Abstract
Hypoxia of the renal medulla is a possible precursor to the onset of acute renal failure in humans and therefore an understanding of the factors influencing the oxygenation status within the renal medulla is very important. Blood oxygenation level-dependent (BOLD) magnetic resonance imaging (MRI) has been shown to non-invasively evaluate intra-renal oxygenation levels of the renal medulla in humans. A newly implemented three-dimensional (3-D) multiple gradient-recalled echo sequence, which permits examination of temporal responses to physiological or pharmacological stimuli, was used to monitor changes in intra-renal oxygenation status during water diuresis. Five healthy, young subjects (22±1.2 years) took part in the study. BOLD MRI data were acquired before and after water loading. Studies were repeated on a separate day after the subjects were pretreated with naproxen. Water diuresis significantly improved renal medullary oxygenation levels in all subjects (pre-waterload =30.3 1/s vs post-waterload 22.8 1/s); however, the temporal response was found to be subject dependent. In the presence of cyclooxygenase (COX) inhibition by naproxen, the improvement in oxygenation during water diuresis was completely abolished (pre-waterload =27.5 1/s vs post-waterload 28.5 1/s). Monitoring of temporal responses for the first time during water loading allowed for an appreciation of subject dependence. Comparison of the temporal response in terms of slopes demonstrated a significant difference between the waterload studies with and without naproxen (with naproxen =0.056 1/(s min) vs without naproxen =0.25 1/(s min)). The observed effects of naproxen were consistent with previous findings with COX inhibition.
Keywords: BOLD MRI, waterload, 3-D mGRE, oxygenation, 3.0 T, naproxen
The human kidneys, together weighing less than 1% of the total body mass, receive 25% of the cardiac output.1,2 Most of the blood passing through the kidneys is directed to the cortical regions to facilitate glomerular filtration. On the other hand, the medulla operates in a hypoxic environment. This is mainly caused by the countercurrent arrangement of tubules and vessels, which is necessary for the preservation of osmotic gradients that aid in efficient water conservation and urinary concentration.1–6 Loop diuretics (e.g. furosemide) have previously shown the ability to alleviate renal medullary hypoxia, presumably by inhibiting active transport reabsorption along the medullary thick ascending limb.1,7,8 The technique of blood oxygenation level-dependent (BOLD) magnetic resonance imaging (MRI) has been used in the past to non-invasively evaluate intra-renal oxygenation levels.3,5,9–15
For renal BOLD MRI, the parameter R2* is commonly used to quantitatively assess oxygenation changes. R2* (= 1/T2*), the apparent spin–spin relaxation rate, is directly related to the tissue content of deoxyhemoglobin and can be estimated from the signal intensity measurements made at several different echo times. The intensity vs echo time data is fit to a single exponential decay function to determine the rate constant R2*. Decreases in R2* values imply an increase in the oxygenation of hemoglobin and thus improved blood oxygenation.9 Assuming blood oxygenation to be in a dynamic equilibrium with the surrounding tissue oxygenation, estimated changes using BOLD MRI can be interpreted as changes in tissue pO2.
MRI at higher field strengths such as 3.0 T have higher inherent signal-to-noise ratios.16,17 For BOLD MRI, there is increased sensitivity to susceptibility effects at higher field strengths. The BOLD effect has been shown to be proportional to the static magnetic field B0 for large vessels and to for smaller vessels and capillaries.18,19 In theory, R2* values at 3.0 T should be approximately twice those found at 1.5 T, assuming that the observed effects are dominated by relatively large vessels. This was actually found to be true for medullary R2* values.13,20
Promising results have been reported recently using a newly implemented three-dimensional (3-D) version of the multiple gradient-recalled echo (mGRE) sequence for BOLD MRI of the kidney.20 Taking advantage of the relatively fast acquisitions afforded by the 3-D mGRE sequence, a temporal BOLD response was monitored for the first time following injection of furosemide.
Previous studies had documented a response to waterload that was similar to administration of furosemide.3,9 However, a subsequent report suggested that the effects of waterload were less consistent in terms of reproducibility within a subject and between subjects.12 This was thought to be related to the potential differences in the physiological status between subjects and within the same subject at different times. It was also thought that the response to waterload may be less in magnitude compared to furosemide, suggesting a need for higher sensitivity.14 Based on these previous experiences, we sought to use the 3-D mGRE sequence at 3.0 T to examine the temporal response of renal medullary oxygenation levels during water diuresis in a small set of selected young subjects. Based on the premise that this response is related to endogenous prostaglandin (PGE2) stimulation, we also studied the effect of cyclooxygenase (COX) inhibition by naproxen.
RESULTS
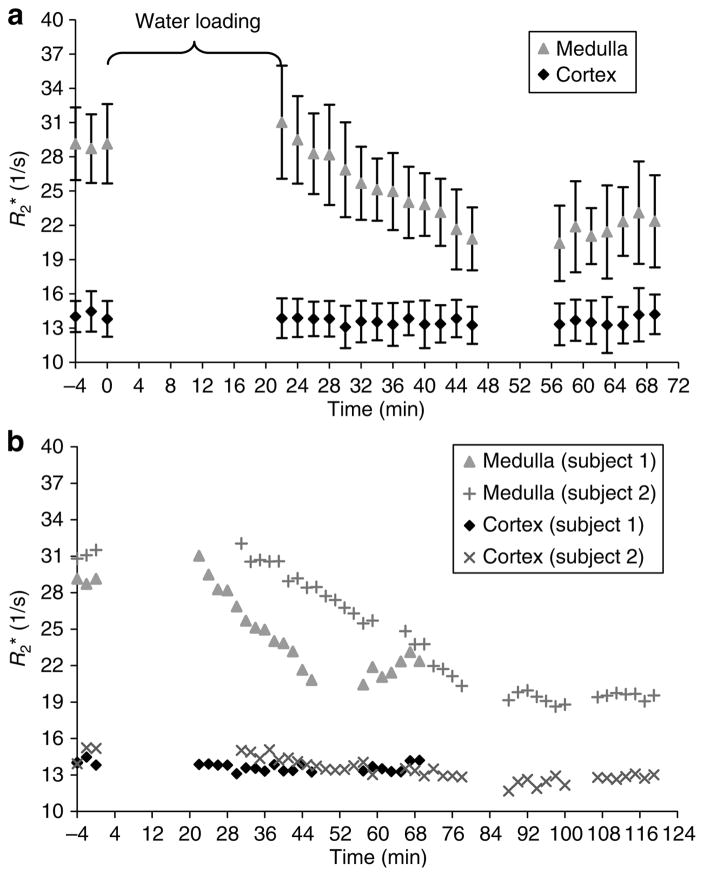
In all five subjects, BOLD MRI measurements (R2*) showed an improvement in the oxygenation levels of the renal medulla post waterload. In all subjects, the medullary R2* values begin to approach those found in the cortex. However, the time to reach the maximum response was variable among the subjects. Figure 1a shows the response observed in a subject who had the fastest response, approximately 30 min to reach the post-waterload equilibrium value. Figure 1b combines the data from the fastest and one of the slowest responses among the study subjects.
Figure 1. Time course of response to waterload.
(a) Time course of response shown for one representative subject. The first three time points represent baseline measurements. For this subject, the temporal response is evident within 30 min post-waterload. The errors bars represent the standard deviation of the pixel data for all of the ROIs used to determine a single time point. (b) Combined plots from subjects no. 1 and no. 2. Note, for subject no. 2, the temporal response is slower, requiring approximately 50 min to reach equilibrium value. In both cases, the R2* values of the medulla begin to approach the values found in the cortex.
All of the values in Tables 1 and 2 are represented as the mean R2* values (1/s). Values in Table 3 are listed as the slope (rate of change in R2* over time) during the post-waterload period for each subject. The pre- and post-waterload data were analyzed using a paired two-tailed Student’s t-test. The differences were considered significant if the P-value was less than 0.05.
Table 1.
Effect of waterload on intra-renal oxygenation levels
| Subject | Cortex |
Medulla |
||
|---|---|---|---|---|
| Pre- waterload R2* (1/s) | Post- waterload R2* (1/s) | Pre- waterload R2* (1/s) | Post- waterload R2* (1/s) | |
| 1 | 14.1 | 13.6 | 29.0 | 21.8 |
| 2 | 14.8 | 12.6 | 31.1 | 19.4 |
| 3 | 14.7 | 13.4 | 29.3 | 22.3 |
| 4 | 13.7 | 14.2 | 31.0 | 25.8 |
| 5 | 15.3 | 13.9 | 31.3 | 24.7 |
| Mean | 14.5 | 13.5 | 30.3 | 22.8† |
| s.d. | 0.6 | 0.6 | 1.1 | 2.5 |
Post-waterload values in the medulla were calculated as the average of the time points when the temporal response reached an equilibrium value.
P<0.05 using a paired two-tailed Student’s t-test when compared to pre-waterload value.
Table 2.
Effect of waterload with naproxen on intra-renal oxygenation levels
| Subject | Cortex |
Medulla |
||
|---|---|---|---|---|
| Pre- waterload R2* (1/s) | Post- waterload R2* (1/s) | Pre- waterload R2* (1/s) | Post- waterload R2* (1/s) | |
| 1 | 14.3 | 14.1 | 28.1 | 28.6 |
| 2 | 14.0 | 13.8 | 28.7 | 30.2 |
| 3 | 14.3 | 13.8 | 25.5 | 26.1 |
| 4 | 14.1 | 14.1 | 27.3 | 28.0 |
| 5 | 14.5 | 13.9 | 27.7 | 29.7 |
| Mean | 14.2 | 13.9 | 27.5 | 28.5† |
| s.d. | 0.2 | 0.2 | 1.2 | 1.6 |
P<0.05 using a paired two-tailed Student’s t-test when compared to pre-waterload value.
Table 3.
Slopes (rate of change in medullary R2* over time) of the temporal responses after water loading (with and without naproxen)
| Subject | Waterload (1/s min) | Waterload with naproxen (1/s min) |
|---|---|---|
| 1 | 0.39 | 0.038 |
| 2 | 0.18 | 0.084 |
| 3 | 0.23 | 0.079 |
| 4 | 0.20 | 0.021 |
| 5 | 0.11 | 0.180 |
| Mean (with subject no. 5) | 0.22 | 0.080 |
| s.d. (with subject no. 5) | 0.10 | 0.062 |
| Mean (without subject no. 5) | 0.25 | 0.056† |
| s.d. (without subject no. 5) | 0.096 | 0.031 |
P<0.05 using a paired two-tailed Student’s t-test when compared to slope value without naproxen pretreatment.
Table 1 details the average R2* values pre- and post-waterload for both the renal cortex and medulla. As the temporal responses were unique for each individual, the post-waterload R2* values were calculated as the average of the time points when the response to waterload had reached an equilibrium plateau. Water diuresis significantly improved medullary oxygenation from the pre-waterload value of 30.3±1.1 1/s (mean±s.d.) to 22.8±2.5 1/s post-waterload (P =0.002). On the other hand, the cortical oxygenation levels did not significantly change (P =0.09) during water loading (pre-waterload =14.5±0.6 1/s vs post-water-load =13.5±0.6 1/s).
Table 2 summarizes the pre- and post-waterload R2* values in both cortex and medulla when pretreated with naproxen. Figure 2 graphically demonstrates the lack of a temporal response for the same subject shown in Figure 1a. Statistically, there was no significant difference (P =0.06) found in the renal cortex (pre-waterload =14.2±0.2 1/s vs post-waterload =13.9±0.2 1/s). Interestingly, the medullary measurements did reach significance (P =0.02) (pre-water-load =27.5±1.2 1/s vs post-waterload =28.5±1.6 1/s), but the post-waterload R2* values were slightly higher than the baseline values.
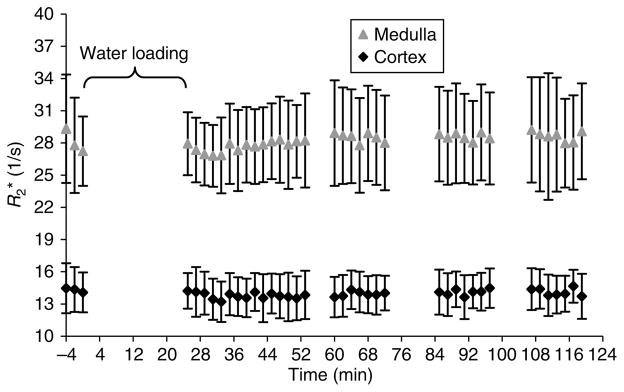
Figure 2. Time course of response to waterload with naproxen shown for the same subject as in Figure 1a.
The first three time points represent baseline measurements. Note that there is no significant change observed in the medullary R2* values post-waterload.
To document the differences in response as shown in Figure 1b in a quantitative fashion, the slope of the R2* vs time curve was estimated. Table 3 summarizes the slopes observed in each subject during water diuresis with and without pretreatment of naproxen. Note the difference in slope to be on the order of a magnitude when comparing the results with and without naproxen.
DISCUSSION
The major outcome of this study is the reconfirmation of our previous observations with BOLD MRI on the effects of waterload and COX inhibition.3 This is reassuring owing to the fact that a subsequent study had found that waterload produced a more variable response between subjects and in the same subject at different times.12 With these previous experiences in mind, we selected relatively young subjects with a smaller age range (21–24 years) for this study in order to minimize any age-related variability. The age group used in this study was slightly younger than in previous water diuresis studies.3,12,14 The present study was also performed at 3.0 T to take advantage of the higher BOLD sensitivity and utilized a newer implementation of the mGRE technique that allows for 3-D data to be acquired within a single breath-hold. Such fast acquisitions facilitated the monitoring of the temporal response during water diuresis without compromising spatial coverage. Our previous studies obtained one set of post-waterload data when the urine flow exceeded 5 ml/min,3,12 based on the premise that this would take into account the different rates of physiological response to waterload between subjects. However, there was no real relevance for the urine flow rate chosen in terms of any known relationship to the observed BOLD MRI response.
The overriding hypothesis for the interest in medullary hypoxia is that the kidneys have evolved to deal with it by developing a number of protective endogenous mechanisms. It is believed that the compromise to one or more of these protective mechanisms may lead to acute renal failure.1,4,8,21–23 Therefore, an efficient method for non-invasively evaluating oxygenation levels in the human kidneys would be highly desirable. One of the well-studied endogenous protective mechanisms is PGE2.24–27 PGE2 is produced in abundance in the renal medullary collecting ducts and interstitial cells. Its major role is to inhibit active transport in the medullary thick ascending limb, reduce the Na-K-ATPase activity in the renal cells, and diminish oxygen consumption while at the same time increasing the blood flow levels in the medulla of the human kidney.25,26 The vasa recta and renal tubular cells which line the medullary thick ascending limb in the outer medulla contain receptors for PGE2.27 It has been previously shown in rats that increases in urinary excretion of PGE2 are directly correlated with reduction in hypoxic damage to the renal medulla, so it is also possible that this association can be observed in humans.26
Using BOLD MRI, we were able to observe improved oxygenation levels in the renal medulla during waterload. Pretreatment with naproxen abolished the BOLD MRI response to water diuresis. It is interesting to note that when pretreated with naproxen, the post-waterload R2* values in the medulla actually increased slightly compared to the baseline values. The exact cause for such an increase in R2* and any significance associated with it are not yet clear. These results support the hypothesis that prostaglandins play a role in the maintenance of oxygenation status of the human renal medulla. The exact mechanism by which prostaglandins influence the medullary pO2 cannot be inferred from BOLD MRI observations alone. This is because BOLD MRI measurements cannot distinguish between oxygen supply and consumption changes. Prostaglandins are associated with both vasodilatory effects and reduce the Na-K-ATPase activity in the renal cells and diminish oxygen consumption.25,26 Currently, there are no techniques available to non-invasively monitor blood flow to the renal medulla that can be used in concert with BOLD MRI in human subjects. However, one could perform microprobe measurements to simultaneously evaluate tissue pO2 and blood flow in animal models.7
As this was the first time that a temporal response was observed following water loading, we compared the slopes (rate of change in R2* over time) of the response as an additional quantitative measure (Table 3). It is clear that the slopes are significantly different between subjects, probably indicating the differences in the physiological status.
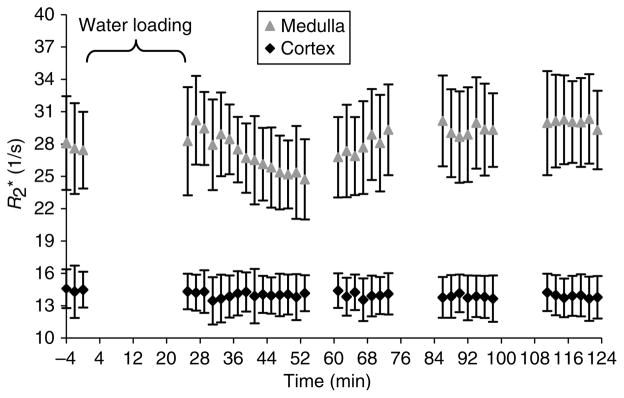
One of the subjects (subject no. 5) took naproxen after the baseline scans were performed instead of before the baseline scans. It is interesting to note that the initial slope for subject no. 5 is higher than the other subjects for the naproxen study. However, the R2* values return to baseline after this initial response to waterload (Figure 3). This is consistent with the action time of oral naproxen. It also suggests that the observed effect of the response to waterload is primarily determined by the dose taken on the morning of the study. This observation is very similar to our previous study with ibuprofen.3 More importantly, the observation supports the ability of the technique to monitor acute drug-induced changes in the regional hemodynamics within the kidney. Because the slopes were variable between subjects, when including subject no. 5 in the statistical analysis, the difference in slopes with and without naproxen did not reach significance (P =0.11). However, when excluding subject no. 5 in the analysis, a significant difference was observed when comparing the slope values with and without pretreatment of naproxen (P =0.038).
Figure 3. Time course of response to waterload with naproxen for subject no. 5 who had ingested naproxen following baseline acquisitions.
In the first 30 min post-waterload, we see a temporal response in the subject. Later, the R2* values in the medulla begin to steadily increase and approach the baseline medullary values. This illustrates the efficacy of the method to monitor acute changes following pharmacological maneuvers.
In conclusion, the 3-D implementation of the mGRE sequence with BOLD MRI allowed us to evaluate renal medullary oxygenation levels during waterload (with and without naproxen). For the first time, we monitored the temporal changes in the medulla post-waterload. We were able to observe a statistically significant improvement in the oxygenation levels (R2*) in the renal medulla following water loading. When pretreated with naproxen, no such improvement was observed. When comparing flow rates with the temporal response times of the different subjects, we feel that a urine flow rate of 5 ml/min is certainly a reliable indicator in determining an appropriate time to perform the post-waterload acquisitions.
MATERIALS AND METHODS
Study plan
Five healthy, young subjects (four female subjects and one male subject, mean age =22±1.2 years) with no prior history of renal disease were recruited to take part in this study. Each subject participated in two different data acquisitions separated by approximately 2 months. At each session, data were acquired pre-and post-waterload with the second study involving COX inhibition. All of the participants gave informed consent according to the protocol approved by the Institutional Review Board before taking part in the study.
The subjects were instructed to abstain from food and water for at least 12 h overnight. All of the subjects were weighed and a sample of urine was collected before being placed in the magnet. Baseline BOLD magnetic resonance images were then acquired in the supine position. The subjects were then taken out of the scanner and a second urine sample was obtained. In order to induce water diuresis, the subjects were asked to drink 20 ml of water per kg of body weight within a 15-min time period. After the water intake, the subjects were once again placed in the scanner and post-waterload scans were performed. Scanning continued for 30 min at a rate of 1 scan every 2 min. The subjects were taken out of the scanner and were once again asked to void in order to monitor the urine flow rate. The subjects went back into the scanner for further post-waterload scans at a rate of 1 scan every 2 min for 15 min. Urine samples were collected after every 15 min scan. These 15 min interval scans continued until both the following conditions were satisfied: first, the urine flow rate had to exceed 5 ml/min; and then one of the subsequent urine samples had to show a decrease in urine flow rate from the previous measurement.
For the COX inhibition study, 500 mg of naproxen was taken by mouth twice a day with meals for the 4 days preceding the study and also once on the morning of the study before the baseline scans. This protocol was similar to the one previously reported with ibuprofen.3
MRI methods
All studies were performed on a short bore of 3.0 T Twin Speed scanner with Excite technology (General Electric Medical Systems, Milwaukee, WI, USA). The scanner had a maximum gradient strength of 23 mT/m and a slew rate of 77 mT/m/ms when using a whole body gradient set. An eight element-coil array was utilized for signal reception. A 3-D mGRE sequence with eight echoes (repetition time/echo time/flip angle/bandwidth/slice/matrix =25.5 ms/1.86–22.9 ms/10°/83.3 kHz/5 mm/256 × 160 and 36–42 cm field of view with 80% phase field of view) was used to acquire data during a single breath-hold of 23 s. Partial Fourier encoding was employed along both the slice (70%) and phase (80%) directions. Ten (coronal) slices were prescribed with two slices on either end not reconstructed in order to avoid wrap-around artifacts.
R2* mapping methods
R2* maps were constructed on an Advantage Workstation (General Electric Medical Systems, Milwaukee, WI, USA) using the FUNCTOOL program by fitting a single exponential function to the signal intensity vs echo time data. Approximately 40 regions of interest (ROIs) covering at least 10 pixels each were drawn on the anatomic templates and R2* values were read off the corresponding R2* maps. The ROIs were carefully placed in the cortex and medulla on all of the six slices that were acquired for both kidneys. Figure 4 helps visualize the placement of representative ROIs on an anatomic template (usually the first image of the eight acquired) and R2* map for one slice. The data from the were averaged in order to obtain a single representative mean value for R2* per subject per time point.
Figure 4. A representative slice (among six slices) acquired with the 3-D mGRE sequence in one subject.
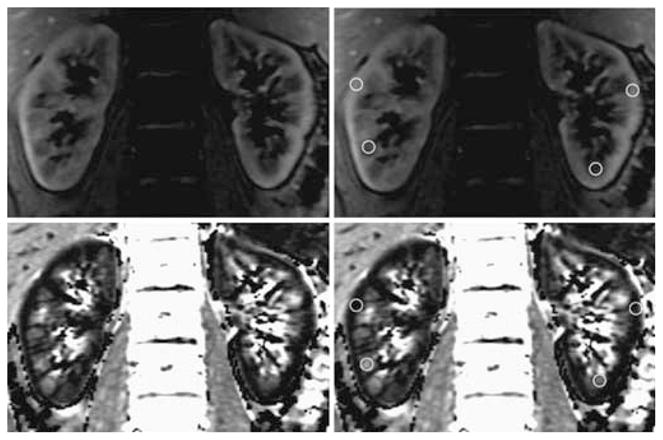
On the top are the anatomical templates and at the bottom the corresponding R2* maps. The images in the right column are same as the left column, but with some representative ROIs marked.
Supplementary Material
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health, DK-53221 (to Pottumarthi V. Prasad). We thank Angel Nickolov from the Center for Outcomes Research at Evanston Northwestern Healthcare for useful consultation regarding statistical analysis.
References
- 1.Brezis M, Rosen S. Hypoxia of the renal medulla – its implications for disease. N Engl J Med. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 2.Kone BC. A ‘BOLD’ new approach to renal oxygen economy. Circulation. 1996;94:3067–3068. doi: 10.1161/01.cir.94.12.3067. [DOI] [PubMed] [Google Scholar]
- 3.Prasad PV, Epstein FH. Changes in renal medullary pO2 during water diuresis as evaluated by blood oxygenation level-dependent magnetic resonance imaging: effects of aging and cyclooxygenase inhibition. Kidney Int. 1999;55:294–298. doi: 10.1046/j.1523-1755.1999.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brezis M, Rosen SN, Epstein FH. The pathophysiological implications of medullary hypoxia. Am J Kidney Dis. 1989;13:253–258. doi: 10.1016/s0272-6386(89)80062-9. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert S, Zuo C, Epstein FH. Effect of specific and non-specific inhibition of COX-2 on renal oxygenation before and after water diuresis. Nephron Physiol. 2005;99:101–104. doi: 10.1159/000083767. [DOI] [PubMed] [Google Scholar]
- 6.Michel CC. Renal medullary microcirculation: architecture and exchange. Microcirculation. 1995;2:125–139. doi: 10.3109/10739689509146761. [DOI] [PubMed] [Google Scholar]
- 7.Brezis M, Agmon Y, Epstein FH. Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol. 1994;267:F1059–F1062. doi: 10.1152/ajprenal.1994.267.6.F1059. [DOI] [PubMed] [Google Scholar]
- 8.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 9.Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94:3271–3275. doi: 10.1161/01.cir.94.12.3271. [DOI] [PubMed] [Google Scholar]
- 10.Prasad PV, Priatna A, Spokes K, Epstein FH. Changes in intrarenal oxygenation as evaluated by BOLD MRI in a rat kidney model for radiocontrast nephropathy. J Magn Reson Imaging. 2001;13:744–747. doi: 10.1002/jmri.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein FH, Prasad P. Effects of furosemide on medullary oxygenation in younger and older subjects. Kidney Int. 2000;57:2080–2083. doi: 10.1046/j.1523-1755.2000.00057.x. [DOI] [PubMed] [Google Scholar]
- 12.Li LP, Storey P, Pierchala L, et al. Evaluation of the reproducibility of intrarenal R2* and ΔR2* measurements following administration of furosemide and during waterload. J Magn Reson Imaging. 2004;19:610–616. doi: 10.1002/jmri.20043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li LP, Vu AT, Li BS, et al. Evaluation of intrarenal oxygenation by BOLD MRI at 3.0 T. J Magn Reson Imaging. 2004;20:901–904. doi: 10.1002/jmri.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuo CS, Rofsky NM, Mahallati H, et al. Visualization and quantification of renal R2* changes during water diuresis. J Magn Reson Imaging. 2003;17:676–682. doi: 10.1002/jmri.10314. [DOI] [PubMed] [Google Scholar]
- 15.Prasad PV, Chen Q, Goldfarb JW, et al. Breath-hold R2* mapping with a multiple gradient-recalled echo sequence: application to the evaluation of intrarenal oxygenation. J Magn Reson Imaging. 1997;7:1163–1165. doi: 10.1002/jmri.1880070633. [DOI] [PubMed] [Google Scholar]
- 16.Haacke EBR, Brown RW, Thompson M, et al. Magnetic Resonance Imaging Physical Principles and Sequence Design. John Wiley & Sons; New York: 1999. Signal, contrast and noise; pp. 343–387. [Google Scholar]
- 17.Edelstein WA, Glover GH, Hardy CJ, Redington RW. The intrinsic signal-to-noise ratio in NMR imaging. Magn Reson Med. 1986;3:604–618. doi: 10.1002/mrm.1910030413. [DOI] [PubMed] [Google Scholar]
- 18.Fisel CR, Ackerman JL, Buxton RB, et al. MR contrast due to microscopically heterogeneous magnetic susceptibility: numerical simulations and applications to cerebral physiology. Magn Reson Med. 1991;17:336–347. doi: 10.1002/mrm.1910170206. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa S, Menon RS, Tank DW, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tumkur SM, Vu AT, Li LP, Prasad PV. Preliminary evaluation of 3D mGRE sequence for BOLD MRI at 3.0 T. Invest Radiol. 2006;41:181–184. doi: 10.1097/01.rli.0000187166.43871.fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindner A, Sherrard DJ. Acute renal failure. N Engl J Med. 1996;335:1320–1321. author reply 1321–1322. [PubMed] [Google Scholar]
- 22.Heyman SN, Brezis M, Reubinoff CA, et al. Acute renal failure with selective medullary injury in the rat. J Clin Invest. 1988;82:401–412. doi: 10.1172/JCI113612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heyman SN, Fuchs S, Brezis M. The role of medullary ischemia in acute renal failure. New Horizons. 1995;3:597–607. [PubMed] [Google Scholar]
- 24.Lear S, Silva P, Kelley VE, Epstein FH. Prostaglandin E2 inhibits oxygen consumption in rabbit medullary thick ascending limb. Am J Physiol. 1990;258:F1372–F1378. doi: 10.1152/ajprenal.1990.258.5.F1372. [DOI] [PubMed] [Google Scholar]
- 25.Jabs K, Zeidel ML, Silva P. Prostaglandin E2 inhibits Na+-K+-ATPase activity in the inner medullary collecting duct. Am J Physiol. 1989;257:F424–F430. doi: 10.1152/ajprenal.1989.257.3.F424. [DOI] [PubMed] [Google Scholar]
- 26.Silva P, Rosen S, Spokes K, et al. Influence of endogenous prostaglandins on mTAL injury. J Am Soc Nephrol. 1990;1:808–814. doi: 10.1681/ASN.V15808. [DOI] [PubMed] [Google Scholar]
- 27.Eriksen EF, Richelsen B, Gesser BP, et al. Prostaglandin-E2 receptors in the rat kidney: biochemical characterization and localization. Kidney Int. 1987;32:181–186. doi: 10.1038/ki.1987.190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.