Abstract
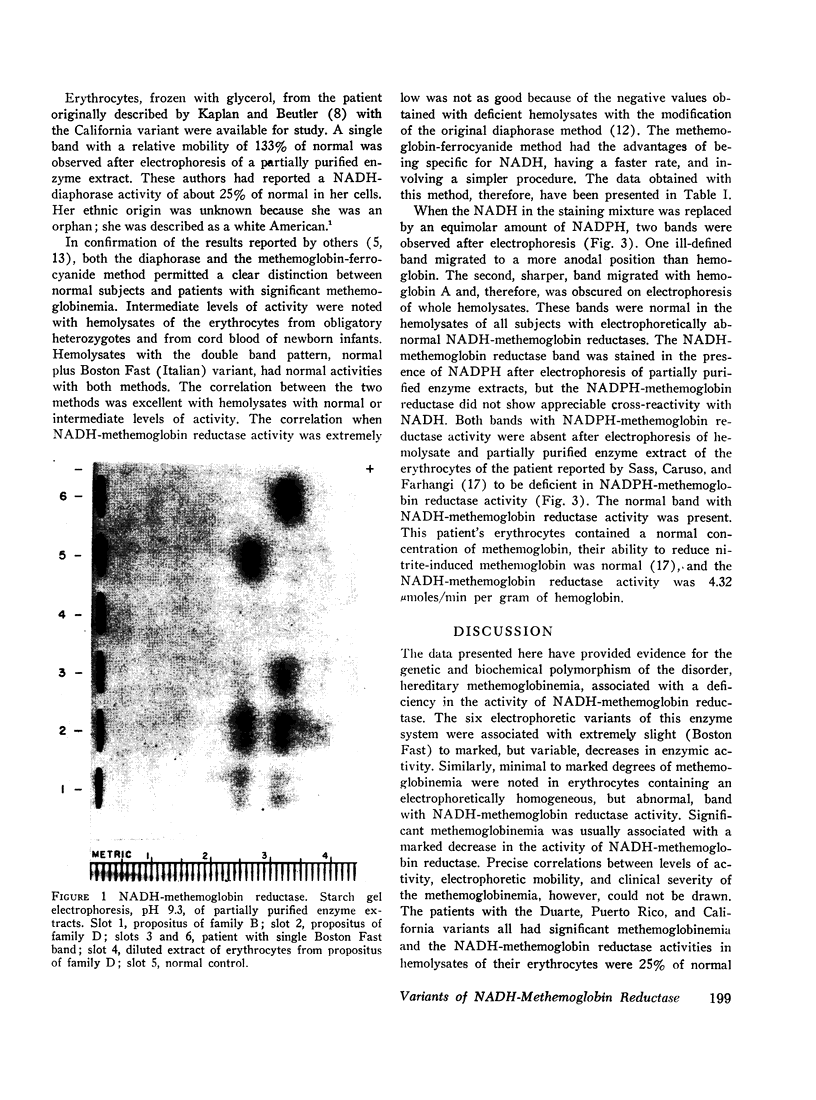
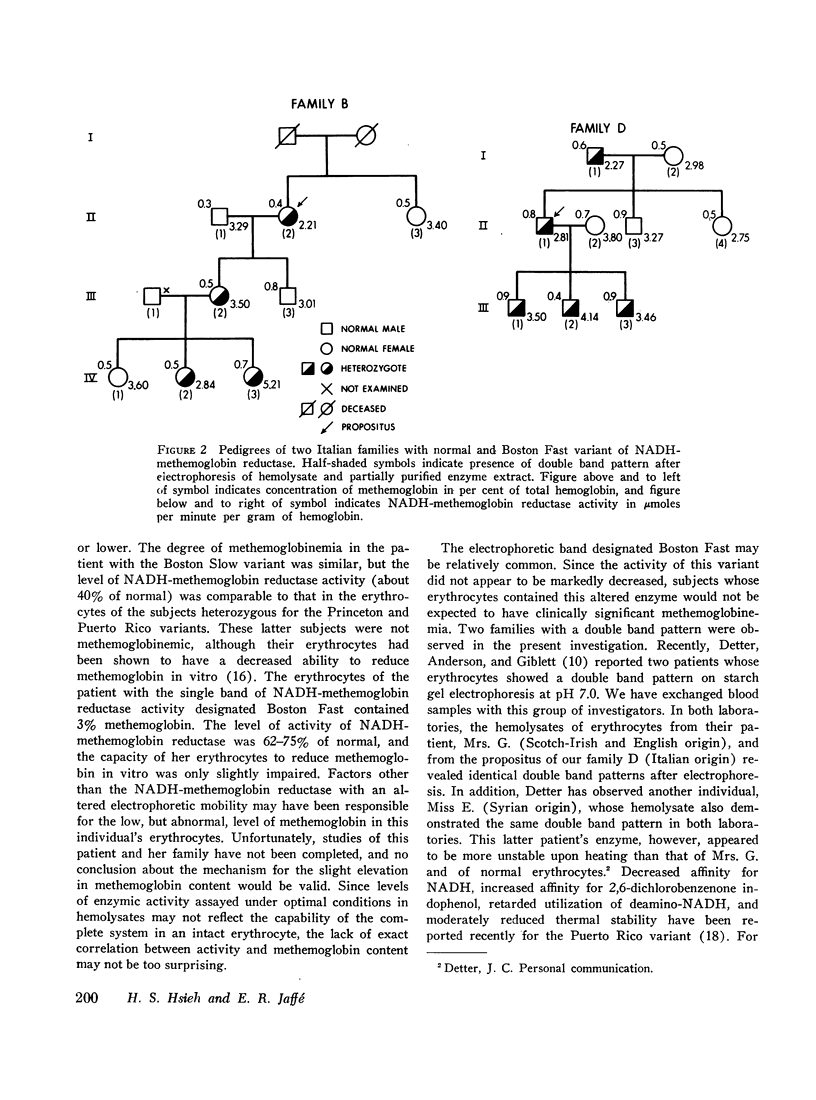
The electrophoretic mobility and activity of NADH-methemoglobin reductase in erythrocytes of patients with hereditary methemoglobinemia, obligatory heterozygotes, and normal subjects were examined. Six distinct electrophoretic variants were found in studies of erythrocytes from members of ten different families. Five variants (Boston Slow, Duarte, Princeton, Puerto Rico, and California) were associated with significant methemoglobinemia and moderate to marked decreases in enzymic activity. Precise correlations between levels of NADH-methemoglobin reductase activity, electrophoretic mobility, and clinical severity of methemoglobinemia, however, could not be drawn. One variant (Boston Fast) was associated with almost normal activity and very minimal methemoglobinemia. Nine members from three generations of two Italian families were found to have two bands with NADH-methemoglobin reductase activity in their erythrocytes, one with normal mobility and one with a mobility identical with that of Boston Fast. No functional or clinical impairment could be attributed to this abnormality. The observations made in this investigation were consistent with an autosomal recessive mode of inheritance of multiple alleles for NADH-methemoglobin reductase. As has been shown to be true for hemoglobin and glucose-6-phosphate dehydrogenase, multiple aberrations in the NADH-methemoglobin reductase of human erythrocytes apparently exist, some with and some without functional consequences.
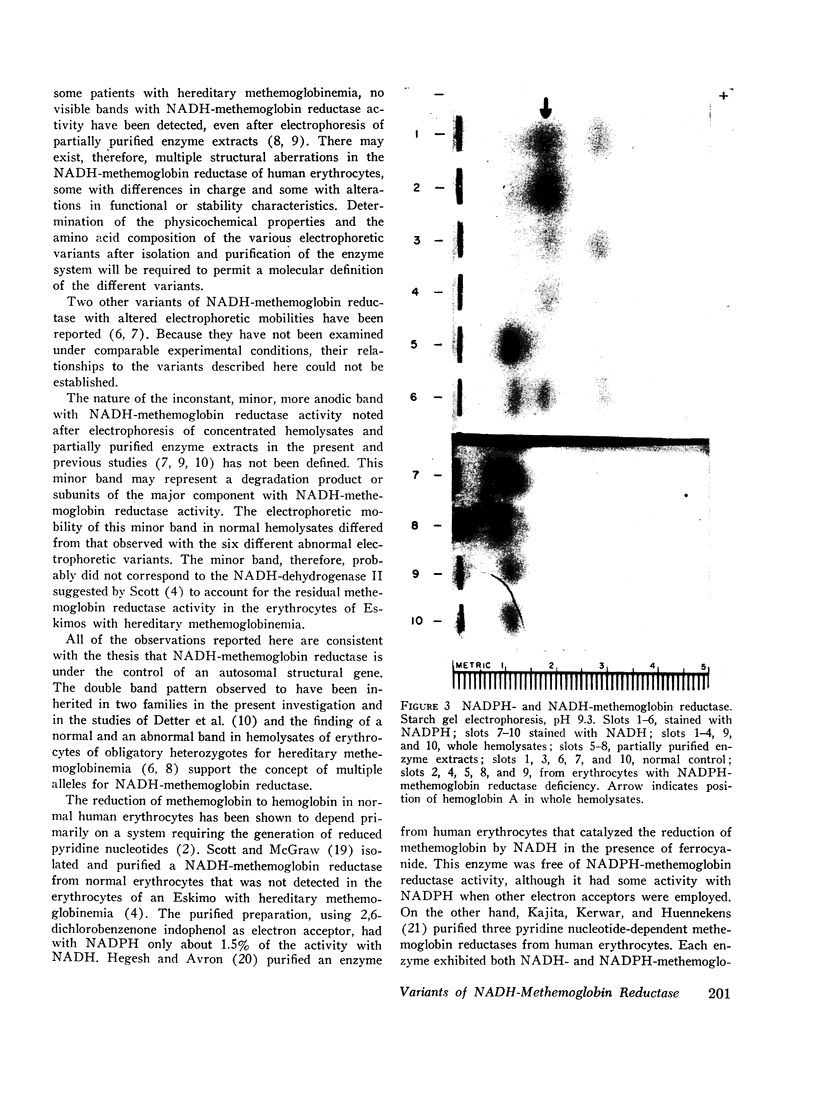
Two bands with NADPH-methemoglobin reductase activity with electrophoretic mobilities distinct from those of the NADH-methemoglobin reductase were found in human erythrocytes. These bands were normal in hemolysates of erythrocytes from patients with hereditary methemoglobinemia, but were absent from the hemolysate of erythrocytes deficient in NADPH-methemoglobin reductase activity. These latter erythrocytes, however, contained normal concentrations of methemoglobin and had a normal ability to reduce methemoglobin in vitro. These observations were most consistent with the thesis that the NADH-methemoglobin reductase, distinct from any NADPH-methemoglobin reductase, was the major system responsible for the reduction of methemoglobin to hemoglobin in human erythrocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom G. E., Zarkowsky H. S. Heterogeneity of the enzymatic defect in congenital methemoglobinemia. N Engl J Med. 1969 Oct 23;281(17):919–922. doi: 10.1056/NEJM196910232811702. [DOI] [PubMed] [Google Scholar]
- Brewer G. J., Eaton J. W., Knutsen C. S., Beck C. C. A starch-gel electrophoretic method for the study of diaphorase isozymes and preliminary results with sheep and human erythrocytes. Biochem Biophys Res Commun. 1967 Oct 26;29(2):198–204. doi: 10.1016/0006-291x(67)90587-6. [DOI] [PubMed] [Google Scholar]
- Detter J. C., Anderson J. E., Giblett E. R. NADH diaphorase: an inherited variant associated with normal methemoglobin reduction. Am J Hum Genet. 1970 Jan;22(1):100–104. [PMC free article] [PubMed] [Google Scholar]
- HENNESSEY M. A., WALTERSDORPH A. M., HUENNEKENS F. M., GABRIO B. W. Erythrocyte metabolism. VI. Separation of erythrocyte enzymes from hemoglobin. J Clin Invest. 1962 Jun;41:1257–1262. doi: 10.1172/JCI104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegesh E., Avron M. The enzymatic reduction of ferrihemoglobin. II. Purification of a ferrihemoglobin reductase from human erythrocytes. Biochim Biophys Acta. 1967;146(2):397–408. doi: 10.1016/0005-2744(67)90224-0. [DOI] [PubMed] [Google Scholar]
- Hegesh E., Calmanovici N., Avron M. New method for determining ferrihemoglobin reductase (NADH-methemoglobin reductase) in erythrocytes. J Lab Clin Med. 1968 Aug;72(2):339–344. [PubMed] [Google Scholar]
- Jaffé E. R. Hereditary methemoglobinemias associated with abnormalities in the metabolism of erythrocytes. Am J Med. 1966 Nov;41(5):786–798. doi: 10.1016/0002-9343(66)90037-4. [DOI] [PubMed] [Google Scholar]
- Kajita A., Kerwar G. K., Huennekens F. M. Multiple forms of methemoglobin reductase. Arch Biochem Biophys. 1969 Mar;130(1):662–673. doi: 10.1016/0003-9861(69)90085-x. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Beutler E. Electrophoresis of red cell NADH- and NADPH-diaphorases in normal subjects and patients with congenital methemoglobinemia. Biochem Biophys Res Commun. 1967 Nov 30;29(4):605–610. doi: 10.1016/0006-291x(67)90529-3. [DOI] [PubMed] [Google Scholar]
- SCOTT E. M., GRIFFITH I. V. The enzymic defect of hereditary methemoglobinemia: diaphorase. Biochim Biophys Acta. 1959 Aug;34:584–586. doi: 10.1016/0006-3002(59)90324-5. [DOI] [PubMed] [Google Scholar]
- SCOTT E. M., McGRAW J. C. Purification and properties of diphosphopyridine nucleotide diaphorase of human erythrocytes. J Biol Chem. 1962 Jan;237:249–252. [PubMed] [Google Scholar]
- SCOTT E. M. Purification of diphosphopyridine nucleotide diaphorase from methemoglobinemic erythrocytes. Biochem Biophys Res Commun. 1962 Sep 25;9:59–62. doi: 10.1016/0006-291x(62)90087-6. [DOI] [PubMed] [Google Scholar]
- Sass M. D., Caruso C. J., Farhangi M. TPNH-methemoglobin reductase deficiency: a new red-cell enzyme defect. J Lab Clin Med. 1967 Nov;70(5):760–767. [PubMed] [Google Scholar]
- Scott E. M. A comparison of two methods of determining DPNH-methemoglobin reductase. Clin Chim Acta. 1969 Mar;23(3):495–498. doi: 10.1016/0009-8981(69)90356-8. [DOI] [PubMed] [Google Scholar]
- West C. A., Gomperts B. D., Huehns E. R., Kessel I., Ashby J. R. Demonstration of an enzyme variant in a case of congenital methaemoglobinaemia. Br Med J. 1967 Oct 28;4(5573):212–214. doi: 10.1136/bmj.4.5573.212. [DOI] [PMC free article] [PubMed] [Google Scholar]