Abstract
First discovered as orphan receptors, liver X receptors (LXRs) were subsequently identified as the nuclear receptor target of the cholesterol metabolites, oxysterols.1 There are 2 LXR receptors encoded by distinct genes: LXRα is most highly expressed in the liver, adipose, kidney, adrenal tissues and macrophages, and LXRβ is ubiquitously expressed. Despite differential tissue distribution, these isoforms have 78% homology in their ligand-binding domain and appear to respond to the same endogenous ligands. Work over the past 10 years has shown that the LXR pathway regulates lipid metabolism and inflammation via both the induction and repression of target genes. Given the importance of cholesterol regulation and inflammation in the development of cardiovascular disease, it is not surprising that activation of the LXR pathway attenuates various mechanisms underlying atherosclerotic plaque development.2 In this minireview we will discuss the impact of the LXR pathway on both cholesterol metabolism and atherosclerosis.
Introduction
LXRs act as “cholesterol sensors”, working in a converse manner to sterol response element binding proteins (SREBPs) to lower cholesterol levels via the increased expression of target genes associated with reverse cholesterol transport, cholesterol conversion to bile acid and intestinal cholesterol absorption. These genes include members of the family of ATP binding cassette (ABC) transporters A1/G1/G5/G8, phospholipid transport protein, apolipoproteins (apo) E/CI/CII/CIV and Cyp7a. In addition, LXRs have been shown to drive fatty acid and triglyceride (TG) synthesis via an upregulation of genes including SREBP1c, fatty acid synthase and acetyl CoA carboxylase, to which the increase in TG levels associated with LXR agonists in vivo has been attributed. Given that raising TG levels could antagonize the otherwise attractive effects of LXR agonists, it was initially unclear whether LXR agonists would be pro- or anti-atherosclerotic in vivo.
Effects of LXR agonists on atherosclerosis
Studies in various models of atherosclerosis have now clearly established that treatment with an LXR agonist results in attenuation of atherosclerosis in vivo (Table 1). Initial studies showed that the synthetic agonist GW3965 inhibited lesion development in both apoE−/− and low density lipoprotein receptor (LDLR)−/− mice.2 Subsequent work has confirmed these findings using a variety of LXR agonists and additional mouse models including the apoE*3 Leiden mouse.3–10 Importantly, the beneficial effect of LXR activation is not sex-specific, as anti-atherosclerotic effects have been observed in both male and female mice. In some studies the attenuation of atherosclerosis was observed in association with a reduction total cholesterol (TC) and/or elevation in high density lipoprotein cholesterol (HDL-C), each associated with reduced cardiovascular risk.2, 3, 6, 8 Studies using the LXR agonists, N,N-dimethyl-3β-hydroxy-cholenamide (DMHCA) or WAY-252623, observed a reduction in atherosclerosis in the absence of effects on SREBP1c and hepatic lipogenesis, while other studies observed an attenuation of atherosclerosis despite an increase TG levels.7, 8, 3 These observations raise the possibility that some of anti-atherosclerotic effects of LXR agonists may be independent of systemic lipid metabolism, and could be attributed to direct actions on the vascular wall.
Table 1.
The effects of LXR agonists/LXR genetic manipulation in mouse models of atherosclerosis
| Treatment | Background, Diet | Findings |
|---|---|---|
| TO9013173 | LDLR−/−, WD | ↓70% lesion area 62% regression of aortic lesion area ↓CD68 and ↑ABCAI mRNA in aortic lesions ↓macrophages and ↑collagen content ↓plasma TC, ↑plasma TG |
| TO9013175 | LDLR−/−, WD | ↓atherosclerosis in innominate artery & RC ↑LC thickness of fibrous cap ↑plasma VLDL-C, TC, TG |
| ATI-8294 | LDLR−/−, WD | ↓atherosclerosis in innominate artery & RC ↑LC thickness of fibrous cap, ↓macrophages no change in plasma TGs, TCs |
| TO9013176 | LDLR−/−, AD | ↓aortic root atherosclerosis ↑plasma HDL-C, lesion ABCAI |
| GW39652 | LDLR−/−, WD | ↓en face atherosclerosis: 53% males; 34% females; ↓35% aortic root lesion area ↓plasma TC |
| GW39652 | apoE−/−, chow | ↓aortic root atherosclerosis: 47% males ↑plasma TG, lesion ABCAI; ↓plasma VLDL-C |
| WAY-2526237 | LDLR−/−, HFHC | ↓en face atherosclerosis No change serum TC, TG, |
| TO90131710 | apoE−/−, HFHC | ↓64.2% en face atherosclerosis 58.3% regression en face atherosclerosis ↑plasma TG, TC, HDL-C levels ↑NPC1, ABCAI in aorta, liver, intestine |
| DMHCA8 | apoE−/−, WD | ↓en face & aortic root lesions ↓plasma TG, TC (females) |
| TO9013179 | apoE*3Leiden, WD, RD | ↓aortic root lesion number, severity (WD) ↓E-selectin, ICAM-1, CD44 (WD) Promotes regression (RD) ↑caspase-3, BAX, CCR7, ABCAI,/GI (RD) |
| Nil12 | LXRαβ−/−, chow | Lipid accumulation in aortic root ↓serum TG, HDL-C, ↑LDL-C |
| Nil13 | LXRα−/−apoE−/−, WD | ↑en face and aortic root lesion area ↑peripheral cholesterol accumulation |
| GW396513 | LXRα−/−apoE−/−, WD | ↓en face and aortic root lesion area ↓peripheral cholesterol accumulation no change in plasma TG levels |
| Nil14 | LXRα−/−LDLR−/−, LXRβ−/−LDLR−/−, WD | ↑en face and aortic root lesion area (LXRα−/−) NS effect on atherosclerosis (LXRβ−/−) |
| T090131714 | LXRα−/−LDLR−/−, LXRβ−/−LDLR−/−, WD | ↓aortic root lesion area in both strains ↓en face atherosclerosis in LXRβ−/−LDLR−/− only |
| LXRα−/−LDLR−/− | LDLR−/−, WD | ↑en face atherosclerosis |
| BMT, LXRα+/+, LDLR−/− BMT14 | LXRα−/−LDLR−/−, WD | ↓en face atherosclerosis |
| LXRαβ−/− BMT16 |
ApoE−/− or LDLR−/− | ↑aortic atherosclerosis (both strains) ↑cholesterol accumulation in isolated macrophages no change in plasma TC |
| LXRαβ−/− BMT T09013173 |
LDLR−/−, WD | ↑aortic lesion area (vehicle) No effect of T0901317 on lesion area |
| Macrophage LXRα Tg17 |
LDLR−/−, SSD | ↓83% lesion area in braciocephalic artery ↑ability to efflux and ↓production of iNOS in isolated macrophages no change in plasma lipids, TC or TG |
ABC, ATP binding cassette transporter; AD, atherogenic; apo, apolipoprotein; CCR, chemokine receptor; HDL-C, high density lipoprotein cholesterol; HFHC, high fat, high cholesterol; ICAM, intracellular adhesion molecule; iNOS, inducible nitric oxide synthase; LC, left coronary related sinus; LPS, lipopolysaccharide; LXR, liver X receptor; NPC1, Niemann pick C1 protein; NS, not significant; RC, right coronary related sinus; RD, regressive cholesterol-depleted diet; SSD, semi-synthetic diet, 0.02% cholesterol; TC, total cholesterol; TG, triglyceride; Tg, transgenic; VLDL-C, very low density lipoprotein cholesterol; WD, western diet;
Indeed, treatment with an LXR agonist was also associated with modulation of the plaque per se in many studies, attenuating inflammatory gene expression and E-selectin, intracellular adhesion molecule (ICAM)-1, interleukins and fibrous cap thickness.2, 9 Interestingly, Levin et al demonstrated that, despite an increase in TG levels, T0901317 was associated with not only a reduction but also a regression of atherosclerotic lesions.3 Similar effects were demonstrated by Dai et al10, 11 who also reported a concomitant increase in Neumann Pick C1 mRNA and protein expression in the aorta, liver and intestine which the authors suggested was responsible for the reduction in atherosclerosis10. Verschuren et al also demonstrated that in addition to mediating athero-protective effects, TO901317 was associated with regression of atherosclerotic plaque, suggesting that LXRs not only attenuate pathways associated with lesion progression but promote modulation of the plaque itself, resulting in a reversal of plaque accumulation.9 This is highly relevant to the clinical setting in which individuals commonly have established lesions prior to presentation for treatment of cardiovascular disease.
Gene deletion studies further support an anti-atherosclerotic role for LXR. Whereas little phenotype was observed upon deletion of either LXRα or LXRβ in wild type mice fed a chow diet for 18 months, deletion of both isoforms together in one study was associated with reduced serum TGs and HDL-C and increased cholesterol content of LDL particles.12 These double knockout mice exhibited lipid accumulation in the aortic root in the subendothelium and in lipid-laden foam cells, demonstrating that even in the absence of pro-atherogenic stimuli, namely elevated dietary cholesterol, the absence of both LXR isoforms results in the initiation of atherosclerosis.
Studies by Bradley et al examined the relative contribution of the LXR isoforms to atherosclerosis in the setting of hypercholesterolemia.13 They demonstrated that LXRα deficiency on an apoE−/− background was associated with accumulation of cholesterol in peripheral tissues and accelerated atherosclerosis both en face and at the aortic root, suggesting that LXRβ is not sufficient to compensate for LXRα deletion in the context of hypercholesterolemia. However, upon activation of LXRβ via administration of GW3965, cholesterol accumulation and atherosclerosis were attenuated without the concomitant increase in plasma TG levels seen in LXR+/+ApoE−/− treated mice. More recently, Bischoff et al performed similar studies on LDLR−/− mice.14 LXRα deletion was associated with an increase in en face and aortic root atherosclerosis, as well as decreased plasma TC and TG. Little effect was seen on these parameters with LXRβ deletion. Upon administration of T0901317, TG were increased in LXRβ but not LXRα mice and no change in plasma TC was seen in either group. Aortic root lesions were reduced by T0901317 in both LXRα−/− and LXRβ−/− strains, however, in contrast to Bradley et al, T0901317 did not attenuate en face atherosclerosis in LXRα−/−LDLR−/− mice. Interestingly, isolated macrophages from LXRα−/− mice in this study demonstrated reduced upregulation of ABCA1 and ABCG1 mRNA expression in response to T0901317 compared to those from LXRβ−/− mice. These findings suggest a particularly important role for LXRα in maintaining cholesterol homeostasis in the setting of hypercholesterolemia.
LXRs and macrophages
Uptake of modified lipids, primarily modified LDL such as oxidized LDL (oxLDL), via scavenger receptors on macrophages is critical to the formation of foam cells. Subsequent accumulation leads to the formation of fatty steaks and ultimately advanced atherosclerotic lesions. It is well established that LXRs antagonize this process by promoting cholesterol efflux via the upregulation of the ABC family transporters resulting in enhanced reverse cholesterol transport.15 Indeed, one would anticipate that enhanced RCT accounts for much of the anti-atherogenic effects observed with LXR agonists. A important role for the macrophage LXR pathway in atherosclerosis susceptibility was established by Tangirala and colleagues who showed that transplantation of bone marrow lacking LXRα and LXRβ expression into apoE−/− and LDLR−/− recipient mice strongly increased lesion development.16 Moreover, isolated LXRαβ null macrophages displayed increased accumulation of cholesterol. The importance of the LXR pathway in macrophages on the development of atherosclerosis is also supported by work demonstrating that overexpression of LXRα in a macrophage-specific manner in LDLR−/− mice was associated with a reduction in atherosclerosis in the absence of changes in plasma lipid levels.17
Levin and colleagues have further reported that TO901317 had no effect on atherosclerotic lesion development in LDLR−/− mice with bone marrow devoid of LXR, suggesting that most of the atherosclerotic protection afforded by LXR agonists are derived from effects on hematopoeitic cells.3 However, T0901317 was only administered for 6 weeks in this study, and thus one might speculate that other effects may have been seen over a longer treatment period. In contrast to these studies, Bischoff et al recently reported that LDLR−/− mice transplanted with LXRα−/−LDLR−/− bone marrow exhibit increased en face atherosclerosis, however, this was not as great as the level of atherosclerosis in global LXRα−/−LDLR−/− mice receiving the same bone marrow, suggesting that LXRα deficiency in extra-hematopoietic cells are also involved in the development of atherosclerosis.14 This was further confirmed by studies that restored LXRα expression in hematopoetic cells via BMT into LXRα−/−LDLR−/− mice. This manipulation attenuated atherosclerosis but not to the level seen in LDLR−/− mice. These studies raise the possibility that LXRs may exert anti-atherogenic effects on cell types other than macrophages critical to the development of atherosclerotic plaques, perhaps including liver, intestine, endothelial cells and smooth muscle cells (see below).
Anti-Inflammatory effects of LXR
LXRs can influence macrophage biology not only via modulation of lipid metabolism but also via effects on innate immunity. The release of cytokines from macrophages results in recruitment of monocytes, cross-talk with T cells, perpetuates cellular activation and further promotes atherosclerotic lesion development.18 The anti-inflammatory effect of LXRs were first described by Joseph and colleagues who demonstrated that LXR activation attenuated E.coli or lipopolysaccharide (LPS)-induced expression of pro-inflammatory molecules, including interleukin (IL)-6, inflammatory nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 in macrophages from wild type but not LXR null mice.19 Interestingly, mice deficient in any of these molecules exhibit increased atherosclerosis, suggesting that the powerful anti-inflammatory effects of LXRs may contribute to their anti-atherosclerotic effects. Mechanistically, the anti-inflammatory effects of LXR have been attributed to nuclear inhibition of NF-kB signaling via a process known as transrepression.20 Subsequent studies demonstrated that LXR also attenuates expression of the NF-kB target gene MMP-9 both in vivo and in vitro.19, 21 MMP-9 has been shown to be localized to macrophage-rich areas within atherosclerotic lesions and is associated with enhanced extracellular matrix degradation, influencing smooth muscle cell migration, neointima formation and plaque instability. The regulation of MMP-9 by LXR is consistent with the observed increase in collagen content in plaques of LXR ligand treated LDLR−/− mice.3
Integration of lipid metabolism and immunity via the LXR pathway was further demonstrated in studies by Castrillo et al who demonstrated that bacterial pathogens such as E.coli and influenza A, which signal via the TLR-3/4 pathway, can downregulate LXR signaling, resulting in reduced ABC transporter expression and efflux of cholesterol, an effect known to exacerbate atherosclerotic lesions formation.22 More recent studies demonstrate that LXRs can also modulate the TLR2/TLR4/MyD88 pathway. 23 C.pneumoniae-induced atherosclerosis, which can be attenuated by TRL2, TLR4 or MyD88 deficiency, was accelerated in the absence of LXRα. Infected LXRα−/−apoE−/− mice exhibited increased atherosclerosis with lesions rich in dendritic cells and cholesterol as well as and higher plasma IL-6 levels compared to LXR+/+apoE−/− mice or uninfected LXRα−/−apoE−/−mice.
Another recent discovery was that LXR can also promote apoptotic cell clearance. Phagocytosis of apoptotic cells results in LXR activation due to cholesterol loading, leading to an increase of the LXR target gene and apoptotic cell receptor Mer.24 LXR activation by apoptotic cells was shown to promote further clearance of apoptotic cells and to concomitantly suppress inflammatory pathways.24 In contrast, LXR null macrophages were defective in their ability to induce Mer expression and phagocytose apoptotic cells, and exhibited an induction of the pro-inflammatory mediators IL-1β and MCP-1. Interestingly, loss of Mer expression and defective apoptotic cell clearance have both previously been linked to accelerated atherosclerosis25, 26. Together, these studies illustrate that LXR regulates a number of immune and inflammatory pathways that have the potential to modulate atherosclerotic lesion development.
LXRs and Endothelial Cells
The importance of the endothelium in the initiation of atherosclerotic plaque development has been well described. Indeed, endothelial cells have been demonstrated to be metabolically active cells capable of responding to the surrounding environment by modulating expression of cell surface receptors and releasing soluble agents that influence the subendothelial layer. The effects of the LXR pathway on the endothelium have been less well studied than other cell types, yet it is possible that they may contribute to the anti-atherosclerotic effects mediated by LXR agonists. Endothelial cells express at least LXRβ, and it has been reported that synthetic LXR agonists can mediate anti-inflammatory and anti-adhesive effects in this tissue. As in other cells, activation of LXR results in upregulation of ABCA1 in the endothelium.27, 28 Conversely, oxLDL, both minimally and extensively modified, swas shown to attenuate ABCA1 expression as well as the production of the endogenous LXR ligand, 27-hydroxycholesterol.29 Interestingly, the expression of LXRs as well as their target genes appear to be differentially expressed throughout the aorta. In the atherosclerotic prone arch, a region of turbulent flow, LXR was found to be expressed 5-fold lower than in the thoracic aorta, a region of laminar flow.30 In vitro studies confirmed a direct upregulation of LXRα and LXRβ as well as their targets, ABCA1, lipoprotein lipase and apoE in response to high, but not low, laminar flow. These studies suggest that in areas of high flow, as seen in healthy arteries, upregulated LXR expression could mediate anti-atherosclerotic effects. Interestingly, laminar shear stress has also been shown to upregulate stearoyl-CoA desaturase (SCD)-1, another LXR target gene and the rate limiting enzyme in the conversion of saturated fatty acids (FA) to monounsaturated FA.31, 32 Accumulation of non-esterified fatty acids is associated with endothelial dysfunction via lipotoxic, apoptotic and pro-inflammatory effects. In human aortic endothelial cells T0901317 was associated with increased SCD-1 expression and attenuated palmitate-induced lipotoxicity, apoptosis and IL-6 and IL-8 expression.33
As mentioned above, LXRs are known not only for their induction of target genes, but also their transrepressive effects. LXRs can mediate anti-inflammatory effects via interference with the TLR pathway, however much of these effects have been characterized in macrophages.19 T0901317 and GW3965 were found to attenuate LPS-induced expression of ICAM-1 and vascular cell adhesion endothelial (VCAM)-1 in human umbilical vein and artery endothelial cells.34 Similar effects were observed in vivo, with administration of T0901317 to ApoE*Leiden mice associated with an attenuation of levels of ICAM as well as E-selectin and CD44 in the vessel wall.9
LXRs and Vascular Smooth Muscle Cells
Smooth muscle cells (SMCs) play a critical role in the vasculature, regulating contractile function. In the setting of vascular disease, SMCs are involved in plaque stabilization, migrating to form a fibrous cap over the plaque, preventing it from rupture. LXRβ and perhaps low levels of LXRα are expressed in human coronary artery smooth muscle cells and limited studies in VSMCs have demonstrated that LXRs can influence proliferation, contractility, apoptosis and calcification.35–37 Blaschke et al demonstrated that the LXR ligand T1317 attenuated VSCM proliferation and that administration of this agent protected against neointima formation following balloon injury.35 Interestingly, angiotensin II (AT) has been shown to promote proliferation as well as vasoconstriction, fibrosis, inflammation and formation of reactive oxygen species and advanced glycation endproducts. Inhibition of this pathway via AT type 1 receptor (AT1R) antagonism is associated with reduced atherosclerotic lesions.38 Both T0901317 and 22(R)-hydroxycholesterol attenuated AT type 1 receptor (AT1R) mRNA and protein, which was associated with a subsequent reduction of downstream signaling.39 Moreover, in Sprague Dawley rats, treatment with GW3965 blunted AT-induced increases in blood pressure in the absence of changes in heart rate. Other effects mediated by AT were not assessed. 25-hydroxycholesterol was shown to upregulate skeletal muscle LIM 1 protein in aortic smooth muscle cells, associated with an increase in α-smooth muscle actin and the cell cycle regulator p27Kip1, suggestive of enhanced differentiation.40 This is in contrast to the abovementioned findings with T1317, however the effect of synthetic LXR agonist and the requirement for LXR expression was not examined, raising the possibility of an LXR-independent mechanism. Finally, LXR ligands, both endogenous and synthetic, have been shown to affect vascular calcification, although whether this may contribute plaque stabilization or promote their rupture remains to be established.37, 41, 42
LXR-Dependent Mechanism for Control of Cholesterol Uptake
As outlined above, the function of LXR in cellular cholesterol efflux and ABC transporter expression has long been appreciated. Recent work has uncovered a novel mechanism by which LXR also modulates cellular cholesterol accumulation.43 Zelcer et al demonstrated that in the setting of high cellular cholesterol LXR induces the expression of an E3 ubiquitin ligase termed Idol (Inducible Degrader of the LDLR). Idol post-translationally modifies LDLR, resulting in its degradation and subsequent attenuation of LDL cholesterol binding and uptake. The LXR-Idol pathway provides a complement to the the SREBP2 pathway which increases LDLR transcription under conditions of low cholesterol to enhance LDL cholesterol uptake. Interestingly, the LXR-Idol pathway appears to operate in many different cell types, including macrophages, hepatocytes and fibroblasts. Similar effects were seen in vivo, with administration of GW3965 associated with upregulation of Idol expression in various tissues including macrophages, spleen and liver. In vitro studies demonstrated that co-transfection of Idol and LDLR was associated with enhanced ubiquitination and degradation of the LDLR via a lysosomal pathway. Adenoviral expression of Idol in wild type mice resulted in elevated plasma LDL cholesterol levels, essentially mimicking the phenotype of the LDLR−/− mice, one of the most common models of atherosclerosis.
Subsequent studies have revelaed that Idol also targets the 2 most closely related members of the LDLR family, VLDLR and ApoER2, for degradaion in a similar manner to that of LDLR.44 Interestingly, the drosophila Idol homolog, DNR-1, was also able to degrade human LDLR indicating that Idol is an evolutionarily conserved mechanism for regulation of lipid uptake. Many questions remain to be answered, including whether there is compensation by the SREBP pathway, how Idol interacts with LDLR and which other proteins are involved in the degradation of LDLR/VLDLR/ApoER2. Future studies will no doubt address many of these issues. Given that LDL carries ~70% of the cholesterol in the plasma, and that elevated LDL cholesterol levels are associated with increased coronary heart disease, the discovery of a new pathway that regulates LDL cholesterol levels may have therapeutic implications as a novel drug target.
Conclusion
The last 10 years has seen major advances in our understanding of LXR biology. Numerous studies have revealed that LXRs lie at the intersection of lipid metabolism, innate immunity and inflammation, all pathways fundamental to the development of atherosclerotic lesions and cardiovascular disease. Future studies will continue to assess whether manipulation of these pathway may have utility in the treatment of cardiovascular disease.
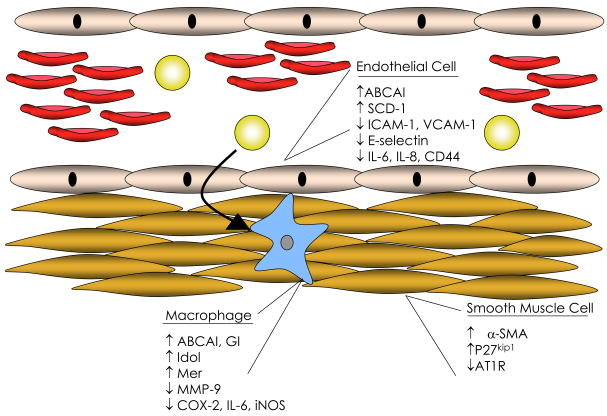
Figure 1. Effects of LXR agonist on cells of the vascular wall.
ABC, ATP binding cassette transport; AT1R, angiotensin II receptor subtype 1; COX, cyclooxygenase; ICAM, intracellular adhesion molecule; Idol, inducible degrader of LDLR; IL, interleukin; iNOS, inducible nitric oxide synthase; MMP, matrix metalloproteinase; SCD, stearoyl-CoA desaturase; SMA, smooth muscle actin; VCAM, vascular cell adhesion molecule;
References
- 1.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 2.Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, Noh G, Goodman J, Hagger GN, Tran J, Tippin TK, Wang X, Lusis AJ, Hsueh WA, Law RE, Collins JL, Willson TM, Tontonoz P. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin N, Bischoff ED, Daige CL, Thomas D, Vu CT, Heyman RA, Tangirala RK, Schulman IG. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler Thromb Vasc Biol. 2005;25:135–142. doi: 10.1161/01.ATV.0000150044.84012.68. [DOI] [PubMed] [Google Scholar]
- 4.Peng D, Hiipakka RA, Dai Q, Guo J, Reardon CA, Getz GS, Liao S. Antiatherosclerotic effects of a novel synthetic tissue-selective steroidal liver X receptor agonist in low-density lipoprotein receptor-deficient mice. J Pharmacol Exp Ther. 2008;327:332–342. doi: 10.1124/jpet.108.142687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng D, Hiipakka RA, Reardon CA, Getz GS, Liao S. Differential anti-atherosclerotic effects in the innominate artery and aortic sinus by the liver X receptor agonist T0901317. Atherosclerosis. 2009;203:59–66. doi: 10.1016/j.atherosclerosis.2008.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terasaka N, Hiroshima A, Koieyama T, Ubukata N, Morikawa Y, Nakai D, Inaba T. T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. FEBS Lett. 2003;536:6–11. doi: 10.1016/s0014-5793(02)03578-0. [DOI] [PubMed] [Google Scholar]
- 7.Quinet EM, Basso MD, Halpern AR, Yates DW, Steffan RJ, Clerin V, Resmini C, Keith JC, Berrodin TJ, Feingold I, Zhong W, Hartman HB, Evans MJ, Gardell SJ, DiBlasio-Smith E, Mounts WM, LaVallie ER, Wrobel J, Nambi P, Vlasuk GP. LXR ligand lowers LDL cholesterol in primates, is lipid neutral in hamster, and reduces atherosclerosis in mouse. J Lipid Res. 2009;50:2358–2370. doi: 10.1194/jlr.M900037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kratzer A, Buchebner M, Pfeifer T, Becker TM, Uray G, Miyazaki M, Miyazaki-Anzai S, Ebner B, Chandak PG, Kadam RS, Calayir E, Rathke N, Ahammer H, Radovic B, Trauner M, Hoefler G, Kompella UB, Fauler G, Levi M, Levak-Frank S, Kostner GM, Kratky D. Synthetic LXR agonist attenuates plaque formation in apoE−/− mice without inducing liver steatosis and hypertriglyceridemia. J Lipid Res. 2009;50:312–326. doi: 10.1194/jlr.M800376-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verschuren L, de Vries-van der Weij J, Zadelaar S, Kleemann R, Kooistra T. LXR agonist suppresses atherosclerotic lesion growth and promotes lesion regression in apoE*3Leiden mice: time course and mechanisms. J Lipid Res. 2009;50:301–311. doi: 10.1194/jlr.M800374-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Dai XY, Ou X, Hao XR, Cao DL, Tang YL, Hu YW, Li XX, Tang CK. The effect of T0901317 on ATP-binding cassette transporter A1 and Niemann-Pick type C1 in apoE−/− mice. J Cardiovasc Pharmacol. 2008;51:467–475. doi: 10.1097/FJC.0b013e31816a5be3. [DOI] [PubMed] [Google Scholar]
- 11.Ou X, Dai X, Long Z, Tang Y, Cao D, Hao X, Hu Y, Li X, Tang C. Liver X receptor agonist T0901317 reduces atherosclerotic lesions in apoE−/− mice by up-regulating NPC1 expression. Sci China C Life Sci. 2008;51:418–429. doi: 10.1007/s11427-008-0054-4. [DOI] [PubMed] [Google Scholar]
- 12.Schuster GU, Parini P, Wang L, Alberti S, Steffensen KR, Hansson GK, Angelin B, Gustafsson JA. Accumulation of foam cells in liver X receptor-deficient mice. Circulation. 2002;106:1147–1153. doi: 10.1161/01.cir.0000026802.79202.96. [DOI] [PubMed] [Google Scholar]
- 13.Bradley MN, Hong C, Chen M, Joseph SB, Wilpitz DC, Wang X, Lusis AJ, Collins A, Hseuh WA, Collins JL, Tangirala RK, Tontonoz P. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J Clin Invest. 2007;117:2337–2346. doi: 10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bischoff ED, Daige CL, Petrowski M, Dedman H, Pattison J, Juliano J, Li AC, Schulman IG. Non-redundant roles for LXRalpha and LXRbeta in atherosclerosis susceptibility in low density lipoprotein receptor knockout mice. J Lipid Res. 2010;51:900–906. doi: 10.1194/jlr.M900096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci U S A. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tangirala RK, Bischoff ED, Joseph SB, Wagner BL, Walczak R, Laffitte BA, Daige CL, Thomas D, Heyman RA, Mangelsdorf DJ, Wang X, Lusis AJ, Tontonoz P, Schulman IG. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci U S A. 2002;99:11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teupser D, Kretzschmar D, Tennert C, Burkhardt R, Wilfert W, Fengler D, Naumann R, Sippel AE, Thiery J. Effect of macrophage overexpression of murine liver X receptor-alpha (LXR-alpha) on atherosclerosis in LDL-receptor deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:2009–2015. doi: 10.1161/ATVBAHA.108.175257. [DOI] [PubMed] [Google Scholar]
- 18.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 19.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 20.Ghisletti S, Huang W, Jepsen K, Benner C, Hardiman G, Rosenfeld MG, Glass CK. Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes Dev. 2009;23:681–693. doi: 10.1101/gad.1773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castrillo A, Joseph SB, Marathe C, Mangelsdorf DJ, Tontonoz P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J Biol Chem. 2003;278:10443–10449. doi: 10.1074/jbc.M213071200. [DOI] [PubMed] [Google Scholar]
- 22.Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003;12:805–816. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- 23.Naiki Y, Sorrentino R, Wong MH, Michelsen KS, Shimada K, Chen S, Yilmaz A, Slepenkin A, Schroder NW, Crother TR, Bulut Y, Doherty TM, Bradley M, Shaposhnik Z, Peterson EM, Tontonoz P, Shah PK, Arditi M. TLR/MyD88 and liver X receptor alpha signaling pathways reciprocally control Chlamydia pneumoniae-induced acceleration of atherosclerosis. J Immunol. 2008;181:7176–7185. doi: 10.4049/jimmunol.181.10.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Diaz M, Gallardo G, de Galarreta CR, Salazar J, Lopez F, Edwards P, Parks J, Andujar M, Tontonoz P, Castrillo A. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ait-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Leseche G, Cohen PL, Tedgui A, Mallat Z. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1429–1431. doi: 10.1161/ATVBAHA.108.169078. [DOI] [PubMed] [Google Scholar]
- 26.Tabas I, Seimon T, Timmins J, Li G, Lim W. Macrophage apoptosis in advanced atherosclerosis. Ann N Y Acad Sci. 2009;1173 (Suppl 1):E40–45. doi: 10.1111/j.1749-6632.2009.04957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norata GD, Ongari M, Uboldi P, Pellegatta F, Catapano AL. Liver X receptor and retinoic X receptor agonists modulate the expression of genes involved in lipid metabolism in human endothelial cells. Int J Mol Med. 2005;16:717–722. [PubMed] [Google Scholar]
- 28.Liao H, Langmann T, Schmitz G, Zhu Y. Native LDL upregulation of ATP-binding cassette transporter-1 in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2002;22:127–132. doi: 10.1161/hq1201.101772. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y, Liao H, Xie X, Yuan Y, Lee TS, Wang N, Wang X, Shyy JY, Stemerman MB. Oxidized LDL downregulates ATP-binding cassette transporter-1 in human vascular endothelial cells via inhibiting liver X receptor (LXR) Cardiovasc Res. 2005;68:425–432. doi: 10.1016/j.cardiores.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Zhu M, Fu Y, Hou Y, Wang N, Guan Y, Tang C, Shyy JY, Zhu Y. Laminar shear stress regulates liver X receptor in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:527–533. doi: 10.1161/ATVBAHA.107.143487. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Hao M, Luo Y, Liang CP, Silver DL, Cheng C, Maxfield FR, Tall AR. Stearoyl-CoA desaturase inhibits ATP-binding cassette transporter A1-mediated cholesterol efflux and modulates membrane domain structure. J Biol Chem. 2003;278:5813–5820. doi: 10.1074/jbc.M208687200. [DOI] [PubMed] [Google Scholar]
- 32.Qin X, Tian J, Zhang P, Fan Y, Chen L, Guan Y, Fu Y, Zhu Y, Chien S, Wang N. Laminar shear stress up-regulates the expression of stearoyl-CoA desaturase-1 in vascular endothelial cells. Cardiovasc Res. 2007;74:506–514. doi: 10.1016/j.cardiores.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peter A, Weigert C, Staiger H, Rittig K, Cegan A, Lutz P, Machicao F, Haring HU, Schleicher E. Induction of stearoyl-CoA desaturase protects human arterial endothelial cells against lipotoxicity. Am J Physiol Endocrinol Metab. 2008;295:E339–349. doi: 10.1152/ajpendo.00022.2008. [DOI] [PubMed] [Google Scholar]
- 34.Morello F, Saglio E, Noghero A, Schiavone D, Williams TA, Verhovez A, Bussolino F, Veglio F, Mulatero P. LXR-activating oxysterols induce the expression of inflammatory markers in endothelial cells through LXR-independent mechanisms. Atherosclerosis. 2009;207:38–44. doi: 10.1016/j.atherosclerosis.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Blaschke F, Leppanen O, Takata Y, Caglayan E, Liu J, Fishbein MC, Kappert K, Nakayama KI, Collins AR, Fleck E, Hsueh WA, Law RE, Bruemmer D. Liver X receptor agonists suppress vascular smooth muscle cell proliferation and inhibit neointima formation in balloon-injured rat carotid arteries. Circ Res. 2004;95:e110–123. doi: 10.1161/01.RES.0000150368.56660.4f. [DOI] [PubMed] [Google Scholar]
- 36.Leik CE, Carson NL, Hennan JK, Basso MD, Liu QY, Crandall DL, Nambi P. GW3965, a synthetic liver X receptor (LXR) agonist, reduces angiotensin II-mediated pressor responses in Sprague-Dawley rats. Br J Pharmacol. 2007;151:450–456. doi: 10.1038/sj.bjp.0707241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu JJ, Lu J, Huang MS, Geng Y, Sage AP, Bradley MN, Tontonoz P, Demer LL, Tintut Y. T0901317, an LXR agonist, augments PKA-induced vascular cell calcification. FEBS Lett. 2009;583:1344–1348. doi: 10.1016/j.febslet.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calkin AC, Giunti S, Sheehy KJ, Chew C, Boolell V, Rajaram YS, Cooper ME, Jandeleit-Dahm KA. The HMG-CoA reductase inhibitor rosuvastatin and the angiotensin receptor antagonist candesartan attenuate atherosclerosis in an apolipoprotein E-deficient mouse model of diabetes via effects on advanced glycation, oxidative stress and inflammation. Diabetologia. 2008;51:1731–1740. doi: 10.1007/s00125-008-1060-6. [DOI] [PubMed] [Google Scholar]
- 39.Imayama I, Ichiki T, Patton D, Inanaga K, Miyazaki R, Ohtsubo H, Tian Q, Yano K, Sunagawa K. Liver X receptor activator downregulates angiotensin II type 1 receptor expression through dephosphorylation of Sp1. Hypertension. 2008;51:1631–1636. doi: 10.1161/HYPERTENSIONAHA.107.106963. [DOI] [PubMed] [Google Scholar]
- 40.Kang MA, Jeoung NH, Kim JY, Lee JE, Jung UJ, Choi MS, Lee WH, Kwon OS, Lee H, Park YB. Up-regulation of skeletal muscle LIM protein 1 gene by 25-hydroxycholesterol may mediate morphological changes of rat aortic smooth muscle cells. Life Sci. 2007;80:460–467. doi: 10.1016/j.lfs.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 41.Saito E, Wachi H, Sato F, Seyama Y. 7-ketocholesterol, a major oxysterol, promotes pi-induced vascular calcification in cultured smooth muscle cells. J Atheroscler Thromb. 2008;15:130–137. doi: 10.5551/jat.e556. [DOI] [PubMed] [Google Scholar]
- 42.Watson KE, Bostrom K, Ravindranath R, Lam T, Norton B, Demer LL. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 1994;93:2106–2113. doi: 10.1172/JCI117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 2009;325:100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong C, Duit S, Jalonen P, Out R, Scheer L, Sorrentino V, Boyadjian R, Rodenburg KC, Foley E, Korhonen L, Lindholm D, Nimpf J, van Berkel TJ, Tontonoz P, Zelcer N. The E3-ubiquitin ligase idol induces the degradation of the low-density lipoprotein receptor family members VLDLR and APOER2. J Biol Chem. 2010 doi: 10.1074/jbc.M110.123729. [DOI] [PMC free article] [PubMed] [Google Scholar]