Abstract
Oxygenated hemoproteins are known to react rapidly with nitric oxide (NO) to produce peroxynitrite (PN) at the heme site. This process could lead either to attenuation of the effects of NO or to nitrosative protein damage. Peroxynitrite is a powerful nitrating and oxidizing agent that has been implicated in a variety of cell injuries. Accordingly, it is important to delineate the nature and variety of reaction mechanisms of PN reactions with heme proteins. In this Forum we survey the range of reactions of PN with heme proteins, with particular attention to myoglobin and cytochrome c. While these two proteins are textbook paradigms for oxygen binding and electron transfer, respectively, both have recently been shown to have other important functions that involve nitric oxide and peroxynitrite. We have recently described direct evidence that ferrylMb and NO2 are both produced during the reaction of PN and metmyolgobin (metMb). Kinetic evidence indicates that these products evolve from initial formation of a caged radical intermediate [FeIV=O .NO2]. This caged pair reacts mainly via internal return with a rate constant kr to form metMb and nitrate in an oxygen rebound scenario. Detectable amounts of ferrylMb are observed by stopped-flow spectrophotometry, appearing at a rate consistent with the rate, kobs of heme-mediated PN decomposition. Freely-diffusing NO2, which is liberated concomitantly from the radical pair (ke), preferentially nitrates myoglobin Tyr103 and added fluorescein. For cytochrome c, Raman spectroscopy has revealed that a substantial fraction of cytochrome c converts to a β-sheet structure, at the expense of turns and helices at low pH. It is proposed that a short β-sheet segment, comprising residues 37-39 and 58-61, extends itself into the large 37-61 loop when the latter is destabilized by protonation of H26, which forms an anchoring H-bond to loop residue P44. This conformation change ruptures the Met80-Fe bond, as revealed by changes in ligation-sensitive Raman bands. It also induces peroxidase activity with the same temperature profile. This process is suggested to model the apoptotic peroxidation of cardiolipin by cytochrome c.
Introduction. The biochemistry of peroxynitrite
This Forum, with its focus on nitric oxide, provides an opportunity to review the roles of reactive oxygen and nitrogen species and the variety of interactions of these species with metalloproteins. Hydrogen peroxide, superoxide ion, nitric oxide, nitrogen dioxide and peroxynitrite ion all react with biological target molecules. Some of these interactions are carefully orchestrated aspects of signaling events within and among cells, others are part of the cell-killing machinery of the immune system, and some are pathological events and may lie at the root of many diseases. Metalloproteins can be altered with loss or gain of function as a result of these small reactive molecules. DNA can be cleaved and lipid components can be oxidized or nitrated. The interactions of these species with each other can be sensed by the cell, resulting in a variety of responses including gene regulation and transcription. Indeed, there is accumulating evidence that the molecular dance of reactive oxygen and nitrogen species is central to the life and death cellular decisions in homeostasis or the initiation of apoptosis. New families of metallopharmaceuticals are emerging that serve both to probe the nature and mechanisms of these events and to effect the outcome. It is with this perspective that we look at the mechanisms of heme protein interaction with the biological oxidant, peroxynitrite anion (ONOO-) and its conjugate acid, peroxynitrous acid (ONOOH).
The formation of peroxynitrite within cells and tissue from the reaction of cellular nitric oxide (NO) with the superoxide radical was first proposed by Beckman nearly two decades ago. Since then, peroxynitrite (PN) has become the focus of intense research alongside the development of nitric oxide chemistry. Peroxynitrite is a powerful nitrating and oxidizing agent. PN is produced by the reaction of NO and superoxide (O .-2) ion at diffusion-controlled rates (~ 1×1010 M-1 s-1).1, 2 Superoxide ion is both a byproduct of respiration and a component oxidant of the immune defense.3 Under normal physiological conditions, the superoxide ion will be intercepted by superoxide dismutases (SODs). However, when nitric oxide concentrations reach ~10 μM in the vicinity of activated phagocytes, the target flux for .NO with O2.- becomes ~105 s-1 which is greater than that estimated for SOD (2.3×104 s-1. Thus, under such conditions, the combination of .NO with O2.- becomes competitive with the dismutation of O2.- by SODs and affords PN. The signature events of PN production within cells are protein and lipid nitrations and these have been found to be an early event in the etiology of a variety of pathologies.
The sites of PN formation are assumed to be spatially associated with the source of superoxide, such as the plasma NADPH oxidases or the mitochondrial respiratory complexes. This insight derives from the fact that O2.- is also a short-lived radical. Moreover, superoxide has restricted diffusion across membranes due to the low pKa (4.7) of the conjugate acid, the hydroperoxyl radical (HO2.). Superoxide can be generated from two major sources: phagocytic NADPH oxidase (NOX)4 and mitochondria respiratory chain.5 Activated NOXs in phagocytes of various tissues assemble in membranes and reduce oxygen to superoxide. This generation of superoxide by NOXs is vital for effective host defense, but also affords a pathway to form peroxynitrite by combining with nitric oxide produced by the inflammatory cells.2, 6 Superoxide produced by complexes I and III in the mitochondrial electron transport chain (ETC) is released asymmetrically to both sides of the mitochondrial inner membrane with different roles in these subcellular environments.5 By contrast, NO is highly diffusive and relatively stable (the diffusion coefficient of nitric oxide has been reported to be 3300 μm2/sec).7 Although PN has not been directly detected in vivo, analytical methods are improving8 and numerous diagnostic footprints of PN formation have been found in a variety of cell types, including macrophages,9 neutrophils10, 11 and cultured endothelial cells.12, 13 All of these cell types can generate nitric oxide concurrently with superoxide.
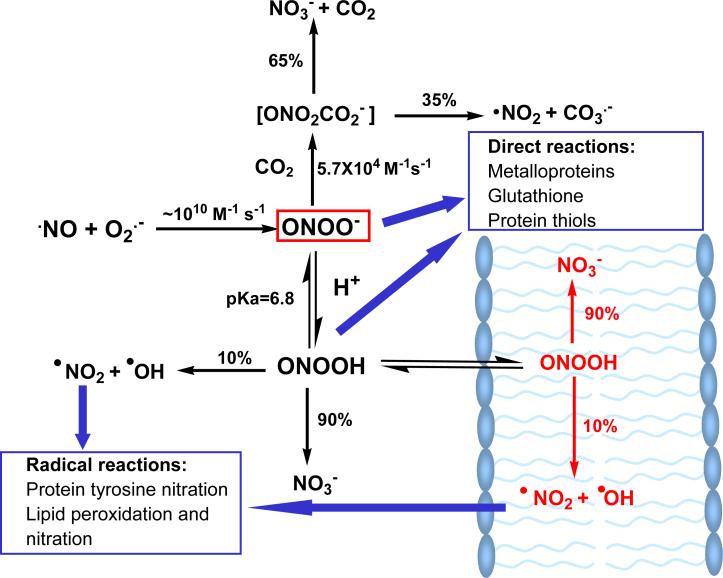
Peroxynitrite anion exists in equilibrium with its protonated form, peroxynitrous acid, with a pKa of 6.8.14 A summary of the reactions of PN is presented in Figure 1. The rates of PN production in specific compartments have been estimated to be as high as 50-100 μM min-1 in vivo.15 The steady-state PN concentrations are estimated to be in the nanomolar range.16, 17 Another important feature of PN is its ability to diffuse readily across lipid membranes.18 The calculated permeability coefficient for PN is 8.0×10-4 cm s-1, which is comparable to that of H2O and is approximately 400 times greater than that of superoxide. This high permeability means that once formed, PN can diffuse a distance larger than the diameter of a typical cell, a characteristic that makes PN an extremely effective oxidant in executing damage even far from its origin. Thus, the reaction of NO with O2- creates a highly transportable and highly reactive oxidant. In vitro experiments have shown oxidation of thiols, sulfides, transition metal centers, ascorbate, olefins, benzene, phenols and other aromatics by PN; these reactions have been thoroughly discussed in a recent review.19 Moreover, PN exhibits a wide range of reactivity that results in the modification of many cellular targets. These modifications to enzymes, cellular DNA and lipids often lead to loss of enzyme activity, and damage to the integrity of DNA and cell membranes (Figure 1). Damage to DNA and lipids can eventually lead to cellular apoptosis.20-22
Figure 1.
Reactions of peroxynitrite in aqueous and lipid phases. Modified from reference 35.
Significantly, PN can induce posttranslational modifications of proteins through nitration of tyrosine, oxidation of methionine and nitrosylation of cysteine residues.23, 24 Tyrosine nitration has been detected in vivo in a large number of proteins during development, oxidative stress, and aging.25 Proteomic methods have been utilized to identify specific protein nitration targets both in vivo and in cell culture models. For example, Stuehr et al. identified more than 40 nitrotyrosine-immunopositive proteins 26 in inflammatory cell models; Castenga et al. 27 applied proteomic approaches to determine specific targets of protein oxidation in brains suffering from Alzheimer's disease and successfully identified six protein targets.
Among protein targets in vivo, metalloproteins are particularly sensitive to the impairments induced by PN. As early as 1992, Ischiropoulos et al. found that bovine Cu, Zn superoxide dismutase reacts with PN to form a stable, yellow protein-bound adduct identified as nitrotyrosine.28 Zou et al. later showed that prostacyclin synthase, a heme-thiolate enzyme essential for regulation of vascular tone, is nitrated and inactivated by submicromolar concentrations of PN.29 Daiber et al. reported findings for NADH-NO reductase (P450NOR) showing that the reaction with PN can effectively catalyze phenol nitration without attacking its own tyrosine residues, whereas P450BM-3 undergoes self-nitration as well as phenol nitration.30, 31 The mitochondrial electron transfer protein cytochrome c displays significant changes in redox properties upon nitration.32, 33 Tyrosine hydroxylase (a non-heme iron protein), which is the initial and rate-limiting enzyme in the biosynthesis of dopamine, is also inactivated by PN via tyrosine nitration and cysteine modification.34
The biochemistry and pathophysiology of peroxynitrite have been thoroughly reviewed.2, 23, 35-37 In this forum we present a discussion regarding the chemical mechanisms of heme protein/PN reactions, with particular attention to myoglobin, cytochrome P450 and cytochrome c.
Interaction of peroxynitrite with heme proteins
Myoglobin and hemoglobin
Myoglobin (Mb) and hemoglobin (Hb) are best known for their oxygen storage and transport functions.38 In the last decade, the idea that Mb and Hb may function as scavengers of reactive nitrogen species (RNS), such as nitric oxide (NO) has been a subject of great interest. It has been recently realized that Mb is an efficient intracellular scavenger of NO, regulating nitric oxide homeostasis in the cardiac and skeletal muscles and thereby protecting mitochondrial respiration.39 This novel function of Mb is based on the rapid and irreversible reaction of ferrous oxygenated Mb (oxyMb) with NO, yielding ferric oxidized Mb (metMb) and nitrate (NO3-).40-42 The detailed mechanism of this process has not yet been fully elucidated, but it is clear that the complexity and importance of the myoglobin-NO interaction continues to develop. Indeed, the overexpression of myoglobin has recently been shown to be an early event in the etiology of epithelial cancers.43 OxyHb was found to bind NO and form nitrosylated thiols.44
The ferric forms of hemoglobin and myoglobin have been shown to catalyze the isomerization of PN to nitrate.45-48 The catalytic rate constant determined by our group at pH 7.6 and 25 °C for metMb is ca. 1.0×104 M-1 s-1.45 Herold et al. measured the kcat of metMb to be 7.7 × 104 M-1 s-1 at pH 7.0 and 20 °C.49 The pH-dependence of the catalytic rate constants indicates that HOONO is the species that reacts with the FeIII center of the proteins. Two mechanisms regarding metMb/PN have been proposed as summarized in Scheme 1. In both mechanisms, the first step is initiated by replacing the sixth ligand H2O by HOONO, forming a transient intermediate MbFeIIIOONO.50 EPR and UV-vis data possibly supporting MbFeIIIOONO as the intermediate of the reaction of NO with oxyMb at alkaline pH has been presented.40, 51, 52 However, since the MbFeIIIOONO complex could not be detected at neutral pH, it probably decays to other intermediates at a very fast rate. Indeed, Raman41 and kinetic data (vide infra)53 have supported an alternative FeIII-nitrato formulation for the observed transient species, supporting a very rapid O-O bond cleavage in MbFeIIIO-ONO.
Scheme 1.
Mechanisms for metMb-catalyzed decomposition of peroxynitrite.
The mechanism describing MbFeIIIOONO decay proposed by Herold et al. proceeds by a direct rearrangement of MbFeIIIOONO and yields metMb and 100% nitrate.46, 49 Additionally, they estimated the rate constant of this process (A) in Scheme 1 to be 205 ± 5 s-1.40 The other mechanism, proposed by our group, suggests the formation of a transient radical cage intermediate [FeIV=O .NO2] followed by .NO2 cage escape (D) or rebound (C).45 The early evidence favoring this idea was the observation of myoglobin nitration and the nitration of fluorescein. This is the key difference in the two processes since in the latter, freely diffusing NO2 will emerge from the encounter of NO with oxyMb. This mechanism will be confirmed and delineated in more detail below.
Goldstein et al. have reported measurements of the dissociation rate of nitrate from the nitrato-complex, MbFeIIIONO2, which was generated by pulse radiolysis from the reaction of ferrylMb and NO2.53 The value for the rate constant was 190 ± 20 s-1 at pH 9.7 and 20 °C, which is virtually identical to the rate reported by Herold for the assumed MbFeIIIONOO decay.40 This similarity implies that the observed decay of MbFeIIIOONO by Herold et al. actually comprises two fast steps: the first is the rapid conversion to MbFeIIIONO2 via ferrylMb and NO2 (C); the second one is the release of NO3- from MbFeIIIONO2 (E). Accordingly, the putative MbFeIIIOONO intermediate probably has not yet been observed. Recent freeze-quench Raman data reported by Yukl, et al. appear to support the nitrato-iron(III) formulation of the observed intermediate.41
We have recently reported a detailed study of the peroxynitrite myoglobin reaction.54 The central conclusions of that work were that a caged radical intermediate [FeIV=O .NO2] is indeed formed from MbFeIIIONOO during the metMb/PN interaction. This caged pair [FeIV=O .NO2] mainly rebounds to form metMb and nitrate in an oxygen rebound scenario, while freely diffusing .NO2 is liberated concomitantly from the radical pair and preferentially nitrates Tyr103 in horse heart myoglobin (Scheme 1, pathway D).
The key result was the direct observation of a UV-vis spectral transient characteristic of MbFeIV=O that appeared at a rate commensurate with the rate of the PN-Mb reaction (Figure 2). The data indicate a mechanism that involves rate limiting O-O bond homolysis in a peroxynitrito-FeIII-heme species (MbFeIII-O-ONO) to afford a caged [FeIV=O .NO2] intermediate (Scheme 1), as we had originally suggested in our initial report.45 Overall the process is analogous to the process we have described for synthetic iron and manganese porphyrins.55-57 The major pathway to close the catalytic cycle is cage collapse of [FeIV=O .NO2] to form an FeIII-heme and nitrate, a process analogous to ferryl oxygen rebound to carbon radical intermediates.58
Figure 2.
Direct spectroscopic detection of ferrylMb formation from the metMb and PN reaction, showing the Soret (A) and (B) Q band regions. (A), from reference 54.
The results also show that .NO2 can diffuse away from the heme site to induce nitration elsewhere. To confirm this latter pathway we employed fluorescein as a chemical trap of .NO2. The yield and kinetics of both ferrylMb and FlNO2 formation were used to quantitate the degree of dynamic cage escape of .NO2 into the protein interior and the medium during the catalytic decomposition of PN. Here the signature spectral changes upon nitration of fluorescein provide a very sensitive and informative measure of the flux of free-diffusing NO2. As shown in Figure 3, the extent of NO2 escape from the active site has a very large effect on the yield of fluorescein nitration. The data shows that myoglobin catalyzes the nitration of fluorescein, with the initial rate of nitrofluorescein nitration increasing with the concentration of Mb. Kinetic simulations confirm the observed data showing that 1) myoglobin accelerates the rate of fluorescein nitration in the early stages of the react and 2) the predicted yield of nitrofluorescein decreases much less than would be expected if all of the NO2 was captured by ferrylMb and converted to nitrate. Further, 3) the simulations reproduced the observed crossing of [Fl-NO2] vs time data plots at ~ 2.5 s for all concentrations of Mb. Significantly, all three of these features of the data are absent in simulations that did not allow for cage escape of NO2.
Figure 3.
(A) Experimental and simulated time courses of nitrofluorescein formation. Experiments (E) and simulations (S) were done under the same reaction conditions: 30 μM Fl, 520 μM PN, 0, 20 and 50 μM metMb. From top to bottom: 0 μM metMb (a, E in black and S in blue), 20 μM metMb (b, E in black and S in red), 50 μM metMb (c, E in black and S in green), 20 μM metMb with ke = 0 s-1 (d, S in dash line and red) and 50 μM metMb with ke = 0 s-1 (e, S in dash line and blue). (B) The formation and decay of ferrylMb during the reaction of 10 μM metMb and 100 μM PN in the absence (black) and presence (red) of 10 μM fluorescein. The structural figure illustrates cage escape of NO2 (reference 54).
Overall, the observed behavior of NO2 within the internal Mb cavities is reminiscent of the recombination and escape scenarios observed for photo-dissociated O2, NO and CO and is further evidence of directed, small molecule processing within the myoglobin structure.59 The details of such diffusive cage phenomena within heme proteins have been extensively studied by photophysical techniques that allow the rapid formation of the dissociated heme-ligand intermediate and spectrophotometric monitoring of ligand rebinding.60, 61 For example, the kinetics of NO rebinding to deoxyMb have been shown to be biphasic. This feature of the data has been interpreted to be the result of very rapid NO recombination from the distal pocket within 10 ps and a slower (200 ps) binding process of NO molecules from the Xe cavities.62 Statistical mechanics simulations have shown that 5% of the NO molecules were still found in the distal pocket and Xe cavities even after 1 ns.63 The kinetic barrier to this slower recombination phase has been associated with entropic considerations, solvent effects and the dynamics of conformational gating for diffusive return to the distal pocket.62, 64, 65
The results for the reaction of myoglobin with peroxynitrite indicate that an NO2 capture and escape scenario also applies. The NO2 formed at the active site by O-O bond homolysis in MbFeIIIOONO is the rate-limiting step can react with the ferryl oxygen to form nitrate (kr). Overall, this is a one-electron oxidation of nitrogen with concomitant oxygen transfer from iron. In competition with this cage recombination, .NO2 can retreat into the protein interior (ke). Moreover, Figure 3 illustrates that Tyr103 is preferentially nitrated, as was unambiguously determined from mass spectroscopic peptide sequence analysis on the purified mono-nitroMb. This nitration selectivity is likely caused by better solvent accessibility of this tyrosine as well as its co-planar donor-to-acceptor orientation with the heme.66 Interestingly, the degree of cage escape of NO2 observed for the myoglobin reaction is similar to the behavior of alkyl peroxynitrites upon rearrangement to alkyl nitrates.67
The extent of ferrylMb build-up and its time dependence can be understood in terms of a situation described by competitive cage collapse and cage escape (Scheme 2). The observed behavior reflects the balance between the oxidation rate k1, the fraction of NO2 cage escape ke/(ke + kr) and the reduction rate, k3. The rates of the reaction cycle (k1 and k3) were measured directly.45, 54 Kinetic simulations of the measured rates and yields according to Scheme 2 showed about 10% cage escape of NO2 (ke/kr = 0.10). FerrylMb can also be reduced by electron transfer from Tyr10368 and reaction with freely-diffusing NO2 from the medium by a reencounter mechanism (k2).69
Scheme 2.
The core catalytic cycle of metMb-catalyzed peroxynitrite decay.
The unambiguous observation of the ferrylMb intermediate provides strong confirmation of the proposed mechanism, in which the transient intermediate [FeIV=O .NO2] can either proceed to cage escape or rebound to form nitrate and ferric Mb. The decomposition of PN catalyzed by water soluble iron(III) porphyrins (e.g., FeTMPS and FeTMPyP) affords significant amounts of oxoFeIV porphyrins. 56, 70 In particular, the fast rebound of NO2 to FeIVTMPS accounts for an important pathway for oxoFeIV reduction to FeIII. The rate constant of this rebound can be as fast as 1.7 × 107 M-1 s-1.70
Biological Implications
The results we have described for the reaction of PN with metMb are pertinent to the discussion regarding the roles of oxyMb and oxyHb in trapping NO in vivo,52, 71 since the two processes intersect at the Fe(III)-OONO intermediate. Recently, this NO dioxygenase activity has been extended to other myoglobin-like proteins, such as neuroglobin,72 cytoglobin71 and bacterial hemoglobins.73 One would expect, a priori, that ferrylMb and NO2 would be formed to the same extent from the oxyMb/NO reaction as from metMb and PN. Olson and Gardner have described a thorough analysis of the isotopic content of nitrate resulting from the reaction of 18O2-oxyMb with NO.74 The results showed 99% dioxygenation of NO to afford 18O2-16O-nitrate via rebound to ferrylMb. Significantly, when we simulated the fate of ferrylMb and NO2 formed from the single-turn-over reaction of 18O2-oxyMb with NO using the same kinetic scheme described above (Scheme 2) and the known rate constant for oxyMb + NO,40 we also find only ~1% singly labeled nitrate, quantitatively consistent with the experimental result. The cage recombination mechanism affords little singly-labeled nitrate in the oxyMb/NO reaction because the concentration of NO2 is low under these conditions, making the bimolecular dimerization-hydrolysis pathway inefficient compared to NO2 rebound and tyrosine nitration.
Theoretical exploration of heme protein/PN intermediates
As seen from the preceding discussion, the goal of our dissection of the Mb-PN reaction has been to identify the major intermediates and measure the rates of the various transformations. Bloomberg et al. studied the oxyMb/NO interaction using hybrid density functional theory.75 They found that the free energy barrier to O-O bond cleavage in the FeIII-O-ONO intermediate for myoglobin was only 5.2 kcal/mol and formation of the [FeIV=O .NO2] intermediate was exergonic by 6.6-11.2 kcal/mol, with the range depending on the entropy considerations. Oxygen rebound to form nitrate was found to be highly exergonic (-24.7 kcal/mol) with a negligible enthalpic barrier to capture of NO2 by FeIV=O. The computed free energy barrier to this NO2 rebound was found to lie between 2.7 and 7.2 kcal/mol depending on how the entropy of NO2 was treated. The detection of ferrylMb and freely diffusing NO2 suggests that the larger of the calculated exothermicities for NO2 formation and the larger of the entropy barriers, both resulting from the more loosely bound NO2, are closer to the real situation. Interestingly, Crespo et al. recently calculated the reaction energy (ΔE) of FeIIIOONO homolysis to Fe(IV)=O and NO2 as well as that of FeIV=O and NO2 rebound to form FeIIIONO2 on the basis of a Mycobacterium tuberculosis truncated hemoglobin model 76. Both processes are exergonic. Combined with the analysis of spin and Mulliken populations, the FeIIIOONO complex was favorable to break homolytically into FeIV=O and .NO2 pair. These calculations also show that the FeIV=O and NO2 rebound reaction to form FeIIIONO2 (-18.0 kcal/mol) is much more exergonic than FeIIIOONO homolysis to FeIV=O and NO2 (-8.1 kcal/mol), which implicates the dominance of the rebound pathway compared with the diffusion pathway. Thus, The experimental results we have reported for the metMb PN reaction are in remarkable agreement with the calculated reaction energy landscape.
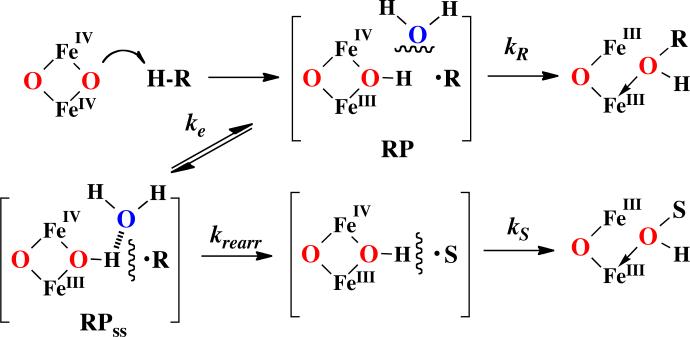
In further analogy to this stochastic behavior of incipient radical pairs, we have recently shown that cage escape competes with cage recombination during the C-H bond hydroxylation reaction of the diiron hydroxylase AlkB.77 The results suggest that caged radical pair phenomena may be more general in metalloenzyme catalysis. The realization here is that fluxional dynamics at the diiron active site can lead to a solvent separated radical pair in the course of C-H hydroxylation (Scheme 3). Radical clock probes have revealed radical lifetimes in the range of 10-100 ps for cytochrome P45078 to 10 ns for AlkB.77 These longer lifetimes allow for significant motion of the intermediate radical within the substrate-binding cavity. Moreover, the kinetics of rapid, diffusional cage escape of the intermediate radical dictates that clock timing will be affected. In particular, slower radical clock probes may show as much or more rearrangement than faster clocks if cage escape for those radicals is competitive with rebound to form products. Indeed, exactly this situation occurs during the hydroxylation of norcarane and bicyclohexane by AlkB. The radical rearrangement of the 2-norcaranyl radical is 10-fold faster than that of the 2-bicyclo[3.1.0]hexyl radical, yet almost two-fold more rearrangement was observed for the slower probe. The lesson here is that while intermediate radicals formed during substrate hydroxylation can be detected by such diagnostic probes, one should not expect a strict ordering of substrate rearrangement according to solution or gas phase rearrangement rates for those molecules.
Scheme 3.
Radical cage dynamics, with cage escape (ke) from the initial radical pair (RP) to form a solvent separated radical pair (RPss) competing with oxygen rebound (kr), provide a precedented explanation for the facilitated rearrangement of some radical probes (reference 77).
Cytochrome P450, Prostaglandin Synthase, Chloroperoxidase and Peroxynitrite
Thiolate-ligated heme proteins, including various cytochrome P450s (CYP), nitric oxide synthase (NOS), prostacyclin synthase (PGI2), chloroperoxidase (CPO), etc., have been shown to interact with PN and to be inactivated by PN-mediated nitration. Importantly, these nitrated or nitroslyated heme proteins appear to be of pathological significance and are detected in Parkinson's and Alzheimer's diseases, neurodegenerative diseases and cardiovascular disorders.35
Among the earliest observations of PN damage to heme proteins was the report by Ullrich et al. of the PN-induced inactivation of PGI2 synthase,29 a thiolate-ligated heme protein involved in the inflammatory response and platelet accumulation in humans. In subsequent work, they investigated the interactions and mechanisms of PN with P450cam, P450nor, chloroperoxidase (CPO), microperoxidase (MP-11) and P450BM3.30, 31, 79, 80 Combining stopped-flow experiments and phenol nitration results, it was concluded that a FeIV=O (compound II) species was a common intermediate in all five reactions via a mechanism analogous to that of metMb (Scheme 1: path B, C, D and E). Although the rate constants (kcat) for these PN-P450 reactions were not reported, kinetic comparisons of PN decay in the presence of different heme proteins were carried out and showed a reactivity sequence of P450nor > P450BM3 > MP-11 > HRP (horseradish peroxidase).80 Since the kcat for HRP was measured to be ~ 106 M-1 s-1, the kcat of P450nor and P450BM3, though not determined, can be estimated to be around 107 M-1 s-1.
Recently, the identification and characterization of the P450-PN intermediates has stirred another wave of interest toward understanding the underlying mechanisms of P450-PN reactions. Based on the results from Ullrich25, 26, and Floris et al.,81 it was believed that PN could be used to generate P450 compound II (Cpd II) in high yield, and that under some conditions P450 Cpd II was more stable than CPO Cpd II. The reaction kinetics of CPO with PN were recently reported by Gebicka and Didik.82 This paper reports CPO-catalyzed PN decay and the appearance of an intermediate, suggested to be CPO Cpd II on the basis of its visible spectrum in the Soret region, within 10 ms when 1 μM CPO was reacted with 10 μM PN at pH 5. The value of the bimolecular reaction rate of ferric CPO and PN was reported to be 107 M-1 s-1, faster than the reaction with its natural substrate, hydrogen peroxide. The pH dependence of the decay rate indicated that, as with myoglobin, peroxynitrous acid, HOONO, was the reacting species. Interestingly, the authors reported that most of the PN disappeared during the conversion of Cpd II back to ferric CPO. However, the three candidates to mediate the Cpd II reduction reaction, .NO2, nitrite ion and PN, were not able to fully rationalize the whole process.82
Newcomb et al. have reported that the CPO/PN intermediate and a relatively stable CYP119/PN intermediate could serve as a platform from which to generate the elusive P450 compound I (Cpd I) by laser flash photolysis (Scheme 4).83 The CPO/PN intermediate, with a Soret λmax at 433 nm, arose and decayed within 400 ms, while the CYP119 species was longer lived, taking 40 s to decay. It was noted that decay of the CYP119-PN intermediate to the resting ferric protein was dependent upon the intensity of the spectrometer light beam. This CYP119 intermediate was assigned the compound II FeIV=O formulation. Laser flash photolysis of the intermediate led to another transient in about 5% conversion that had a less intense and blue-shifted Soret λmax at ~ 400-410 nm and a weak Q-band at 640-670 nm, typical of a porphyrin cation radical. The resting ferric protein absorbs at 417 nm. On this basis the LFP intermediate was assigned to be the compound I of CYP119 with the usual oxoFeIV-porphyrn cation radical formulation. Paradoxically, however, this intermediate was observed to be notably unreactive toward substrates, in surprising contrast to the known reactivity83 of the well-characterized CPO compound I.84-86
Scheme 4.
Cytochrome P450 Compound I formation by laser flash photolysis, modified from ref 83.
Soon afterward, Green et al. reported results characterizing the P450BM3-PN intermediate as an iron(III)-nitrosyl (FeIII-NO) complex, not FeIV=O (or FeIV-OH) on the basis of Mössbauer spectroscopy and supporting density functional theory (DFT) calculations.87 The Mössbauer sample was prepared by the freeze-quench technique with the PN-P450bm3 mixture being quenched after 450 ms. A critical piece of the experimental evidence for the FeIII-NO assignment was the diamagnetism of the P450BM3-PN intermediate. More recently, Newcomb et al. reported XAS data of the CYP119-PN intermediate.88 From a comparison of the results to XAS data for the authentic nitrosyl complex, they concluded that the CYP119-PN intermediate is a protonated ferryl, FeIV-OH. This hydroxoiron(IV) porphyrin formulation had previously found in the Cpd II of CPO and P450s by Green et al. 89-91 It is to be noted that the Fe-N (NO) bond length of the authentic ferric nitrosyl and the Fe-O (OH) bond distance of the CYP119-PN intermediate were found to be the same (1.82 Å) from the EXAFS data. Further, the Fe-S (thiolate) of both species were also the same (2.24 Å). There was, however, a discernable difference in the XANES near edge features of the two samples. While such a long Fe-X bond distance is expected for FeIV-OH, ferric nitrosyls typically have much shorter FeIII-N (NO) bond ditances.92, 93 Kinetic studies of the decomposition of PN-generated CYP119 Cpd II were reported under conditions where the nitrosyl complex is apparently stable. P450 ferric nitrosyls are unstable to air, however, and some modes of PN decomposition afford oxygen. Newcomb, et al. offer an explanation for the discrepancy between the two labs observations. Nitrite, which is a contaminant in all peroxynitrite solutions, was reported to react with the ferric CYP119 protein to form the nitrosyl over a period of 100s. This, however, appears to be much longer than the 450 ms mixing time in the method described for the formation of the P450BM3 ferric-nitrosyl from PN reported by Green.87 So this issue appears not to be resolved. In the most recent reports, Newcomb et al. describe the formation of CYP119 compound I in high conversion, also via LFP of the CYP119-PN intermediate.94, 95 Now, however, the compound I is reactive toward substrates but has a spectrum very much like the resting ferric protein. Clearly, there is much more to do to characterize the spectral transients in these P450 reactions. On balance, the consensus view that P450 compound I is a particularly reactive, thiolate ligated, oxoiron(IV) porphyrin cation radical similar to the compound I of chloroperoxidase appears to be correct.96-99
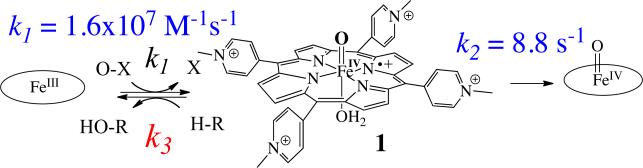
It is particularly pertinent in this regard that a highly reactive compound I model compound has recently been described in our laboratories.100 The UV-vis spectral features of this intermediate, which include a broad, long wavelength absorbance, indicate that it is an oxoiron(IV) porphyrin cation radical. Key to the observation of this species was use of a water-soluble porphyrin with 4-N-methylpyridinium substituents on the meso positions. The results show that [OFeIV-4-TMPyP+] (1), which is accessed chemically from a peroxide precursor, is orders of magnitude more reactive than the well-studied tetramesityl analogs.101-104 By mapping the rate constants observed for C-H bond cleavage by 1 onto the Brønsted-Evans-Polanyi relationship105, 106 for similar substrates, the BDE of H-OFeIV-4-TMPyP can be estimated to be ~100 kcal/mol (Figure 4), much stronger than the values estimated for H-OFeIVTMP (92 kcal/mol)103 and H-OFeIIITPFPP (~86 kcal/mol).107 The second-order rate constant for C-H bond cleavage in xanthene by this complex exceeded 106 M-1 s-1, and 1 showed a rate constant for ethylbenzoic acid hydroxylation similar to that reported recently by Newcomb, et al. for ethyl benzene with CYP119 and chloroperoxidase compound I.108
Figure 4.
Rates of hydrogen abstraction by [O=FeIV-4-TMPyP+] (1) reveal a 100 kcal/mol BDE for [H-OFeIV-4-TMPyP+] (reference 100).
An important advantage in this analysis was the finding that the reaction of FeIII-4-TMPyP with m-chloroperoxybenzoic acid was very fast (k1=1.6×107 M-1s-1), affording a nearly complete conversion to 1 within 10 ms in the stopped-flow experiment (Scheme 5). An additional advantage was the clear and quantitative conversion of 1 to a relatively stable ferryl species (k2=8.8 s-1). Substrate reactivity could be assessed by measuring k3 and by monitoring the ratio of FeIII and FeIV species produced during the reaction using global analysis techniques.
Scheme 5.
Kinetic partitioning of [OFeIV-4-TMPyP+] (1) between spontaneous decay to OFeIV-4-TMPyP (k2) and substrate reaction (k3) resulting in the formation of FeIII-4-TMPyP.
It is of considerable interest to consider why [O=FeIV-4-TMPyP+] (1) should be so reactive. Indeed, in water solvent, one would expect the oxo-group lone pairs to be solvated with hydrogen bonds. The hydrogen abstraction transition state depicted in Figure 4 would require prior desolvation of the oxidant. We have shown that electron-withdrawing meso-substituents dramatically decrease the reactivity of oxoMnV porphyrins,109 due to stabilization of the singlet d2 ground state with respect to the triplet and quintet manifolds, stabilization of the porphyrin HOMO and the unique centrosymmetric trans-dioxo ligand arrangement.110 Clearly, the situation for iron described here is markedly different. We have suggested that a relatively high redox potential for porphyrin ring oxidation for FeIII-4-TMyP would destabilize the cation radical, making this compound I model compound a stronger oxidant. Further, efficient transmission of the electron deficit to the metal core is signaled by the low pKa of the axial water in diaquaFeIII-4-TMyP (5.5), while the corresponding pKa of diaquaFeIIITMPS is 7.5.111 In this scenario, the high kinetic reactivity observed for [O=FeIV-4-TMPyP+] (1) may result in part from a decrease of the energy level of the iron d(x2-y2) orbital and spin state crossing phenomena into this orbital in the course of the reaction.106, 110, 112-114 The results show that even subtle charge modulation surrounding the heme center of cytochrome P450 could result a highly reactive P450 compound I.
Cytochrome c, Peroxynitrite and Apoptosis
Cytochrome c (cyt c) is a small globular heme protein present at high concentrations (~ 1 mM) in the intermembrane space of mitochondria.115 The primary role of cyt c is to shuttle electrons between respiratory complex III (cytochrome bc1 complex) and complex IV (cytochrome c oxidase), where oxygen is reduced to water. However, possible alternative functions of cyt c have been recognized recently. As cyt c is abundant in the mitochondrial intermembrane space, it is a potential target of the diffusible PN generated within or near the mitochondria. Cyt c nitration by PN in vitro has been previously studied by Radi et al.32, 33 The direct reactivity of cyt c3+ with PN was not as pronounced as other heme proteins we have so far. The inactivity of cyt c3+ with PN can be attributed to the inaccessible heme cavity, which contains a six-coordinate heme iron. However, ferro cyt c is oxidized to ferri cyt c by PN with a reaction rate constant 2.3×105 M-1 s-1.116 Significantly, the binding of cyt c to cardiolipin, a unique phospholipid in the mitochondrial membranes, converts cyt c from a six-coordinate heme iron into a five-coordinate iron form117-121 and thereby activates its interaction with PN via a metMb-like peroxidase mechanism.54 Another intriguing enzymatic role for cyt c that has been suggested recently is its possible involvement in the biosynthesis of fatty acid amides, which are now recognized as signaling molecules.122-124
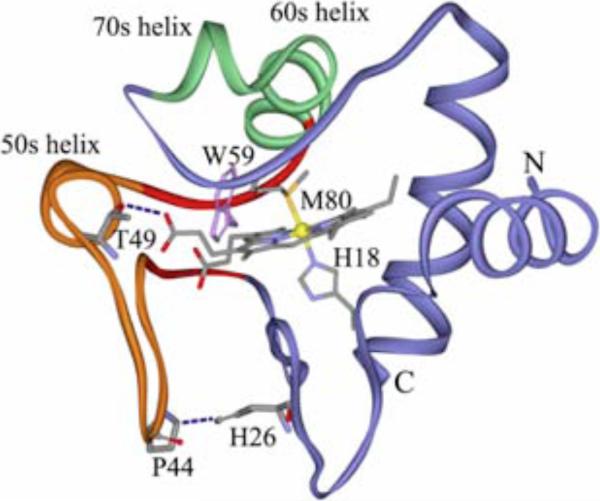
Raman spectroscopy has revealed that a substantial fraction of cyt c converts to a β-sheet structure, at the expense of turns and helices at low pH.125 It is proposed that a short β-sheet segment, comprising residues 37-39 and 58-61, extends itself into the large 37-61 loop when the latter is destabilized by protonation of H26, which forms an anchoring H-bond to loop residue P44 (Figure 5). This conformation change ruptures the Met80-Fe bond, as revealed by changes in ligation-sensitive Raman bands. It also induces peroxidase activity with the same temperature profile. We found that peroxidase activity is indeed induced, with a T near 54m °C when cyt c is heated at pH 3. Peroxidase activity was negligible at pH 7, regardless of temperature. Thus, peroxidase activity and β-sheet formation are directly correlated. This process is suggested to model the apoptotic peroxidation of cardiolipin by cyt c.
Figure 5.
Ribbon diagram of cyt c, highlighting the 40s Ω loop (orange) and the 37-61 foldon with its 37-39/58-61 β-sheet neck (red). A conformational switch involving protonation of H26 and disruption of the H26...P44 H-bond is proposed to displace the heme via the propionate H-bonds, inducing Met80-Fe bond rupture (reference 125).
We have studied the interactions of PN with a cyt c/liposome complex. In the presence of bovine heart cardiolipin-containing (bhCL) liposomes, cyt c was observed to catalyze PN decomposition. A linear dependence was observed for the rate of PN decay, kobs= ksp + kcat[cyt c-CL]0 (Figure 6A). The slope of kobs vs [cyt c-bhCL] gave a kcat value of 5.0 × 103 M-1 s-1 at pH 7.4 and 25 °C. In this set of experiments, the ratios of bhCL and cyt c were kept 100:1, while the concentrations of cyt c and bhCL were varied simultaneously. The liposomes used in our experiments were composed of both cardiolipin and DOPC (1:1), however, no catalysis of PN decomposition was seen when cyt c was mixed with liposomes that contained only DOPC (L/P = 200:1) (Figure 6A). Clearly, the unique dianionic charge and four unsaturated fatty acid tails of CL cause this striking change of cyt c function.
Figure 6.
(A, left panel) Pseudo-first-order rate constants for the decay of peroxynitrite vs the concentrations of cyt c/lipid complexes. Round circle: CL-containing liposomes (CL: DOPC=1:1, CL/cyt c= 100:1), triangle: DOPC only (DOPC/cyt c= 200:1). (B, right panel) Tyrosine nitration yields of cyt c by PN under different conditions. 1. cyt c + PN, 2. cyt c + PN+CL (DOPC: CL: cyt c= 25: 25:1), 3. Cyt c+ PN+ CL (DOPC: CL: cyt c=100:100:1), 4. Cyt c + PN+DOPC (DOPN:cyt c= 200:1). 5, 6, 7 are 1, 2, 3 with 1.2 mM CO2, respectively.
To determine the nitration yields of cyt c in membranes, both aqueous and cardiolipin-associated reactions of cyt c (20 μM) with PN (1 mM) were carried out using the stopped-flow spectrometer as a mixing device. We first estimated the yields of nitrated cyt c in the absence of lipid vesicles by electrospray mass spectrometry (ESI-Q-TOF). Reaction of 20 μM cyt c with 1 mM PN resulted in (20 ± 3) % nitrated cyt c by comparing the peak areas corresponding to unreacted and modified peaks. In the presence of a physiological concentration of CO2 (1.2 mM), the total nitration yield (including some dinitration) increased to (46 ± 6)%. Since lipid-bound cyt c solutions did not ionize well in the ESI source, cyt c tyrosine nitration yields in the presence of lipids were quantified by trypsin digestion followed by HPLC-MS/MS analysis (details in experimental section). As seen in Figure 6B, when the ratio of cyt c and CL was 1:25, the nitrated tyrosine yield increased moderately from (20 ± 3) % to (26 ± 2) %. When the ratio of cyt c and CL increased to 1: 100, the nitration yield enhanced dramatically to (45 ± 1) %, more than twice the amount observed in the absence of cardiolipin. By contrast, in the presence of only DOPC, the nitrotyrosine yield of cyt c was still ca. 20%. Interestingly, in the presence of 1.2 mM CO2, the extent of nitration was always around 50%, whether CL was present or not.
In contrast to the inertness of native cyt c during PN decay, the bimolecular rate constant kcat of bhCL-bound cyt c on PN decay was measured to be 5000 M -1 s-1 (L/P = 100:1). This value is about half of the kcat for metMb at pH 7.4 and 25 °C. Moreover, the nitration yields of cyt c by PN at a high bhCL/cyt c ratio of 100:1 were 2.5-fold higher than that of cyt c alone in aqueous solution. It is therefore tempting to propose that bhCL-bound cyt c has been converted to an effective PN activator and catalyzes the formation of .NO2 via a metMb-like mechanism.54 The presence of physiological concentration of CO2 (1.2 mM) in the bhCL liposome solutions abolished the catalytic effect and resulted in ~ 50 % nitration yields in all CO2-containing samples. This observation is expected as PN reacts with CO2 at a rate of 3 × 104 M-1 s-1,126 six times faster than with the heme of cyt c. In the presence of 1.2 mM CO2 and 20 μM cyt c/CL complex (L/P=100:1), over 90% PN flux would react with CO2 and affords NO2 and CO3.-3. Both radicals can nitrate tyrosine residues efficiently via tyrosyl radical formation. Hence, the catalysis of nitration by CO2 outcompetes the one by cyt c/bhCL complex, leading to almost identical yields of CO2-involved cyt c nitration in both absence and presence of CL. Additionally, CO3-. anion radical is known to be not permeable to lipid membranes.127
Metalloporphyrins that decompose peroxynitrite
As mimics for metalloproteins, especially cytochrome P450s, water-soluble metalloporphyrin reactions with PN have been examined carefully by our group55-57 and the Monsanto group.128 Porphyrins bearing charged ligands, such as the cationic Mn- and Fe-TMPyP or the anionic sulfonate complexes, have been the focus of most PN decomposition catalyst studies. In this review, the reaction mechanisms of two representative metalloporphyrins with PN will be briefly discussed. Water-soluble manganese(III) porphyrins react rapidly with PN, forming a stable oxoMnIV species and .NO2.55 Unlike the proposed radical cage [FeIV=O .NO2] formed in metMb-PN interactions, the generated .NO2 does not rebound with oxoMn rapidly, but diffuses into the solvent and nitrates exogenous substrates. In the meantime, this high valent oxoMnIV is reduced very slowly to MnIII. However, addition of biological antioxidants such as ascorbate and Trolox dramatically increased the turnover of PN decomposition through reduction of oxoMn(IV), with a rate on the same order of magnitude as the formation of oxoMnIV by PN (Scheme 6). Therefore, concurrent administration of reducing agents with Mn porphyrins may be an avenue for their pharmacological application.
Scheme 6.
Manganese porphyrin-antioxidant redox couples as PN decomposition system modified from ref 55.
Stern et al. have shown that [FeIIITMPyP] and [FeIIITMPS] display profound activity as “peroxynitrite isomerases” 128, converting ONOO- to NO3-. Lee et al. showed that FeIIITMPyP reacts rapidly with ONOO- to afford oxoFeIVTMPyP and .NO2.56 In analogy to Mn porphyrins, this oxoFeIVTMPyP appears to be relatively unreactive toward .NO2 and nitrite, but can catalyze the conversion of excess ONOO- to NO3- with a rate constant of 1.8×106 M-1 s-1. This represents a very significant advantage for the development of therapeutics based on this type of metallporphyrin reactivity since the .protein nitration due to PN can be drastically reduced. FeIIITMPS and PN interactions also involve the oxidation of FeIIITMPS and give rise to an oxoFeIV species, with a concomitant formation of .NO2, Shimanovich et al. found a fast rebound of NO2 to the oxoFeIV accounts for a preferential pathway for the reduction of oxoFeIV in the catalytic circle. 57 The presence of this “rebound” process sets FeIIITMPS apart from manganese porphyrins in that it can catalytically and cleanly decompose PN to harmless products without the aid of additional reducing agents (Scheme 7). Surprisingly, MnIII and FeIII-TMPyP are both able to efficiently catalyze the dismutation of the ONOO- precursor superoxide (O2.-) into O2 and H2O2 55, 56. Significantly, recent developments of a second-generation catalyst, based on Fe-2-TMPyP, led to the discovery of the highly reactive analog FP-15.129, 130 In several studies, this compound has been shown to be capable of reducing myocardial infarct size and reactive hyperemia as well as other pathological conditions, including diabetic neuropathy.129, 131-133 These very promising developments are an excellent example of ‘unintended’ favorable outcomes of basic research in bioinorganic chemistry that offer promise of far reaching impact in the health sciences.
Scheme 7.
Mechanism of PN interactions with FeTMPS and FeTMPyP, modified from references 55-57.
The catalytic rate constants for PN decomposition by heme proteins and Mn/Fe porphyrins are summarized in Table 1.
Table 1.
Reaction of PN with heme proteins and porphyrins (all pHs are around pH 7.4).
| Protein/Porphyrin | Rate constant, M-1 s-1 | Reference |
|---|---|---|
| Oxymyoglobin | 5.4 × 104 | 134 |
| Metmyoglobin | 1.0 × 104 | 45 |
| Oxyhemoglobin | 8.8 × 104 | 134 |
| Myeloperoxidase | 6.2 × 106 | 81 |
| Lactoperoxidase | 3.3 ×105 | 81 |
| Chloroperoxidase | 7.1 × 105 | 82 |
| Cytochrome c2+ | 2.3 ×105 | 116 |
| CL-bound cyt c 3+ (L/P=100) | 5.0 × 103 | this work |
| Horseradish Peroxidase | 3.2 ×106 | 81 |
| Microperoxidase 11 | ~ 106 | 80 |
| Cytochrome P450nor | ~107 | 79 |
| Cytochrome P450bm-3 | ~ 107 | 80 |
| Nitric Oxide Synthase | 2.2 ×105 | 135 |
| MnTMPyP | 3.2 ×106 | 55 |
| FeTMPS | 3.0 × 105 | 57 |
| FeTMPyP | 5.0 ×107 | 56 |
| FP15 | 2.0 ×107 | 130 |
| CO2 | 3.4 × 104 | 136 |
Summary and Conclusions
Reactions of heme proteins with PN are complex, depending on the type of heme protein, the concentration of PN and the specific reaction conditions. In biological systems, the level of CO2 also has to be taken into account. Considering the bimolecular rate constants between heme proteins and PN as shown in Table 1, the presence of CO2 at 1.2 mM definitely competes with oxyMb, oxyHb or their ferric forms to react with PN, whereas the influence of CO2 on P450s might not be as significant. The turnover of histidine-ligated heme proteins by PN may all share the same Cpd II intermediate, which can arise from a precursor radical cage [FeIV=O .NO2] as illustrated for myoglobin in Scheme 1. Upon the concurrent liberation of the nitrating .NO2 (ke), Cpd II can be observed distinctly. Meanwhile, the unreleased NO2 may recombine or rebound to Cpd II and “collapse” into the FeIII state and NO3- (kr). Depending on the ratio of ke/kr, the catalytic effect of heme protein on PN-mediated nitration can be reversed; difficulty in trapping Cpd II during the reaction may also rise. The ratio of ke/kr is determined by the sequence and conformation of the protein as well as the configuration of the heme. Nonetheless, thiolate ligated heme proteins such as CPO and P450 may also lead to ferric nitrosyls, as reported by Green et al.87 Identification of heme protein/PN intermediates has generally been heavily dependent on stopped-flow UV-Vis spectroscopy, and it is therefore prudent to examine and confirm those intermediates with additional techniques, such as kinetic criteria for diagnostic reactions, Mössbauer spectroscopy, X-ray absorption spectroscopies, etc. The understanding of these complex reactions between heme proteins and PN provides diverse avenues to develop novel and powerful porphyrin-based therapeutics.
Experimental Section
Materials
Horse heart cytochrome c (type C-2506, >99%), butylated hydroxytoluene (BHT) and sodium hydroxide were purchased from Sigma. Hydrogen peroxide (30%) and perchloric acid (70%) were obtained from J.T. Baker. Diethylenetriamine-pentaacetic acid (DTPA) was obtained from Alfa Aesar. Bovine heart cardiolipin (bhCL) and 1,2-dieleoyl-sn-glycero-3-phosphocholine (DOPC) were purchased from Avanti Polar Lipids Inc. Standard nitrotyrosine containing peptide KY*IPGTLGK was custom synthesized by Sigma Genosys.
Preparation of Vesicles
Small unilamellar vesicles (SUV) were prepared by sonication as previously described.137 Briefly, mixtures of bhCL and DOPC powders were dissolved in chloroform and transferred into 5 mL test tubes. The thin films of lipid were deposited on the walls of the test tubes after evaporating the solvent with a stream of argon, and were then left under high vacuum for 6 hours. The dried lipid films were hydrated in phosphate buffer (100 mM or 50 mM, 0.1mM DTPA) at room temperature for 1 hr. The hydrated bhCL/DOPC (1:1) or DOPC-containing lipid solutions were sonicated with a probe tip sonicator (Branson) in an ice-water bath for 5 min. After sonication, all samples were settled for 10 min. The vesicle suspensions were centrifuged at 12 000 r.p.m. for 5 min to remove the sonicator tip debris. Minimal exposure of lipids to light was ensured throughout the above procedures.
Peroxynitrite
PN was synthesized from hydrogen peroxide and nitrous acid as described138 using a sp250i syringe pump (KD scientific). To avoid any contamination with bicarbonate from ambient air, all the reagents for PN synthesis were degassed with argon thoroughly before mixing; the synthesized PN was later collected and kept under argon as well. Contaminating hydrogen peroxide was reduced to less than 5 % (molar ratio) of peroxynitrite by manganese dioxide (10 mg/mL at 4 °C for 15 min); MnO2 was removed through a 0.2 μm membrane syringe filter (PALL). Peroxynitrite concentrations were determined at 302 nm (ε = 1670 M-1 cm-1).139 The nitrite and nitrate content in peroxynitrite were estimated by Griess reagent and nitrate reductase purchased from Cayman. Peroxynitrite was decomposed in 0.14 M HClO4 prior to the above assay in that 90% of peroxynitrite decomposes into nitrate under acidic conditions.140 The concentrations of hydrogen peroxide in the peroxynitrite before and after manganese dioxide treatment were measured by PeroXOquant Quantitative peroxide assay kit (Pierce biotechnology) as instructed. The H2O2 measurements were performed after decomposing peroxynitrite at neutral pH phosphate buffer (0.2M, pH 7.2). Peroxynitrite solutions were prepared by diluting the stock solution immediately before use with 0.01 M NaOH to achieve the required concentrations. All the solutions involving the reactions with PN were purged with argon vigorously before mixing.
Cytochrome c Nitration
Nitration of cyt c3+ was performed by reaction with PN in the absence and presence of physiological concentration of CO2. To prepare pure cyt c3+, a small amount of potassium ferri cyanide (Sigma) was added to dissolved cyt c in 0.1 M (pH 7.2) phosphate buffer to oxidize the possible remaining cyt c2+ in solution. The solution was subsequently purified chromatographically over an Econo-Pac 10DG column (Bio-Rad) using a 0.1M phosphate buffer solution (pH 7.2) as eluant. The concentration of cyt c was determined by measuring the absorbance at 340 nm (ε340 = 21.4 mM-1 cm-1 and 550 (ε550 = 8 mM-1 cm-1).139
To achieve efficient and reproducible nitration of cyt c by synthetic PN, cyt c and PN were mixed by stopped-flow apparatus. Before reacting CL-bound cyt c with PN, cyt c was incubated with bhCL/DOPC (1:1) or DOPC only liposomes in 100 mM phosphate buffer for 5 min while being purged with argon. The mixings were performed with varied amounts of PN basic solutions and cyt c with or without liposome solutions in 100 mM phosphate buffer (pH 7.4, 0.1 mM DTPA). The nitrated cyt c solutions were collected at the outlet of the stopped-flow. BHT solution (final concentration: 50 μM) was added immediately into each resulting solution to quench any further radical reactions. The resulting solutions (200 μL) were subject to a protein dialysis by D-tube dialyzer (Calbiochem) against 50 mM ammonium biocarbonate (50 mM, pH 8) buffer for tyrpsin digestion and HPLC-tandem MS analysis. Meanwhile, 50 μL of each mixing solutions without liposomes was dialyzed against doubly deionized water using Slide-A-Lyzer Mini dialysis units (Pierce, MWCO 7000) for HPLC-chip-Q-TOF MS (Agilent) analysis.
Trypsin Digestion and LC/MS/MS peptide mapping
. Control and PN-treated liposome-bound cyt c samples were digested with trypsin (w/w = 1:1, Promega) at 37 °C for 24 hr. Known amount nitrotryosine-containing peptide KY*IPGTLGK was spiked into the control solution after digestion to determine the time delay between UV-Vis diode array detection and mass analyzer. All the digested samples were immediately analyzed by ThermoFinnigan LCQ DECA XP Plus ion trap mass spectrometer equipped with a Surveyor PDA UV-Vis detector. Digested samples were analyzed on a C-12 reverse phase column (Jupiter, 4 μ, 150 × 2.00 mm, Phenomenex) using a gradient elution described as follows. The solvent system consisted of solvent A (0.15 % TFA, 5 % acetylnitrile and H2O) and solvent B (0.1 % trifluoroacetic acid, 80 % acetylnitrile and H2O). The elution starts with an isocratic elution with 2 % B in the first 3 min followed by a linear gradient to 45 % B over 57 min and to 98 % over 4 min at a flow rate 0.2 mL/min. The ESI conditions were recorded as follows: sheath gas, 90 arbitrary units; auxiliary gas, 30 arbitrary units; spray voltage, 3.5 kV; capillary temperature, 200 °C; capillary voltage, 45 V and tube lens offset, 25V. Data were acquired in positive mode using Xcalibur software package (Thermo) with SEQUEST data base search. Combining SEQUEST search with manual evaluation, different nitrated peptides absorbing at 365 nm were identified unambiguously by tandem MS. The tyrosine nitration yields of cyt c under different conditions were computed by normalizing the total peak areas of nitrotyrosine peptides in LC chromatogram to an intact peptide GITWK found in all the chromatograms. The values of cyt c nitration yields in the presence of liposomes were calculated by comparing with the total nitration yields of cyt c without liposomes estimated by HPLC-Q-TOF as described.54
Stopped-Flow Kinetic Analysis
Stopped-flow kinetic studies were carried out using a Hi-Tech SF-61DX2 double mixing stopped-flow spectrophotometer (Hi-Tech, Salisburg, UK). The mixing time of the instrument is less than 2 ms and the mixing cell is 1cm long. The reactions were monitored either at a selected wavelength (single wavelength mode) or by using a diode array detector to record the entire visible range at each time point. For all the studies of the reactions under CO2-free conditions, the stopped-flow lines were washed with argon-purged doubly deionized water immediately before the experiments. Thoroughly degassed solutions of cyt c, liposome and PN were transferred in Hamilton gastight syringes right before the mixing. Several shots were collected as fast as possible. With all these efforts, essentially no CO2 was involved during the mixing of cyt c and PN. The traces were averaged from at least three shots. Kinetic data was analyzed and fitted on KinetAssyst software provided by Hi-Tech. Calculations and data treatment were performed using Microsoft Excel 2000 and a commercial graphics and data analysis software (Origin 7.5; MicroCal Inc.). In CO2-involed experiments, ~ 2.5 mM HCO3- was premixed with liposomes followed by mixing with other reactants in open air syringes by the stopped-flow apparatus.
HPLC-chip Q-TOF MS analysis
Nitrated protein mixtures were thoroughly dialyzed (Slide-A-Lyzer mini Dialysis units, 7,000 MWCO, Pierce) against water prior to further analysis by an Agilent 6510 LC Q-TOF MS. This Q-TOF was coupled with an Agilent HPLC-chip (G4240-62001, Zorbax 300SB-C18) for sample loading and separation. The LC-chip solvents were 0.1% formic acid/3% ACN/97% H2O v/v/v (solvent A) and 0.1% formic acid/10% H2O/90% ACN v/v/v (solvent B). Protein samples were diluted in solvent A before injection. The online LC-chip separation employed a gradient from 3 to 90% solvent B in 6 min and then 90 to 100% B in 8 min to 10 min, followed by 100% B for 5 min. The flow rate was 0.5 μl/min. Proteins eluted from HPLC-chip were directly injected into the coupled Q-TOF MS with a nanoelectrospray ionization source. MS data were acquired and processed using Agilent Masshunter workstation and included qualitative analysis software.
Acknowledgements
. We are grateful for support of this research by the National Institutes of Health (2R37 GM036298).
REFERENCES
- 1.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–4. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrer-Sueta G, Radi R. ACS Chem. Biol. 2009;4:161–177. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- 3.Beckman JS, Koppenol WH. Am. J. Physiol. 1996;271:C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 4.Lambeth JD. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 5.Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Free Radical Biol. Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 6.Winterbourn CC. Nat. Chem. Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 7.Malinski T, Taha Z, Grunfeld S, Patton S, Kapturczak M, Tomboulian P. Biochem. Biophys. Res. Commun. 1993;193:1076–1082. doi: 10.1006/bbrc.1993.1735. [DOI] [PubMed] [Google Scholar]
- 8.Tiscornia A, Cairoli E, Marquez M, Denicola A, Pritsch O, Cayota A. J. Immunol. Methods. 2009;342:49–57. doi: 10.1016/j.jim.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Ischiropoulos H, Zhu L, Beckman JS. Arch. Biochem. Biophys. 1992;298:446–51. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 10.Carreras MC, Pargament GA, Catz SD, Poderoso JJ, Boveris A. FEBS Lett. 1994;341:65–8. doi: 10.1016/0014-5793(94)80241-6. [DOI] [PubMed] [Google Scholar]
- 11.Rohn TT, Nelson LK, Sipes KM, Swain SD, Jutila KL, Quinn MT. J. Leukocyte Biol. 1999;65:59–70. doi: 10.1002/jlb.65.1.59. [DOI] [PubMed] [Google Scholar]
- 12.Mayer B, Schrammel A, Klatt P, Koesling D, Schmidt K. J. Biol. Chem. 1995;270:17355–60. doi: 10.1074/jbc.270.29.17355. [DOI] [PubMed] [Google Scholar]
- 13.Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A. Free Radical Biol. Med. 2001;30:463–88. doi: 10.1016/s0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- 14.Radi R, Beckman JS, Bush KM, Freeman BA. J. Biol. Chem. 1991;266:4244–50. [PubMed] [Google Scholar]
- 15.Alvarez MN, Piacenza L, Irigoin F, Peluffo G, Radi R. Arch. Biochem. Biophys. 2004;432:222–32. doi: 10.1016/j.abb.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Nalwaya N, Deen WM. Chem. Res. Toxicol. 2005;18:486–93. doi: 10.1021/tx049879c. [DOI] [PubMed] [Google Scholar]
- 17.Quijano C, Romero N, Radi R. Free Radical Biol. Med. 2005;39:728–41. doi: 10.1016/j.freeradbiomed.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Marla SS, Lee J, Groves JT. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14243–8. doi: 10.1073/pnas.94.26.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szabo C. Toxicol. Lett. 2003;140-141:105–12. doi: 10.1016/s0378-4274(02)00507-6. [DOI] [PubMed] [Google Scholar]
- 20.Burney S, Niles JC, Dedon PC, Tannenbaum SR. Chem. Res. Toxicol. 1999;12:513–20. doi: 10.1021/tx980254m. [DOI] [PubMed] [Google Scholar]
- 21.Burney S, Caulfield JL, Niles JC, Wishnok JS, Tannenbaum SR. Mutat. Res. 1999;424:37–49. doi: 10.1016/s0027-5107(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 22.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Free Radical Biol. Med. 2002;33:1451–64. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein S, Merenyi G. Methods Enzynol. 2008;436:49–61. doi: 10.1016/S0076-6879(08)36004-2. [DOI] [PubMed] [Google Scholar]
- 24.Landino LM. Methods Enzymol. 2008;440:95–109. doi: 10.1016/S0076-6879(07)00805-1. [DOI] [PubMed] [Google Scholar]
- 25.Bartesaghi S, Ferrer-Sueta G, Peluffo G, Valez V, Zhang H, Kalyanaraman B, Radi R. Amino acids. 2007;32:501–15. doi: 10.1007/s00726-006-0425-8. [DOI] [PubMed] [Google Scholar]
- 26.Aulak KS, Miyagi M, Yan L, West KA, Massillon D, Crabb JW, Stuehr DJ. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12056–61. doi: 10.1073/pnas.221269198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesbery WR, Butterfield DA. J. Neurochem. 2003;85:1394–401. doi: 10.1046/j.1471-4159.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 28.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS. Arch. Biochem. Biophys. 1992;298:431–7. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 29.Zou MH, Ullrich V. FEBS Lett. 1996;382:101–4. doi: 10.1016/0014-5793(96)00160-3. [DOI] [PubMed] [Google Scholar]
- 30.Daiber A, Herold S, Schoneich C, Namgaladze D, Peterson JA, Ullrich V. Eur. J. Biochem. 2000;267:6729–39. doi: 10.1046/j.1432-1033.2000.01768.x. [DOI] [PubMed] [Google Scholar]
- 31.Daiber A, Schoneich C, Schmidt P, Jung C, Ullrich V. J. Inorg. Biochem. 2000;81:213–20. doi: 10.1016/s0162-0134(00)00110-0. [DOI] [PubMed] [Google Scholar]
- 32.Cassina AM, Hodara R, Souza JM, Thomson L, Castro L, Ischiropoulos H, Freeman BA, Radi R. J. Biol. Chem. 2000;275:21409–15. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 33.Batthyany C, Souza JM, Duran R, Cassina A, Cervenansky C, Radi R. Biochemistry. 2005;44:8038–8046. doi: 10.1021/bi0474620. [DOI] [PubMed] [Google Scholar]
- 34.Kuhn DM, Sadidi M, Liu X, Kreipke C, Geddes T, Borges C, Watson JT. J. Biol. Chem. 2002;277:14336–42. doi: 10.1074/jbc.M200290200. [DOI] [PubMed] [Google Scholar]
- 35.Szabo C, Ischiropoulos H, Radi R. Nat. Rev. Drug Discovery. 2007;6:662–80. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 36.Pacher P, Beckman JS, Liaudet L. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groves JT. Curr. Opin. Chem. Biol. 1999;3:226–35. doi: 10.1016/S1367-5931(99)80036-2. [DOI] [PubMed] [Google Scholar]
- 38.Rossifanelli A, Antonini E, Caputo A. Adv. Protein Chem. 1964;19:73–222. doi: 10.1016/s0065-3233(08)60189-8. [DOI] [PubMed] [Google Scholar]
- 39.Brunori M. Trends Biochem. Sci. 2001;26:209–210. doi: 10.1016/s0968-0004(01)01824-2. [DOI] [PubMed] [Google Scholar]
- 40.Herold S, Exner M, Nauser T. Biochemistry. 2001;40:3385–95. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 41.Yukl ET, de Vries S, Moenne-Loccoz P. J. Am. Chem. Soc. 2009;131:7234–7235. doi: 10.1021/ja9026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doyle MP, Hoekstra JW. J. Inorg. Biochem. 1981;14:351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 43.Flonta SE, Arena S, Pisacane A, Michieli P, Bardelli A. Am. J. Pathol. 2009;175:201–206. doi: 10.2353/ajpath.2009.081124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gow AJ, Luchsinger BP, Pawloski JR, Singel DJ, Stamler JS. Proc. Natl. Acad. Sci. U.S.A. 1999;96:9027–32. doi: 10.1073/pnas.96.16.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bourassa JL, Ives EP, Marqueling AL, Shimanovich R, Groves JT. J. Am. Chem. Soc. 2001;123:5142–3. doi: 10.1021/ja015621m. [DOI] [PubMed] [Google Scholar]
- 46.Herold S, Shivashankar K, Mehl M. Biochemistry. 2002;41:13460–72. doi: 10.1021/bi026046h. [DOI] [PubMed] [Google Scholar]
- 47.Gardner PR. J. Inorg. Biochem. 2005;99:247–266. doi: 10.1016/j.jinorgbio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Gardner PR, Gardner AM, Martin LA, Salzman AL. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10378–10383. doi: 10.1073/pnas.95.18.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herold S, Shivashankar K. Biochemistry. 2003;42:14036–46. doi: 10.1021/bi0350349. [DOI] [PubMed] [Google Scholar]
- 50.Schopfer MP, Mondal B, Lee D-H, Sarjeant AAN, Karlin KD. J. Am. Chem. Soc. 2009;131:11304–11305. doi: 10.1021/ja904832j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herold S. FEBS Lett. 1999;443:80–84. doi: 10.1016/s0014-5793(98)81345-8. [DOI] [PubMed] [Google Scholar]
- 52.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. Free Radical Biol. Med. 2004;36:685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein S, Merenyi G, Samuni A. J. Am. Chem. Soc. 2004;126:15694–701. doi: 10.1021/ja046186+. [DOI] [PubMed] [Google Scholar]
- 54.Su J, Groves JT. J. Am. Chem. Soc. 2009;131:12979–12988. doi: 10.1021/ja902473r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JB, Hunt JA, Groves JT. J. Am. Chem. Soc. 1998;120:6053–6061. [Google Scholar]
- 56.Lee JB, Hunt JA, Groves JT. J. Am. Chem. Soc. 1998;120:7493–7501. [Google Scholar]
- 57.Shimanovich R, Groves JT. Arch. Biochem. Biophys. 2001;387:307–17. doi: 10.1006/abbi.2000.2247. [DOI] [PubMed] [Google Scholar]
- 58.Groves JT. Proc. Natl. Acad. Sci. U.S.A. 2003;100:3569–74. doi: 10.1073/pnas.0830019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frauenfelder H, McMahon BH, Austin RH, Chu K, Groves JT. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2370–4. doi: 10.1073/pnas.041614298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jongeward KA, Magde D, Taube DJ, Marsters JC, Traylor TG, Sharma VS. J. Am. Chem. Soc. 1988;110:380–387. [Google Scholar]
- 61.Austin RH, Beeson KW, Eisenstein L, Frauenfelder H, Gunsalus IC. Biochemistry. 1975;14:5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- 62.Ionascu D, Gruia F, Ye X, Yu A, Rosca F, Beck C, Demidov A, Olson JS, Champion PM. J. Am. Chem. Soc. 2005;127:16921–16934. doi: 10.1021/ja054249y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nutt DR, Meuwly M. Biophys J. 2006;90:1191–1201. doi: 10.1529/biophysj.105.071522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldbeck RA, Bhaskaran S, Ortega C, Mendoza JL, Olson JS, Soman J, Kliger DS, Esquerra RM. Proc. Natl. Acad. Sci. U.S.A. 2006;103:1254–1259. doi: 10.1073/pnas.0507840103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frauenfelder H, McMahon BH, Fenimore PW. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8615–8617. doi: 10.1073/pnas.1633688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Makinen MW, Schichman SA, Hill SC, Gray HB. Science. 1983;222:929–931. doi: 10.1126/science.6415814. [DOI] [PubMed] [Google Scholar]
- 67.Goldstein S, Lind J, Merenyi G. J. Phys. Chem. A. 2004;108:1719–1725. [Google Scholar]
- 68.Lardinois OM, Ortiz de Montellano PR. Biochemistry. 2004;43:4601–4610. doi: 10.1021/bi036241b. [DOI] [PubMed] [Google Scholar]
- 69.Goldstein S, Merenyi G, Samuni A. J. Am. Chem. Soc. 2004;126:15694–701. doi: 10.1021/ja046186+. [DOI] [PubMed] [Google Scholar]
- 70.Shimanovich R, Hannah S, Lynch V, Gerasimchuk N, Mody TD, Magda D, Sessler J, Groves JT. J. Am. Chem. Soc. 2001;123:3613–3614. doi: 10.1021/ja005856i. [DOI] [PubMed] [Google Scholar]
- 71.Mammen PP, White J, McGrath AJ, Kanatous SB, Horton JW, Garry DJ. Circulation. 2003;108:291–291. [Google Scholar]
- 72.Brunori M, Giuffre A, Nienhaus K, Nienhaus GU, Scandurra FM, Vallone B. Proc. Natl. Acad. Sci. U.S.A. 2005;102:8483–8488. doi: 10.1073/pnas.0408766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ascenzi P, Bocedi A, Bolognesi M, Fabozzi G, Milani M, Visca P. Biochem. Biophy. Res. Commun. 2006;339:450–456. doi: 10.1016/j.bbrc.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 74.Gardner PR, Gardner AM, Brashear WT, Suzuki T, Hvitved AN, Setchell KDR, Olson JS. J. Inorg. Biochem. 2006;100:542–550. doi: 10.1016/j.jinorgbio.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 75.Blomberg LM, Blomberg MRA, Siegbahn PEM. J. Biol. Inorg. Chem. 2004;9:923–935. doi: 10.1007/s00775-004-0585-5. [DOI] [PubMed] [Google Scholar]
- 76.Crespo A, Marti MA, Kalko SG, Morreale A, Orozco M, Gelpi JL, Luque FJ, Estrin DA. J. Am. Chem. Soc. 2005;127:4433–4444. doi: 10.1021/ja0450004. [DOI] [PubMed] [Google Scholar]
- 77.Austin RN, Luddy K, Erickson K, Pender-Cudlip M, Bertrand E, Deng D, Buzdygon RS, van Beilen JB, Groves JT. Angew. Chem. Int. Ed. 2008;47:5232–5234. doi: 10.1002/anie.200801184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Austin RN, Deng DY, Jiang YY, Luddy K, van Beilen JB, Ortiz de Montellano PR, Groves JT. Angew. Chem. Int. Ed. 2006;45:8192–8194. doi: 10.1002/anie.200603282. [DOI] [PubMed] [Google Scholar]
- 79.Mehl M, Daiber A, Herold S, Shoun H, Ullrich V. Nitric Oxide-Biol. Chem. 1999;3:142–152. doi: 10.1006/niox.1999.0217. [DOI] [PubMed] [Google Scholar]
- 80.Zou MH, Daiber A, Peterson JA, Shoun H, Ullrich V. Arch. Biochem. Biophys. 2000;376:149–55. doi: 10.1006/abbi.2000.1699. [DOI] [PubMed] [Google Scholar]
- 81.Floris R, Piersma SR, Yang G, Jones P, Wever R. Eur. J. Biochem. 1993;215:767–75. doi: 10.1111/j.1432-1033.1993.tb18091.x. [DOI] [PubMed] [Google Scholar]
- 82.Gebicka L, Didik J. J. Inorg. Biochem. 2007;101:159–64. doi: 10.1016/j.jinorgbio.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 83.Newcomb M, Zhang R, Chandrasena REP, Halgrimson JA, Horner JH, Makris TM, Sligar SG. J. Am. Chem. Soc. 2006;128:4580–4581. doi: 10.1021/ja060048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davydov R, Osborne RL, Kim SH, Dawson JH, Hoffman BM. Biochemistry. 2008;47:5147–5155. doi: 10.1021/bi702514d. [DOI] [PubMed] [Google Scholar]
- 85.Stone KL, Behan RK, Green MT. Proc. Natl. Acad. Sci. U.S.A. 2006;103:12307–12310. doi: 10.1073/pnas.0603159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stone KL, Behan RK, Green MT. Proc. Natl. Acad. Sci. U.S.A. 2005;102:16563–16565. doi: 10.1073/pnas.0507069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Behan RK, Hoffart LM, Stone KL, Krebs C, Green MT. J. Am. Chem. Soc. 2007;129:5855–5859. doi: 10.1021/ja064590y. [DOI] [PubMed] [Google Scholar]
- 88.Newcomb M, Halgrimson JA, Horner JH, Wasinger EC, Chen LX, Sligar SG. Proc. Natl. Acad. Sci. U.S.A. 2008;105:8179–8184. doi: 10.1073/pnas.0708299105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Green MT, Dawson JH, Gray HB. Science. 2004;304:1653–1656. doi: 10.1126/science.1096897. [DOI] [PubMed] [Google Scholar]
- 90.Green MT. Curr. Opin. Chem. Biol. 2009;13:84–88. doi: 10.1016/j.cbpa.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 91.Behan RK, Hoffart LM, Stone KL, Krebs C, Green MT. J. Am. Chem. Soc. 2006;128:11471–11474. doi: 10.1021/ja062428p. [DOI] [PubMed] [Google Scholar]
- 92.Praneeth VKK, Paulat F, Berto TC, George SD, Nather C, Sulok CD, Lehnert N. J. Am. Chem. Soc. 2008;130:15288–15303. doi: 10.1021/ja801860u. [DOI] [PubMed] [Google Scholar]
- 93.Ellison MK, Scheidt WR. J. Am. Chem. Soc. 1999;121:5210–5219. [Google Scholar]
- 94.Wang Q, Sheng X, Horner JH, Newcomb M. J. Am. Chem. Soc. 2009;131:10629–10636. doi: 10.1021/ja9031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sheng X, Zhang HM, Hollenberg PF, Newcomb M. Biochemistry. 2009;48:1620–1627. doi: 10.1021/bi802279d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spolitak T, Dawson JH, Ballou DP. J. Biol. Chem. 2005;280:20300–20309. doi: 10.1074/jbc.M501761200. [DOI] [PubMed] [Google Scholar]
- 97.Kellner DG, Hung SC, Weiss KE, Sligar SG. J. Biol. Chem. 2002;277:9641–9644. doi: 10.1074/jbc.C100745200. [DOI] [PubMed] [Google Scholar]
- 98.Egawa T, Shimada H, Ishimura Y. Biochem. Biophys. Res. Commun. 1994;201:1464–1469. doi: 10.1006/bbrc.1994.1868. [DOI] [PubMed] [Google Scholar]
- 99.Ortiz de Montellano PR. Chem. Rev. 2009;109 ASAP; DOI: 10.1021/cr9002193. [Google Scholar]
- 100.Bell SR, Groves JT. J. Am. Chem. Soc. 2009;131:9640–9641. doi: 10.1021/ja903394s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Groves JT, Watanabe Y. J. Am. Chem. Soc. 1988;110:8443–8452. [Google Scholar]
- 102.Groves JT, Haushalter RC, Nakamura M, Nemo TE, Evans BJ. J. Am. Chem. Soc. 1981;103:2884–2886. [Google Scholar]
- 103.Pan ZZ, Zhang R, Fung LWM, Newcomb M. Inorg. Chem. 2007;46:1517–1519. doi: 10.1021/ic061972w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Franke A, Fertinger C, van Eldik R. Angew. Chem. Int. Ed. 2008;47:5238–5242. doi: 10.1002/anie.200800907. [DOI] [PubMed] [Google Scholar]
- 105.Mayer JM. Acc. Chem. Res. 1998;31:441–450. [Google Scholar]
- 106.Shaik S, Kumar D, de Visser SP. J. Am. Chem. Soc. 2008;130:10128–10140. doi: 10.1021/ja8019615. [DOI] [PubMed] [Google Scholar]
- 107.Jeong YJ, Kang Y, Han AR, Lee YM, Kotani H, Fukuzumi S, Nam W. Angew. Chem. Int. Ed. 2008;47:7321–7324. doi: 10.1002/anie.200802346. [DOI] [PubMed] [Google Scholar]
- 108.Sheng X, Horner JH, Newcomb M. J. Am. Chem. Soc. 2008;130:13310–13320. doi: 10.1021/ja802652b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jin N, Groves JT. J. Am. Chem. Soc. 1999;121:2923–2924. [Google Scholar]
- 110.De Angelis F, Jin N, Car R, Groves JT. Inorg. Chem. 2006;45:4268–4276. doi: 10.1021/ic060306s. [DOI] [PubMed] [Google Scholar]
- 111.Shimanovich R, Groves JT. Arch. Biochem. Biophys. 2001;387:307–317. doi: 10.1006/abbi.2000.2247. [DOI] [PubMed] [Google Scholar]
- 112.Balcells D, Raynaud C, Crabtree RH, Eisenstein O. Inorg. Chem. 2008;47:10090–10099. doi: 10.1021/ic8013706. [DOI] [PubMed] [Google Scholar]
- 113.Altun A, Shaik S, Thiel W. J. Am. Chem. Soc. 2007;129:8978–8987. doi: 10.1021/ja066847y. [DOI] [PubMed] [Google Scholar]
- 114.Ogliaro F, de Visser SP, Groves JT, Shaik S. Angew. Chem. Int. Ed. 2001;40:2874–2878. [PubMed] [Google Scholar]
- 115.Vanneste WH. Biochim. Biophys. Acta. 1966;113:175–&. doi: 10.1016/s0926-6593(66)80132-7. [DOI] [PubMed] [Google Scholar]
- 116.Thomson L, Trujillo M, Telleri R, Radi R. Arch. Biochem. Biophys. 1995;319:491–7. doi: 10.1006/abbi.1995.1321. [DOI] [PubMed] [Google Scholar]
- 117.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Nat. Chem. Biol. 2005;1:223–32. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 118.Kagan VE, Bayir HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang JF, Stoyanovsky DA, Wipf P, Kochanek PM, Greenberger JS, Pitt B, Shvedova AA, Borisenko G. Free Radical Biol. Med. 2009;46:1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kagan VE, Bayir A, Bayir H, Stoyanovsky D, Borisenko GG, Tyurina YY, Wipf P, Atkinson J, Greenberger JS, Chapkin RS, Belikova NA. Mol. Nutr. Food Res. 2009;53:104–114. doi: 10.1002/mnfr.200700402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jitkaew S, Witasp E, Zhang S, Kagan VE, Fadeel B. J. Leukocyte Biol. 2009;85:427–437. doi: 10.1189/jlb.0408232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Godoy LC, Munoz-Pinedo C, Castro L, Cardaci S, Schonhoff CM, King M, Tortora V, Marin M, Miao Q, Jiang JF, Kapralov A, Jemmerson R, Silkstone GG, Patel JN, Evans JE, Wilson MT, Green DR, Kagan VE, Radi R, Mannick JB. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2653–2658. doi: 10.1073/pnas.0809279106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zaccagnino P, Saltarella M, D'Oria S, Corcelli A, Saponetti MS, Lorusso M. Free Radical Biol. Med. 2009;47:585–592. doi: 10.1016/j.freeradbiomed.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 123.Farrell EK, Merkler DJ. Drug Discovery Today. 2008;13:558–568. doi: 10.1016/j.drudis.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mueller GP, Driscoll WJ. J. Biol. Chem. 2007;282:22364–22369. doi: 10.1074/jbc.M701801200. [DOI] [PubMed] [Google Scholar]
- 125.Balakrishnan G, Hu Y, Oyerinde OF, Su J, Groves JT, Spiro TG. J. Am. Chem. Soc. 2007;129:504–505. doi: 10.1021/ja0678727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lymar SV, Hurst JK. J. Am. Chem. Soc. 1995;117:8867–8868. [Google Scholar]
- 127.Zhang H, Bhargava K, Keszler A, Feix J, Hogg N, Joseph J, Kalyanaraman B. J. Biol. Chem. 2003;278:8969–78. doi: 10.1074/jbc.M211561200. [DOI] [PubMed] [Google Scholar]
- 128.Stern MK, Jensen MP, Kramer K. J. Am. Chem. Soc. 1996;118:8735–8736. [Google Scholar]
- 129.Mabley JG, Liaudet L, Pacher P, Southan GJ, Groves JT, Salzman AL, Szabo C. Mol. Med. 2002;8:581–90. [PMC free article] [PubMed] [Google Scholar]
- 130.Szabo C, Mabley JG, Moeller SM, Shimanovich R, Pacher P, Virag L, Soriano FG, Van Duzer JH, Williams W, Salzman AL, Groves JT. Mol. Med. 2002;8:571–580. [PMC free article] [PubMed] [Google Scholar]
- 131.Radovits T, Seres L, Gero D, Lin LN, Beller CJ, Chen SH, Zotkina J, Berger I, Groves JT, Szabo C, Szabo G. Mech. Ageing Dev. 2007;128:173–81. doi: 10.1016/j.mad.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 132.Drel VR, Pacher P, Vareniuk I, Pavlov IA, Ilnytska O, Lyzogubov VV, Bell SR, Groves JT, Obrosova IG. Int. J. Mol. Med. 2007;20:783–792. [PMC free article] [PubMed] [Google Scholar]
- 133.Drel VR, Pacher P, Vareniuk I, Pavlov IA, Ilnytska O, Lyzogubov VV, Tibrewala J, Groves JT, Obrosova IG. Eur. J. Pharmacol. 2007;569:48–58. doi: 10.1016/j.ejphar.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Exner M, Herold S. Chem. Res. Toxicol. 2000;13:287–93. doi: 10.1021/tx990201k. [DOI] [PubMed] [Google Scholar]
- 135.Marechal A, Mattioli TA, Stuehr DJ, Santolini J. J. Biol. Chem. 2007;282:14101–12. doi: 10.1074/jbc.M609237200. [DOI] [PubMed] [Google Scholar]
- 136.Denicola A, Freeman BA, Trujillo M, Radi R. Arch. Biochem. Biophys. 1996;333:49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- 137.Luo M, Fadeev EA, Groves JT. J. Am. Chem. Soc. 2005;127:1726–1736. doi: 10.1021/ja044230f. [DOI] [PubMed] [Google Scholar]
- 138.Koppenol WH, Kissner R, Beckman JS. Methods Enzymol. 1996;269:296–302. doi: 10.1016/s0076-6879(96)69030-2. [DOI] [PubMed] [Google Scholar]
- 139.Kakinoki K, Maeda Y, Hasegawa K, Kitano H. J. Colloid Interf. Sci. 1995;170:18–24. [Google Scholar]
- 140.Maiti D, Lee DH, Sarjeant AAN, Pau MYM, Solomon EI, Gaoutchenova K, Sundermeyer J, Karlin KD. J. Am. Chem. Soc. 2008;130:6700–6703. doi: 10.1021/ja801540e. [DOI] [PubMed] [Google Scholar]