: 425–432. [DOI] [PubMed] [Google Scholar]
- 50.Schroeder R., London E., Brown D. 1994. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc. Natl. Acad. Sci. USA. 91: 12130–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McIntosh T. J., Vidal A., Simon S. A. 2003. Sorting of lipids and transmembrane peptides between detergent-soluble bilayers and detergent-resistant rafts. Biophys. J. 85: 1656–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antollini S. S., Barrantes F. J. 1998. Disclosure of discrete sites for phospholipid and sterols at the protein-lipid interface in native acetylcholine receptor-rich membrane. Biochemistry. 37: 16653–16662. [DOI] [PubMed] [Google Scholar]
- 53.Antollini S. S., Barrantes F. J. 2002. Unique effects of different fatty acid species on the physical properties of the Torpedo acetylcholine receptor membrane. J. Biol. Chem. 277: 1249–1254. [DOI] [PubMed] [Google Scholar]
- 54.Brown A. C., Towles K. B., Wrenn S. P. 2007. Measuring raft size as a function of membrane composition in PC-based systems: part 1–binary systems. Langmuir. 23: 11180–11187. [DOI] [PubMed] [Google Scholar]
- 55.Brown A. C., Towles K. B., Wrenn S. P. 2007. Measuring raft size as a function of membrane composition in PC-based systems: part II–ternary systems. Langmuir. 23: 11188–11196. [DOI] [PubMed] [Google Scholar]
- 56.Bagatolli L. A., Parasassi T., Fidelio G. D., Gratton E. 1999. A model for the interaction of 6-lauroyl-2-(N,N-dimethylamino)naphthalene with lipid environments: implications for spectral properties. Photochem. Photobiol. 70: 557–564. [PubMed] [Google Scholar]
- 57.Dietrich C., Bagatolli L. A., Volovyk Z. N., Thompson N. L., Levi M., Jacobson K., Gratton E. 2001. Lipid rafts reconstituted in model membranes. Biophys. J. 80: 1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lakowicz J. R. 1999. Principles of Fluorescence Spectroscopy. 2nd ed Kluwer Academic/Plenum Publishers, New York, NY. [Google Scholar]
- 59.De Almeida R. F., Fedorov A., Prieto M. 2003. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys. J. 85: 2406–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown D. A. 2006. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda). 21: 430–439. [DOI] [PubMed] [Google Scholar]
- 61.De Almeida R. F., Loura L. M., Prieto M., Watts A., Fedorov A., Barrantes F. J. 2006. Structure and dynamics of the gammaM4 transmembrane domain of the acetylcholine receptor in lipid bilayers: insights into receptor assembly and function. Mol. Membr. Biol. 23: 305–315. [DOI] [PubMed] [Google Scholar]
- 62.De Almeida R. F., Loura L. M., Prieto M., Watts A., Fedorov A., Barrantes F. J. 2004. Cholesterol modulates the organization of the gammaM4 transmembrane domain of the muscle nicotinic acetylcholine receptor. Biophys. J. 86: 2261–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmed S. N., Brown D. A., London E. 1997. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 36: 10944–10953. [DOI] [PubMed] [Google Scholar]
- 64.Marcheselli V., Daniotti J. L., Vidal A. C., Maccioni H., Marsh D., Barrantes F. J. 1993. Gangliosides in acetylcholine receptor-rich membranes from Torpedo marmorata and Discopyge tschudii. Neurochem. Res. 18: 599–603. [DOI] [PubMed] [Google Scholar]
- 65.Pato C., Stetzkowski-Marden F., Gaus K., Recouvreur M., Cartaud A., Cartaud J. 2008. Role of lipid rafts in agrin-elicited acetylcholine receptor clustering. Chem. Biol. Interact. 175: 64–67. [DOI] [PubMed] [Google Scholar]
- 66.Kalvodova L., Kahya N., Schwille P., Ehehalt R., Verkade P., Drechsel D., Simons K. 2005. Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. J. Biol. Chem. 280: 36815–36823. [DOI] [PubMed] [Google Scholar]
- 67.Mitchell J. S., Brown W. S., Woodside D. G., Vanderslice P., McIntyre B. W. 2009. Clustering T-cell GM1 lipid rafts increases cellular resistance to shear on fibronectin through changes in integrin affinity and cytoskeletal dynamics. Immunol. Cell Biol. 87: 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mantipragada S. B., Horvath L. I., Arias H. R., Schwarzmann G., Sandhoff K., Barrantes F. J., Marsh D. 2003. Lipid-protein interactions and effect of local anesthetics in acetylcholine receptor-rich membranes from Torpedo marmorata electric organ. Biochemistry. 42: 9167–9175. [DOI] [PubMed] [Google Scholar]
- 69.Ramarao M. K., Cohen J. B. 1998. Mechanism of nicotinic acetylcholine receptor cluster formation by rapsyn. Proc. Natl. Acad. Sci. USA. 95: 4007–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pokorny A., Yandek L. E., Elegbede A. I., Hinderliter A., Almeida P. F. 2006. Temperature and composition dependence of the interaction of delta-lysin with ternary mixtures of sphingomyelin/cholesterol/POPC. Biophys. J. 91: 2184–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frazier M. L., Wright J. R., Pokorny A., Almeida P. F. 2007. Investigation of domain formation in sphingomyelin/cholesterol/POPC mixtures by fluorescence resonance energy transfer and Monte Carlo simulations. Biophys. J. 92: 2422–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Veatch S. L., Keller S. L. 2003. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 85: 3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.