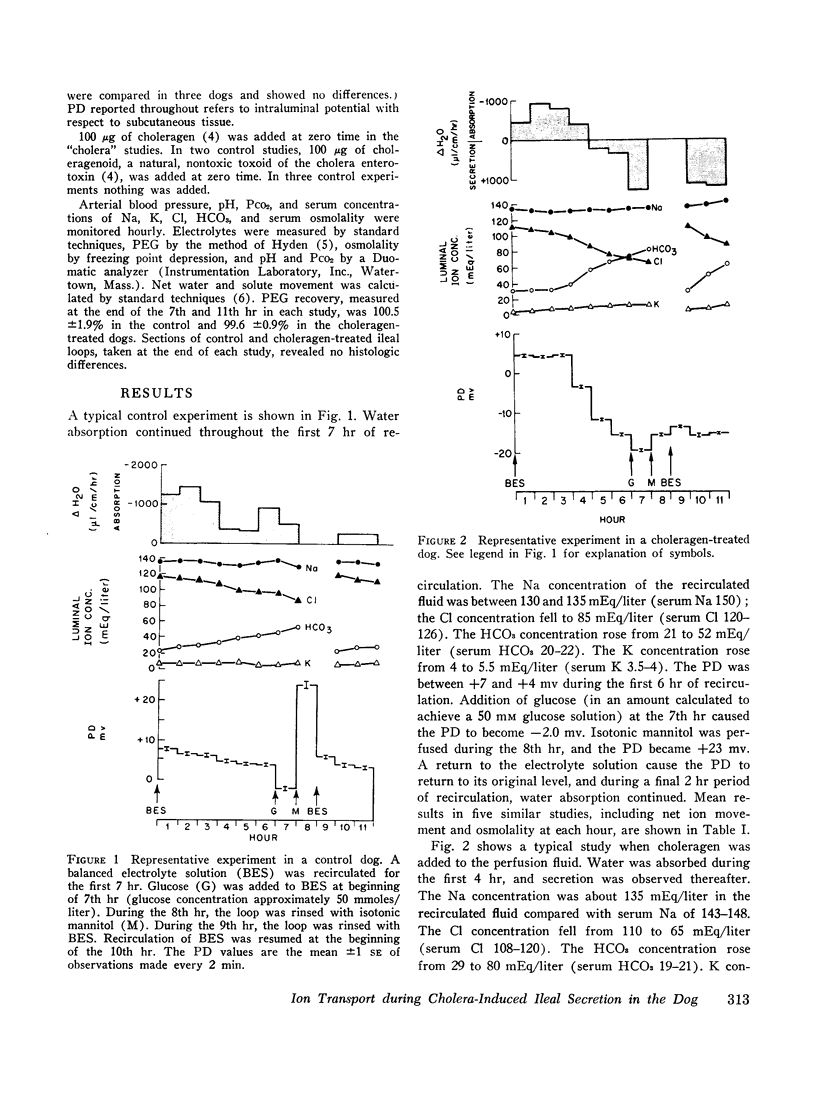
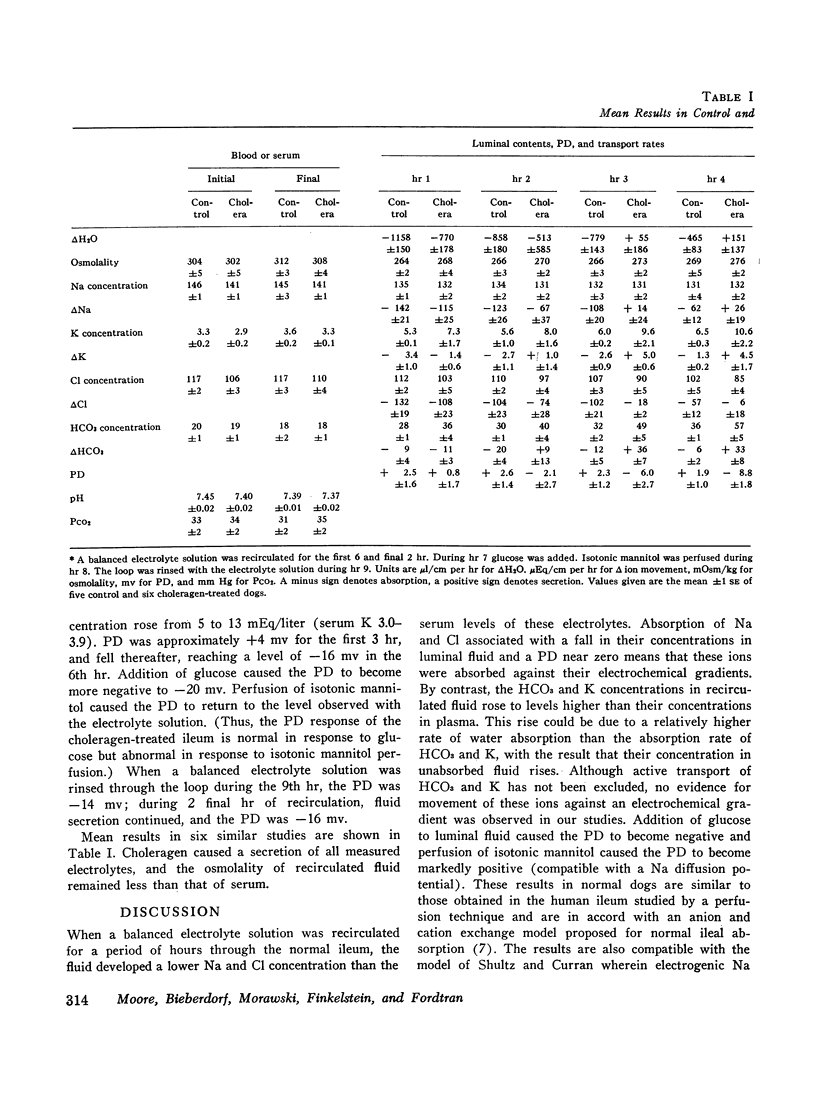
Abstract
To assess the ion transport mechanism by which cholera causes the small bowel to secrete, ion transport rates and electrical potential difference (PD) were determined simultaneously in the normal and choleragen-treated dog ileum in vivo. The results indicate that, during cholera, HCO3 is actively secreted (i.e., against both an electrical and a concentration gradient); Cl is also actively secreted, against a modest electrochemical gradient. Electrogenic pumping of one or both of these anions is probably responsible for an observed PD change of approximately 13 mv (lumen negative). Na secretion can be accounted for entirely by passive ion movement. K secretion can be partly explained by passive diffusion secondary to the negative intraluminal PD; however, its concentration in the secreted fluid is two to three times higher than expected on the basis of passive forces, suggesting a component of active K secretion. The PD response of the choleragen-treated ileum is normal in response to glucose, but there was no PD response to saline-free mannitol perfusion. This suggests that the normal differential permeability of the ileum to anions and cations may be altered by choleragen, although other explanations of this finding are also possible.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter C. C., Greenough W. B., 3rd Response of the canine duodenum to intraluminal challenge with cholera exotoxin. J Clin Invest. 1968 Dec;47(12):2600–2607. doi: 10.1172/JCI105942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. C., Greenough W. B., 3rd, Sack R. B. The relationship of superior mesenteric artery blood flow to gut electrolyte loss in experimental cholera. J Infect Dis. 1969 Feb;119(2):182–193. doi: 10.1093/infdis/119.2.182. [DOI] [PubMed] [Google Scholar]
- Carpenter C. C., Sack R. B., Feeley J. C., Steenberg R. W. Site and characteristics of electrolyte loss and effect of intraluminal glucose in experimental canine cholera. J Clin Invest. 1968 May;47(5):1210–1220. doi: 10.1172/JCI105810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran J. S. Marker perfusion techniques for measuring intestinal absorption in man. Gastroenterology. 1966 Dec;51(6):1089–1093. [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Jr, Carter N. W. The mechanisms of sodium absorption in the human small intestine. J Clin Invest. 1968 Apr;47(4):884–900. doi: 10.1172/JCI105781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran J. S. Speculations on the pathogenesis of diarrhea. Fed Proc. 1967 Sep;26(5):1405–1414. [PubMed] [Google Scholar]
- Hakim A. A., Lifson N. Effects of pressure on water and solute transport by dog intestinal mucosa in vitro. Am J Physiol. 1969 Feb;216(2):276–284. doi: 10.1152/ajplegacy.1969.216.2.276. [DOI] [PubMed] [Google Scholar]
- Iber F. L., McGonagle T., Serebro H. A., Luebbers E., Bayless T. M., Hendrix T. R. Unidirectional sodium flux in small intestine in experimental canine cholera. Am J Med Sci. 1969 Nov;258(5):340–350. doi: 10.1097/00000441-196911000-00005. [DOI] [PubMed] [Google Scholar]
- Patlak C. S., Goldstein D. A., Hoffman J. F. The flow of solute and solvent across a two-membrane system. J Theor Biol. 1963 Nov;5(3):426–442. doi: 10.1016/0022-5193(63)90088-2. [DOI] [PubMed] [Google Scholar]
- Sachar D. B., Taylor J. O., Saha J. R., Phillips R. A. Intestinal transmural electric potential and its response to glucose in acute and convalescent cholera. Gastroenterology. 1969 Mar;56(3):512–521. [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F., Wright E. M. Interpretation of hexose-dependent electrical potential differences in small intestine. Nature. 1967 Apr 29;214(5087):509–510. doi: 10.1038/214509a0. [DOI] [PubMed] [Google Scholar]
- Swallow J. H., Code C. F. Intestinal transmucosal fluxes of bicarbonate. Am J Physiol. 1967 Mar;212(3):717–723. doi: 10.1152/ajplegacy.1967.212.3.717. [DOI] [PubMed] [Google Scholar]
- Turnberg L. A., Bieberdorf F. A., Morawski S. G., Fordtran J. S. Interrelationships of chloride, bicarbonate, sodium, and hydrogen transport in the human ileum. J Clin Invest. 1970 Mar;49(3):557–567. doi: 10.1172/JCI106266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnberg L. A., Fordtran J. S., Carter N. W., Rector F. C., Jr Mechanism of bicarbonate absorption and its relationship to sodium transport in the human jejunum. J Clin Invest. 1970 Mar;49(3):548–556. doi: 10.1172/JCI106265. [DOI] [PMC free article] [PubMed] [Google Scholar]