Abstract
Correlation of leukocyte typing with homograft survival suggests that HL-A typing of white blood cells reflects the histocompatibility factors of the kidney, yet some apparently well-matched kidneys are rejected. The latter results may, in part, reflect inadequacies of typing techniques, incomplete expression of HL-A factors on white blood cells as compared with the cells of the rejected organ, or isoantigens not shared with leukocytes.
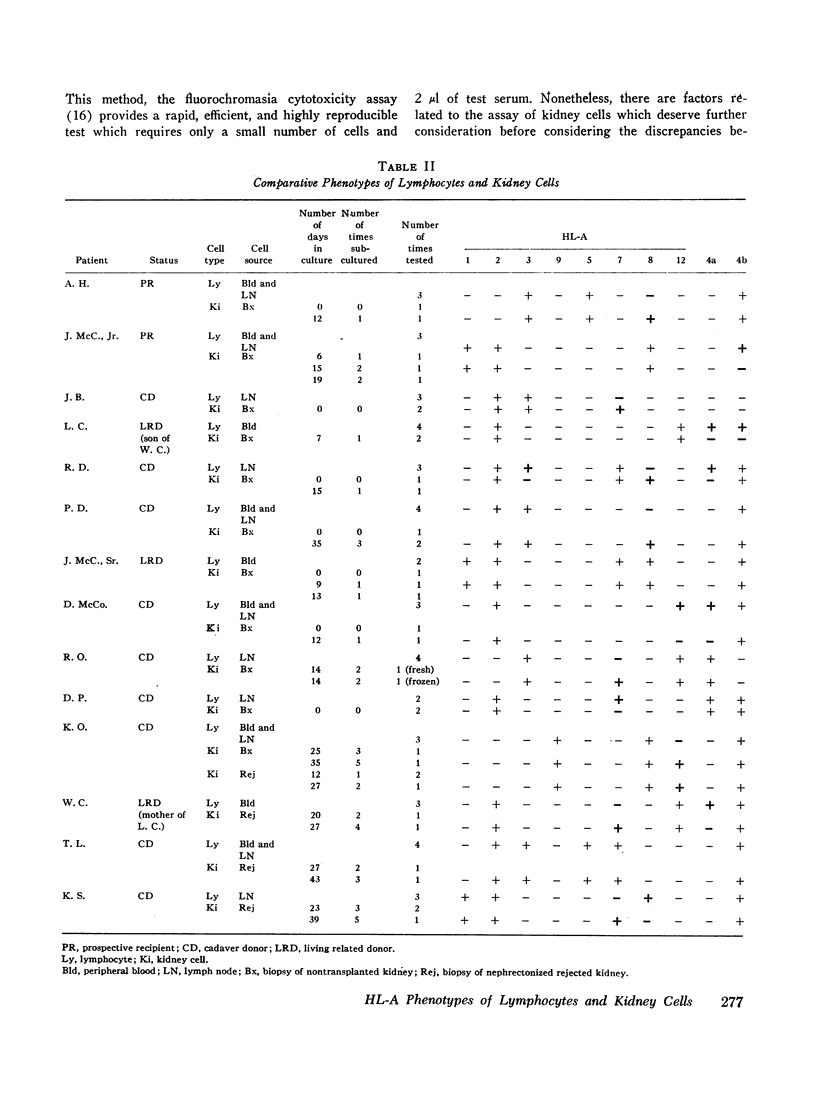
In this study the kidney cells and lymphocytes (from blood or nodes) of 14 individuals were typed for HL-A factors 1, 2, 3, 5, 7, 8, 9, and 12, and factors 4a and 4b by fluorochromasia cytotoxicity. Biopsied kidney cells were prepared with 0.25% trypsin and typed fresh, after varying periods in monolayer culture or after storage in liquid nitrogen, in all cases resulting in cells which were pleomorphic but uniform in reactivity.
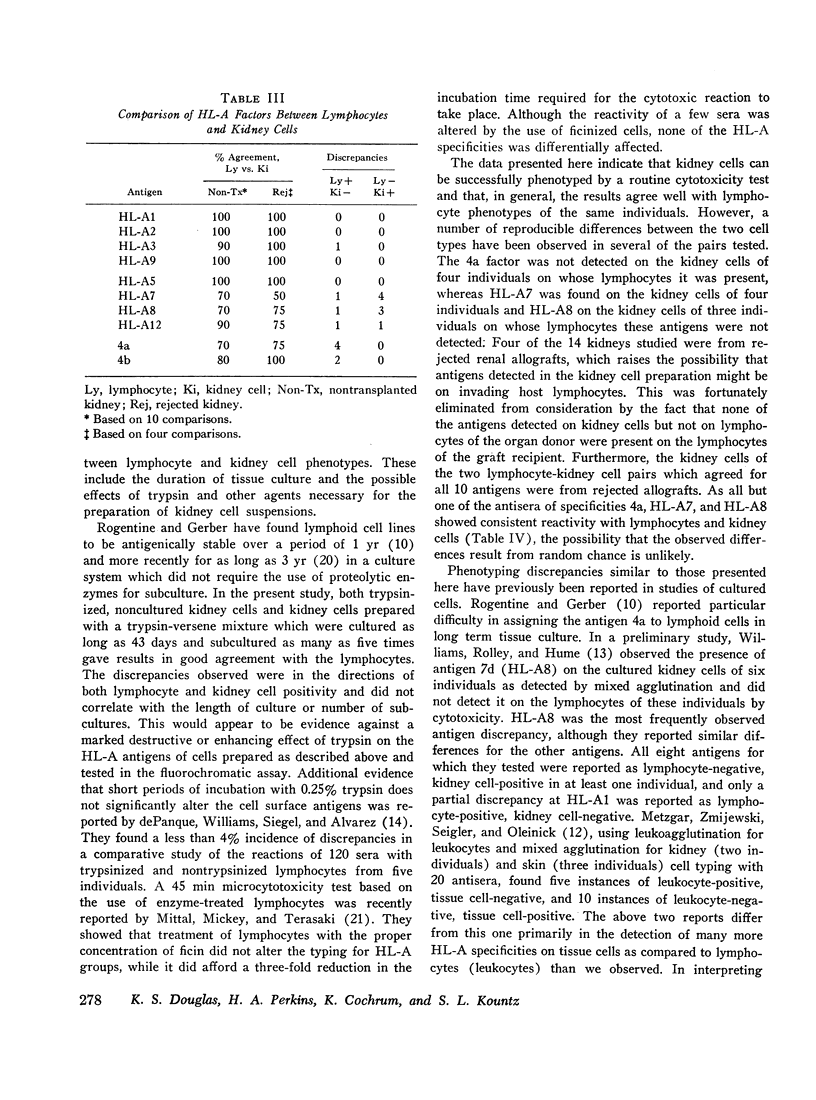
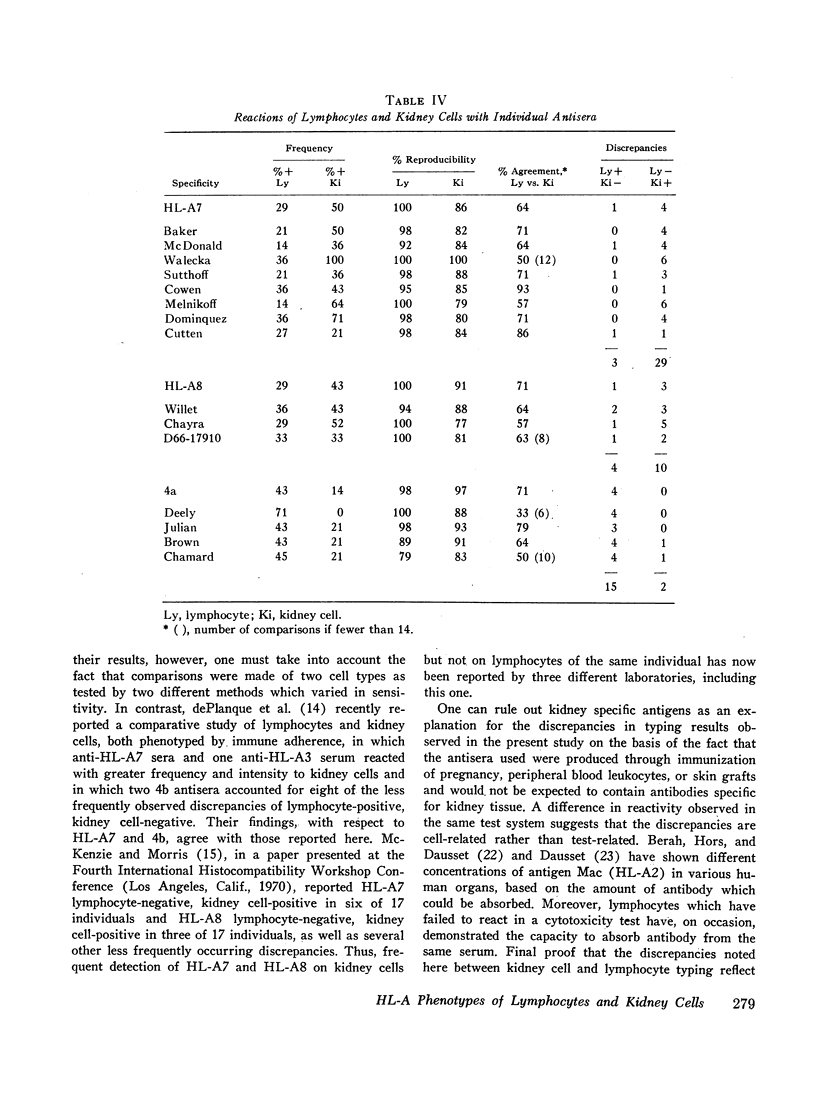
Reproducibility of lymphocyte typing was 99%, and of kidney cell typing, 93%. The 4a factor was detected on the lymphocytes but not the kidney cells of four individuals. HL-A7 and HL-A8, in contrast, were detected on kidney cells and not lymphocytes of four and three individuals, respectively. Results were consistent within the groups of individual sera used to detect each factor. The HL-A factors detected on both kidney cells and lymphocytes never resulted in more than two alleles at each genetic sublocus.
Several examples of post-rejection sera have reacted with donor kidney cells but not with lymphocytes. Kidney cells may thus be useful in compatibility tests to aid in selection of donors for a retransplant.
The ability to store donor kidneys by perfusion provides time to employ kidney cells for typing and in compatibility tests, and the use of a standard cytotoxic assay makes their routine use practical. Typing kidney cells as well as lymphocytes thus offers an approach to more complete and accurate HL-A phenotyping.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belzer F. O., Ashby B. S., Gulyassy P. F., Powell M. Successful seventeen-hour preservation and transplantation of human-cadaver kidney. N Engl J Med. 1968 Mar 14;278(11):608–610. doi: 10.1056/NEJM196803142781108. [DOI] [PubMed] [Google Scholar]
- Cochrum K. C., Kountz S. L. Cytotoxic antibodies following human renal transplantation. Surg Forum. 1969;20:302–304. [PubMed] [Google Scholar]
- Dausset J. Tissue typing and transplantation. Vox Sang. 1969 Apr;16(4):263–278. doi: 10.1111/j.1423-0410.1969.tb04747.x. [DOI] [PubMed] [Google Scholar]
- Kountz S. L., Cochrum K. C., Perkins H. A., Douglas K. S., Belzer F. O. Selection of allograft recipients by leukocyte and kidney cell phenotyping. Surgery. 1970 Jul;68(1):69–76. [PubMed] [Google Scholar]
- Lee H. M., Hume D. M., Vredevoe D. L., Mickey M. R., Terasaki P. I. Serotyping for homotransplantation. IX. Evaluation of leukocyte antigen matching with the clinical course and rejection types. Transplantation. 1967 Jul;5(4 Suppl):1040–1045. doi: 10.1097/00007890-196707001-00038. [DOI] [PubMed] [Google Scholar]
- Metzgar R. S., Flanagan J. F., Zmijewski C. M. Detection of tissue isoantigens on primary and serial heteroploid cell lines of human origin by the mixed agglutination technique. J Immunol. 1965 Sep;95(3):494–500. [PubMed] [Google Scholar]
- Metzgar R. S., Zmijewski C. M., Seigler H. F., Oleinick S. R. A comparison of the leukocyte agglutination and mixed agglutination techniques for detecting human tissue isoantigens. Transplantation. 1968 Jan;6(1):84–90. [PubMed] [Google Scholar]
- Payne R., Bodmer W. F., Troup G. M., Walford R. L. Serologic activities and specificities of eleven human leukocyte antisera produced by planned immunization. Transplantation. 1967 Jul;5(4):597–605. doi: 10.1097/00007890-196707000-00004. [DOI] [PubMed] [Google Scholar]
- Rapaport F. T., Dausset J., Hamburger J., Hume D. M., Dano K., Williams G. M., Milgrom F. Serologic factors in human transplantation. Ann Surg. 1967 Oct;166(4):596–608. doi: 10.1097/00000658-196710000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogentine G. N., Jr, Gerber P. HL-A antigens of human lymphoid cells in long-term tissue culture. Transplantation. 1969 Jul;8(1):28–37. doi: 10.1097/00007890-196907000-00004. [DOI] [PubMed] [Google Scholar]
- Rolley R. T., Williams G. M., Hume D. M. Distribution of leukocyte antigens in human tissues. Surg Forum. 1968;19:241–243. [PubMed] [Google Scholar]
- Shanbrom E., Feingold E., Shepherd L., Walford R. L. Antisera for tissue-typing: the production of cytotoxic and agglutinating antibodies following intradermal leukocyte injections in man. Blood. 1968 Sep;32(3):402–411. [PubMed] [Google Scholar]
- Starzl T. E., Groth C. G., Brettschneider L., Smith G. V., Penn I., Kashiwagi N. Perspectives in organ transplantation. Antibiot Chemother. 1969;15:349–383. doi: 10.1159/000386790. [DOI] [PubMed] [Google Scholar]
- Stickel D. L., Amos D. B., Zmijewski C. M., Glenn J. F., Robinson R. R. Human renal transplantation with donor selection by leukocyte typing. Transplantation. 1967 Jul;5(4 Suppl):1024–1029. [PubMed] [Google Scholar]
- Williams G. M., Rolley R. T., Hume D. M. A comparison of lymphocyte and kidney cell typing in eleven patients. Surg Forum. 1968;19:209–210. [PubMed] [Google Scholar]


